Evaluation of In vivo Efficacy of Aqueous Leaf Extract of Phyllanthus Niruri in Diabetic Hypertensive Rats
- 1. Department of Pharmacology, St John Institute of Pharmacy and Research, India
- 2. S. N. D. College of Pharmacy, India
- 3. School of Pharmaceutical Sciences, Jaipur National University, India
Abstract
Objective: The present study was aimed to evaluate the effects of aqueous leaf extract of the leaves of Phyllanthus niruri on Streptozocin (STZ) induced diabetes in spontaneously hypertensive rats.
Methods: Diabetes was induced by a single intra peritoneal injection (i.p.) of 55 mg/kg, (in 0.1M citrate buffer, pH 4.5) of STZ in Spontaneously Hypertensive Rats (SHR).The extract (600 mg/kg/day) was administered orally in Diabetic Control (DC), SHR and Diabetic-SHR (D-SHR). Body weight of animals were measured weekly throughout the study (28 days). Blood glucose level and total cholesterol level was measured at day 0 after induction of diabetes and at day 28. Arterial blood pressure of all the groups was measured at day 14 and day 28 by tail cuff method. Serum glutamic oxaloacetic transaminase (SGOT) level in all the control and treated groups were measured at the end of experiment.
Results: A decrease in body weight was observed in the case of STZ diabetic control, SHR and D-SHR groups whereas no loss in the weight of animal is observed in the group treated with the aqueous extract of P. niruri. The study showed that a) aqueous leaf extract of P. niruri prevented attenuation of the blood glucose in all the groups; b) no significant fall in total cholesterol levels; c) significant decrease in Mean Arterial Blood Pressure (MABP) and d) decrease in SGOT level.
Conclusion: The findings of the study support the traditional use of P. niruri for the treatment of diabetes and arterial hypertension, and indicate that it may have a beneficial effect in patients with co-existing diabetic hypertension. The results also signify that aqueous extract of P. niruri protects the organs from injury or damage due to high blood pressure.
Keywords
• Diabetic hypertension
• Phyllanthus niruri
• Spontaneously hypertensive rats
• Streptozotocin
• Mean arterial blood pressure
• Serum glutamic oxaloacetic transaminase
Citation
Bharati D, Rawat S, Sharma P, Srivastava B (2015) Evaluation of In vivo Efficacy of Aqueous Leaf Extract of Phyllanthus Niruri in Diabetic Hypertensive Rats. Ann Clin Exp Hypertension 3(3): 1031.
INTRODUCTION
Worldwide, there has been an increase in the prevalence of diabetes mellitus over the past 40 years In the year 2000, it was approximately 2.8 % and is estimated to grow to 4.4 % by 2030, interpreting the rise from 171 million in 2000 to over 350 million in 2030 [1]. Obesity is regarded as one of the factors contributing to the development of diabetes and is increasing day by day in children [2]. Additionally, there is considerable evidence for an increased prevalence of hypertension in diabetic persons [3]. Both hypertension and diabetes predisposes the development of cardiovascular disease (CVD) and renal disease [3,4].The risk of death due to cardiovascular events in persons with type 2 diabetes is three times higher than in the general population. The risks of coronary heart disease, stroke, nephropathy and retinopathy increases substantially by approximately 75%, when hypertension diabetes co-exists in patients. This further contributes to the overall morbidity and mortality of high risk population [4,5]. The substantial overlap between diabetes and hypertension reflects in their etiology and disease mechanisms. Only 42 % of people with diabetes had normal blood pressure and only 56 % of people with hypertension had normal glucose tolerance [6]. In the US population, hypertension occurs in approximately 30 % of patients with type 1 diabetes and in 50 % to 80 % of patients with type 2 diabetes. People with hypertension have almost 2.5 times higher chances of development of type 2 diabetes mellitus compared with peoples with normal blood pressure. The presence of type 2 diabetes mellitus, obesity or any additional CV risk factors leads to complications in the management of hypertension [6].
Since from ages, plants have been the main source of medicines for human beings. Plants contain various phytochemicals which can play an important role in reducing occurrence of several diseases by: i) boosting up various organ functions, ii) acting as antioxidants and iii) supplying necessary nutrients. Numerous traditional healing herbs and their parts have been shown to have medicinal value and can be used to prevent, alleviate or cure several human diseases [7]. It is estimated that 70–80 % of people worldwide rely chiefly on traditional, largely herbal medicine to meet their primary healthcare needs [8,9].There are reports, explaining the effectiveness of plant sterols and stanols for lowering cholesterol and as such, reducing the chances for heart disorders [10]. For cardiovascular diseases plant or herbal treatments have been used in patients with congestive heart failure, systolic hypertension, angina pectoris, atherosclerosis, cerebral insufficiency and venous insufficiency. A major portion of the global population in developing countries still relies on botanical drugs to meet its healthcare needs. The attention paid by health authorities to the use of herbal medicines has increased considerably, both because they are often the only medicine available in less developed areas and because they are becoming a popular alternative treatment in more developed areas. It has also been observed that a number of modern drugs have been derived from plants used by the indigenous people [11].
Phyllanthus niruri L., Phyllanthaceae, is a little shrub found both in tropical and subtropical countries. Several species from this genus are used worldwide in folk medicine for diverse therapeutic purposes. Plant has a long history in herbal medicine systems such as Indian Ayurveda and Chinese Medicine. The whole plant is used as a remedy for several conditions including dysentery, influenza, vaginitis, tumours, diabetes, diuretics, jaundice, kidney stones and dyspepsia. The plant is also useful for treating hepatotoxicity, hepatitis B, hyperglycemia and viral and bacterial diseases [12]. P. niruri has been used in Ayurvedic medicine for over 2000 years and has a wide number of traditional uses for jaundice, gonorrhoea; frequent menstruation and also to cure kidney disorders, hepatitis and diabetes [13-15]. In India, it is a common household remedy for asthma, bronchitis, coughs, extreme thirst, anaemia, jaundice and tuberculosis [16]. Various active constituents to which the biological activity of P. niruri has been attributed include lignans, tannins, coumarins, terpenes, flavonoids, alkaloids, saponins and phenylpropanoids. These active constituents along with common lipids, sterols and flavonols are mainly found in the leaves, stem and roots of the plant [17]. These all phytochemicals present in P. niruri exert different pharmacological actions, for example, a) hepatoprotective [17,18] and anti-viral properties by lignans [19] b) anti-microbial activity by terpenes [20] c) anti-oxidant [21], antileishmanial [22], and anti-inflammatory activities by flavonoids [23], d) antispasmodic activity, [24] smooth muscle relaxation effect by alkaloid [25] e) aldose reductase inhibitory (ARI) activity [26] by glycosides (quercitrin and geraniin). In recent studies, the protein fraction of P. niruri demonstrated protection of liver tissues against oxidative stress in mice [28,29].
Although, P. niruri has found to have beneficial effects in the management of diabetes and hypertension as a traditional remedy, there are very few systematic pharmacological investigations on its aqueous leaf extract. In addition, organ protecting effect of leaves of P. niruri needs to be evaluated. Such a kind of investigations will allow medical practitioners to promote use of P. niruri for simultaneous management of diabetes and associated hypertension (diabetic hypertension). This will eventually reduce the treatment cost and patient compliance as there will be no need of administering two different medications, one for diabetes and the other for hypertension.
Therefore, the present study was focused on investigating the effect of aqueous leaf extract of P. niruri, on arterial blood pressure, blood glucose level and total cholesterol and SGOT level in Diabetic Spontaneously Hypertensive Rats (D-SHR).
MATERIALS AND METHODS
Plant material
The fresh aerial parts of leaves of Phyllanthus niruri were collected from outskirt of Palghar, Maharashtra, India and authenticated by Agharkar Research Institute, Pune, India under the number WP-116/auth. 14-106. The animal usage protocol was approved by the Institutional Animal Ethical Committee (IAEC) for National Toxicology Centre (Protocol No.38 on 15/09/2014) and executed by following the Committee for the Purpose of Control and Supervision of Experiments in Animals (CPCSEA) guidelines.
Preparation of the aqueous extract
The fresh leaves of Phyllanthus niruri were dried under shade and coarsely powdered. Powdered material was subjected to continuous hot percolation (soxhlation) with distilled water. After the exhaustive extraction, the solvent was removed and the crude material obtained was dried in vacuum desiccator.
Animals
The Male Wistar rats and Spontaneous hypertensive rats (SHR) weighing 200-250 grams were procured from in-house animal facility of National Toxicology Centre, Pune. They were housed under standard conditions of temperature and relative humidity with 12 h light/dark cycle. Animals were fed on standard commercial pellet diet and water ad libitum.
Acute oral toxicity study
The Acute oral toxicity test of the extracts was determined prior to the experimentation on animals according to the Organization for Economic Co-operation and Development (OECD) guideline number 423. Female Swiss Albino mice (18- 20 g) were taken for the study and dosed once with 2000 mg/kg of the extracts. The treated animals were monitored for 14 days [30], for general clinical signs and symptoms as well as mortality. No mortality was observed till the end of the study revealing the 2000 mg/kg dose to be safe. Thus, 1/8 th doses of 2000 mg/kg i.e. 600 mg/kg were chosen for subsequent experimentation.
Induction of diabetes
The Male Wistar rats and SHR were kept on fasting overnight and Streptozotocin (Sigma-Aldrich Ltd, Mumbai, India), 55 mg/kg, in 0.1M citrate buffer, pH 4.5 was administered intraperitoneally (i.p.) for the induction of diabetes [31]. The animals were bled through the retro orbital plexus and blood was collected in heparinised tube. The plasma glucose was measured in a biochemical analyser (SFRI BSA-3000 Chemistry analyser) by Glucose oxidase-peroxidase (GOD/POD) method. The rats that developed more than 250 mg/dl of plasma glucose [32] on the 3rd day of induction were selected for the study.
Animal experimentation
In the present study the animals were distributed into eight groups containing six animals each (n=6) in the following manner:
Group I (NC): Normal Control (0.1 M citrate buffer (pH 4.5)).
Group II (NC-AEPN): Normal Control rats treated aqueous extract of P. niruri (600mg/kg/day, p.o.,)
Group III (DC): Diabetic Control (STZ injected rats)
Group IV: D+AEPN (Diabetic rats + aqueous extract of P. niruri 600mg/kg/day, p.o.,).
Group V: SHR
Group VI: SHR+AEPN (Spontaneous Hypertensive rats + aqueous extract of P. niruri 600mg/kg/day, p.o.,).
Group VII: D+SHR (Diabetic Hypertensive rats)
Group VIII: D+SHR+ AEPN (Diabetic Hypertensive rats + aqueous extract of P. niruri 600mg/kg/day, p.o.,).
The study was conducted for 28 days to evaluate the potential of the extracts to lower blood glucose level and mean arterial blood pressure. The body weights of the rats were monitored weekly during the study period.
Biochemical examination
During 28 days study, blood was withdrawn at an interval of 7 days from the retro orbital plexus under anesthesia of the fasted animals. Blood glucose levels were then estimated by GOD/POD method in an auto analyzer using the commercial enzyme estimation kit (Coral Clinical Systems, Crest Biosynthesis, India). Besides this, parameters like total cholesterol, SGOT were monitored by using commercial kit (Coral Clinical Systems, Crest Biosynthesis, India) [33].
Blood pressure measurement
Animals were anesthetized with Ketamine/Xylazine (1:1). Mean arterial blood pressure (MABP) was recorded by tail-cuff method (ADInstruments ML125 NIBP Power Lab), at 14th day and 28th day up to end of the study and expressed as mm Hg.
Histopathology
At the end of the study animals were dissected out and the organs were fixed in 10% formalin for monitoring necropsy or adverse effect of the treatment. The tissues were processed for histopathology starting from trimming, graded alcohol dehydration, embedding in paraffin, sectioning, spreading, fixing in the slides and staining with hematoxylin and eosin. The slides were seen under the microscopy (200 X) for abnormalities and photomicrographs were taken [33].
Statistical analysis
Data were expressed as mean ± SEM of six observations. Statistical analysis was done using one-way analysis of variance followed by post-hoc test, Bonferrani’s test by using Graph pad Prism 5 version. Statistical significance was considered at p < 0.05.
RESULTS
Effect of aqueous extract P. niruri on the body weight of normal, diabetic, SHR and diabetic
SHR rats: Table 1 illustrates the effect of aqueous extract of P. niruri on body weights of severe diabetic control, SHR and D-SHR groups. There was a significant reduction (p < 0.05) in the animal body weight of D-NC and D-SHR compared to the vehicletreated control and SHR groups. As shown in Table 1, daily oral treatment with aqueous extract of P. niruri (600mg/kg) caused dose- and time-related weight gaining effect on the treated rats with the most pronounced and significant (p < 0.05) effect seen in the groups treated with aqueous extract of P. niruri (D + AEPN, SHR + AEPN, D-SHR + AEPN) when compared with the untreated groups (NC, DC, SHR) during the study period.
Effect of aqueous extract of P. niruri on blood glucose and total cholesterol level
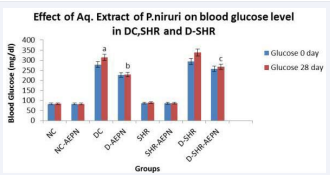
The effect of repeated oral administration of aqueous extract P. niruri on blood glucose and total cholesterol is shown in Table 2. In normal control rats, blood glucose levels observed at 0 and 28 days after treatment with vehicle was almost similar to that of pre-treatment levels. In diabetic control rats there was gradual increase in blood glucose in 14 days and reached its peak at 28th day. However, administration of the most effective dose (600 mg/ kg body weight) of the extract produced a hypoglycaemic effect in treated D + AEPN, D-SHR + AEPN groups. The blood glucose level dropped from 314.5 ± 7.375 mg/dl to 228.2 ± 6.96 mg/dl and from 338.3 ± 8.887 mg/dl to 266.7 ± 8.53 mg/dl after 28 days treatment in D + AEPN and D - SHR + AEPN group respectively (Figure 1).
Figure 1 Effect of aqueous extract of P. niruri on blood glucose level at day 0 and day 28 in DC, SHR and D-SHR. Data were analysed by one way ANOVA followed by Bonferrani’s test. Values are represented as mean ± S.E.M. (n=6); a Value significantly different from NC, (p < 0.05); b Value significantly different from DC (p < 0.05); c Value significantly different from D-SHR, (p < 0.05);
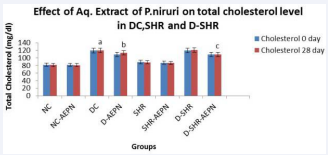
The post-treatment levels of total cholesterol were not significantly lesser than the pre-treatment levels. A fall to 113.3 ± 1.43 mg/dl from 120.3±0.5578 mg/dl in D + AEPN, and to 109.3±3.18 mg/dl from 121.3±1.022 mg/dl in D-SHR + AEPN total cholesterol levels was observed after 28 days of extract treatment (Figure 2). In SHR, blood glucose levels and total cholesterol levels observed at 0 and 28 days after treatment with aqueous extract of P. niruri was almost similar to that of pretreatment levels (p > 0.05).
Figure 2 Effect of aqueous extract of P. niruri on total cholesterol level at day 0 and day 28 in DC, SHR and D-SHR. Data were analysed by one way ANOVA followed by Bonferrani’s test. Values are represented as mean ± S.E.M. (n=6); a Value significantly different from NC, (p < 0.05); b Value significantly different from DC, (p < 0.05); c Value significantly different from D-SHR, (p < 0.05)
Effect of aqueous extract of P. niruri on mean arterial blood pressure
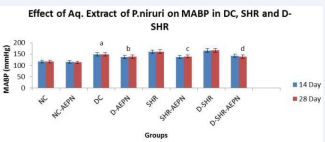
Mean arterial blood pressure was reduced from 160.2 ± 2.386 mmHg to 137.5 ± 3.138 mmHg within 14 days and 161.2 ± 2.120 mmHg to 139.5±1.839 mmHg within 28 days in SHR group treated with aqueous extract P. niruri (600 gm/kg, p.o.) (Table 3, Figure 3).
Figure 3 The effect of aqueous extract of P. niruri treatment on MABP in DC, SHR and D-SHR; after 14 days and 28 days treatment. Data were analysed by one way ANOVA followed by Bonferrani’s test. Values are represented as mean ± S.E.M. (n=6); a Value significantly different from NC, (p < 0.05); b Value significantly different from DC, (p < 0.05); c Value significantly different from SHR, (p < 0.05); d Value significantly different from D-SHR, (p < 0.05); after 14 days and 28 days treatment of aqueous extract P. niruri.
Treatment with aqueous extract P. niruri significantly reduced (p < 0.05) MABP in D + AEPN from 148.3 ± 2.603 mmHg to 137.0 ± 1.506 mmHg within 14 days and 148.7 ± 2.108 mmHg to 138.8 ± 2.774 mmHg within 28 days.
Induction of diabetes in SHR increased MABP to 161.2 ± 2.120 mmHg. Treatment with aqueous extract P. niruri significantly reduced (p < 0.05) MABP in D-SHR from 165.8 ±1.579 mmHg to 142.7 ± 4.169 mmHg within 14 days and 166.5 ± 1.875 mmHg to 138.88 ± 1.990 mmHg within 28 days.
These results showed that the pressure response to aqueous extract P. niruri is significant in hypertensive animals than normotensive, characterizing the antihypertensive activity of aqueous P. niruri extract. These results suggest that the extract shows fall in blood pressure in diabetic hypertensive animals.
Effect of aqueous extract of P. niruri on SGOT after 28 days treatment
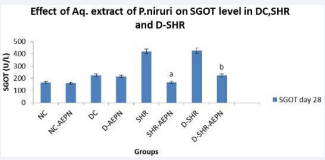
Figure 4 Effect of aqueous extract of P. niruri on SGOT after 28 days treatment in DC, SHR and D-SHR. Data were analysed by one way ANOVA followed by Bonferrani’s test. Values are represented as mean ± S.E.M. (n=6); a Value significantly different from SHR, (P < 0.05); b Value significantly different from D-SHR, (P < 0.05); after 28 days treatment of aqueous extract P. niruri.
The effect of repeated oral administration of aqueous extract P. niruri on SGOT in severe diabetic, SHR and D - SHR is presented in Figure 4. Serum glutamic oxaloacetic (SGOT) level decreased dynamically from 418.7 ± 3.461 U/L to 166.8 ± 8.05 U/L in SHR + AEPN and from 425.7 ± 6.505 U/L to 224.8 ± 1.74 U/L in D – SHR - AEPN, (p < 0.05) after challenge with aqueous extract P. niruri (600 gm/kg, p.o.) for 28 days. SGOT level reached peak during the study in SHR and D-SHR group.
Table 1: Effect of aqueous extract of P. niruri on animal body weight in DC, SHR and D-SHR.
| Body Weight (grams) | |||
| Groups studied | 0 Day | 14 Day | 28 Day |
| NC | 217.5 ± 8.160 | 214.0 ± 6.835 | 229.6 ± 3.527 |
| NC + AEPN 2 | 217.5 ± 8.409 | 34.8 ± 4.214 a | 238.3 ± 4.394a |
| D - NC | 214.0 ± 6.835 | 193.3 ± 6.400 b | 196.2 ± 4.498b |
| D + AEPN | 250.7 ± 8.077 | 255.8 ± 4.126 a | 264.6 ± 6.554a |
| SHR | 233.7 ± 21.5 | 217.8 ± 21.3 b | 216.6 ± 23.8b |
| SHR + AEPN | 240.8 ± 9.031 | 253.3 ± 8.636 a | 276.8 ± 20.45a |
| D - SHR | 241.5 ± 15.64 | 202.5 ± 34.26 b | 194.2 ± 46.21b |
| D – SHR + AEPN | 246.2 ± 8.784 | 243.0 ± 5.550 a | 258.0 ± 11.91a |
| Data were analysed by one way ANOVA followed by Bonferrani’s test. Values are represented as mean ± S.E.M. (n=6); a represents a significant increase at p < 0.05 when compared to values on day 0, while b represent significant decreases at p < 0.05, when compared to values on day 0. | |||
Table 2: Effect of aqueous extract of P. niruri on blood glucose and total cholesterol in DC, SHR and D-SHR.
| 0 Day | 28 Day | |||
| Groups | Glucose | Cholesterol | Glucose | Cholesterol |
| Studied | (mg/dl) | (mg/dl) | (mg/dl) | (mg/dl) |
| NC | 82.17±1.24 | 82.33±1.80 | 83.00±1.36 | 81.50±1.64 |
| NC + AEPN | 81.83±1.30 | 81.67±1.11 | 82.67±1.20 | 81.00±1.41 |
| DC | 278.5±1.17 | 119.5±0.88 | 314.5±7.3a | 120.3±0.55a |
| D + AEPN | 227.3±4.40 | 109.3±2.86 | 228.2±6.96b | 113.3±1.43b |
| SHR | 86.33±2.01 | 89.17±1.47 | 89.50±4.02 | 88.50±1.14 |
| SHR + AEPN | 86.00±0.68 | 87.50±0.99 | 86.17±0.71 | 86.83±0.87 |
| D - SHR | 293.7±3.87 | 119.8±1.24 | 338.3±8.88 | 121.3±1.02 |
| D-SHR + | ||||
| AEPN | 257.8±0.872 | 109.5±2.75 | 266.7±8.53c | 109.3±3.18c |
| Data were analysed by one way ANOVA followed by Bonferrani’s test. Values are represented as mean±S.E.M. (n=6); a Value significantly different from NC, (p < 0.05); b Value significantly different from DC, (p< 0.05); c Value significantly different from D-SHR, (p < 0.05); | ||||
Table 3: Effect of aqueous extract of P. niruri on mean arterial blood pressure in DC, SHR and D-SHR.
| Mean Arterial Blood Pressure (mmHg) | ||
| Groups studied | 14 Day | 28 Day |
| NC | 116.8±1.249 | 117.2±1.352 |
| NC + AEPN | 115.5±1.408 | 113.2±1.558 |
| DC | 148.3±2.603a | 148.7±2.108a |
| D + AEPN | 137.0±1.506b | 138.8±2.774b |
| SHR | 160.2±2.386 | 161.2±2.120 |
| SHR + AEPN | 137.5±3.138c | 139.5±1.839c |
| D - SHR | 165.8±1.579 | 166.5±1.875 |
| D – SHR + AEPN | 142.7±4.169d | 138.88±1.990d |
| Data were analysed by one way ANOVA followed by Bonferrani’s test. Values are represented as mean ± S.E.M. (n=6); a Value significantly different from NC, (p < 0.05); b Value significantly different from DC, (p < 0.05); c Value significantly different from SHR, (p < 0.05); d Value significantly different from D-SHR, (p < 0.05); after 14 days and 28 days treatment of aqueous extract P. niruri. | ||
DISCUSSION
The results obtained through this study showed decrease in body weight in the case of D-NC, SHR and D-SHR control groups while in the group treated with the aqueous extract of P. niruri (600mg/kg), weight loss was reversed. The ability of the aqueous extract to protect body weight loss seems to be as a result of its ability to reduce hyperglycemia as well as high blood pressure. The decrease in plasma glucose and total cholesterol levels was also observed in curative treatment with the plant extract of P. niruri at 600 mg/kg dose. The hypoglycaemic studies of the diabetic animals revealed a maximum fall of 27.45 % in blood glucose level whereas, diabetic SHR animals showed a maximum fall of 21.17 % in blood glucose after 28 days treatment of aqueous extract of P. niruri using the dose of 600 mg/kg body weight. Moreover, no significant fall (p < 0.05) in total cholesterol level in diabetic and diabetic SHR groups was noted when treated with the aqueous extract of P. niruri for 28 days.
The study showed that P. niruri prevents attenuation of the blood glucose and total cholesterol levels induced by the STZ. Further studies will be beneficial to explain the effect of the extract on the metabolic changes.
Results from present study have shown that MABP was significantly elevated in DC by about 30 % compared to nondiabetic control group whereas it was very slightly increased in D-SHR by about 3 % compared to non-diabetic SHR. The increase in MABP following induction of diabetes in rats was in good agreement with a previous study [34]. Common observations in the clinic are diabetes patients generally have higher MABP than non-diabetic patients [35], but it is not clear why not in diabetic SHR. It may reflect the presence of a compensatory response that opposes elevation of MABP in the underlying state of hypertension following induction of diabetes but clearly needs further study. Systolic blood pressure measured by tail cuff method in STZ induced diabetes in SHR treated with aqueous extract of P. niruri exhibited significant decrease in MABP compared with diabetic control, SHR and D-SHR. Decline in mean arterial blood pressure in diabetic rats treated with aqueous extract of P. niruri was 6.66 % which is not significant (p < 0.05). MABP was significantly decreased (p < 0.05) by about 13.47 % in aqueous extract of P. niruri (600 mg/kg body weight) treated group compared with SHR group, 20.85 % in aqueous extract of P. niruri treated group compared with D-SHR group. SGOT is an enzyme found mainly in heart muscle, liver cells, skeletal muscle and kidneys. Injury to these tissues results in the release of the enzyme in blood stream. Elevated levels are found in myocardial infarction, cardiac operations, hepatitis, cirrhosis, acute pancreatitis, acute renal diseases, and primary muscle diseases. In present study, administrations of aqueous extract of P. niruri shows significant (p < 0.05) decrease in SGOT level in diabetic and hypertensive animals. This result signifies that aqueous extract of P. niruri protects the organs from injury or damage due to high blood pressure or myocardial infarction, cardiac operations, primary muscle diseases.
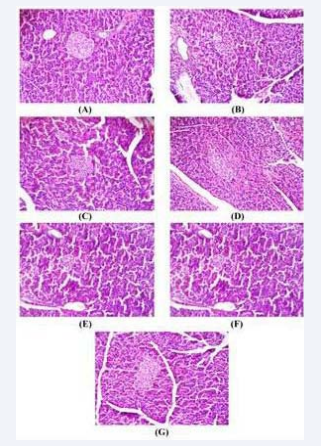
The histological investigations of the pancreatic tissue (Figure 5A) showed that islet cells were normal in their architecture in control group whereas STZ treated group showed reduction in the number and size of islet cells in the pancreas of the diabetic rats (Figure 5B). Similarly spontaneous hypertensive rats moderate changes in size of islets and mild necrosis was observed (Figure 5D). Damaged islets cells and reduced islet size in diabetic hypertensive rats was noted in D+ SHR group (Figure 5F). While figure 5C, 5E and 5G suggests that aqueous extract of P. niruri might have a protective or regenerative property on pancreatic tissue as indicated by the presence of larger and prominent islets cells at the end of 28 days treatment of the diabetic rats, SHR and D-SHR respectively.
Figure 5 Histopathological photomicrographs of pancreas. Histopathological photomicrographs of pancreas in adult rats at 200X magnification and stained with Hematoxylin and Eosin. (A) Normal rat showing normal acini and normal cellular population in islets of Langerhan’s (B) Diabetic control rat showing damaged islets and reduced islet size. (C) Diabetic rats treated with aqueous extract of P. niruri (600 mg/kg) showed almost normal sized islet cells. (D) SHR showed moderate changes in size of islets and mild necrotic changes (E) aqueous extract of P. niruri (600 mg/kg) treated SHR showed normal sized islet cells (F) Diabetic hypertensive rats (D+ SHR) showing damaged islets and reduced islet size. (G) Aqueous extract treated diabetic hypertensive rats (D+ SHR+AEPN) rats at 200 mg/kg showed many large islets of Langerhan’s
Altogether the results obtained in vivo indicated that oral treatment with aqueous extract of P. niruri decrease blood pressure of diabetic and hypertensive rats. The hypotension can be correlated to as indicated by prevention of abnormal vascular reactivity to constrictor and dilator stimuli in the vasculature, reduced MABP. One important observation was the aqueous extract of P. niruri did not interfere with the animal growth throughout the study. This is a positive sign of biosafety of the extract to treat diabetic hypertension without affecting the normal body functions.
The presences of glycoside, flavonoid, saponins, tannin, sterol and carbohydrate in aqueous extract of P. niruri, which are known to be bioactive for the management of hypertension and diabetes, may be responsible for its action. It is well known that certain flavonoids exhibit hypoglycaemic activity and is also known for their ability of beta cell regeneration of pancreas. Sterols have also shown to decrease blood sugar in experimental animal models. The cardiac glycosides have also been proved for its cardio-protective and cardio tonic activity thus, the significant antihypertensive effect of aqueous extract of P. niruri may be due to the presence of more than one antihypertensive principle and their synergistic properties.
To evaluate fast and short-lasting effect of the isolated compounds, new pharmacodynamic and pharmacokinetic studies of the plant chemical constituents should be developed in contrast with the delayed onset of the hypotension and the slow washout after oral administration.
CONCLUSION
In conclusion, the aqueous extract of P. niruri exhibited hypoglycaemic and anti-hypertensive activity in diabetic hypertensive animals. Thus the scientific validation for the traditional claims for anti-hypertensive use of plants may be justified. Further studies are needed to explore the possible mechanism of claimed actions of these plants.
ACKNOWLEDGEMENTS
Authors are thankful to National Toxicological Centre, Pune, India for animal studies, Mr. Albert
W. D’souza, Chairman, Aldel Education Trust and Mr. Thomas Lobo, Campus Director, St. John
Educational Campus for their motivation and support. Dr. Savita J. Tauro, Principal, St John Institute of Pharmacy and Research and Dr. Rahul Kalhapure, University of KwaZulu-Natal, Durban, South Africa are acknowledged for proof reading the manuscript.
REFERENCES
7. Dhar U, Rawal RS, Samant SS, Airi S, Upreti J. People’s participation in Himalayan biodiversity conservation: a practical approach. Current Sci. 1999; 76: 36–40.
8. Farnsworth NR, Soejarto DD. Global importance of medicinal plants. The observation of medicinal plants. Cambridge University Press, Cambridge. 1991; 25–51.
11. Balick JM, Cox PA. Plants, People and Culture: the Science of Ethnobotany, Scientific American Library, New York. 1996; 228.
12. Chopra RN, Nayar SL, Chopra IC. Glosssary of Indian medicinal plants. Catholic Press, Ranchi, CSIR, New Delhi, India. 1986.
25. Kitisin T, Reinli K, Block G. Pharmacological studies Phyllanthus niruri. Sirriaj. Hosp. Gaz. 1952; 4: 641–649.
27. Dixit SP, Achar MP, Thabrew MR. Phyllanthus niruri (Bhumyamalaki) and jaundice in children. J. Natl Integ. Med. Ass. 1982; 25: 269–272.