Recent Articles
-
September 23, 2023 Research Article
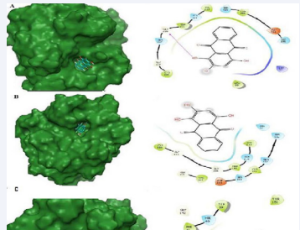
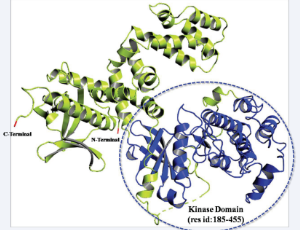 Abstract Human G protein-coupled receptor kinase 2 (GRK2) is emerging as a critical hub in cell signalling cascades and also acts as a pharmaceutical target for treating cardiovascular diseases. Based on sequence similarity, human GRKs are classified into thr.....
Abstract Human G protein-coupled receptor kinase 2 (GRK2) is emerging as a critical hub in cell signalling cascades and also acts as a pharmaceutical target for treating cardiovascular diseases. Based on sequence similarity, human GRKs are classified into thr.....