Effects of Hydration of Geosynthetic Clay Liners (GCLs) with Groundwater on their Hydraulic Performance
- 1. Department of Civil and Environmental Engineering, Florida A&M University, USA
- 2. Wood Environmental and Infrastructure Solutions, USA
ABSTRACT
A detailed study was performed to gain knowledge on prehydration of commercially available geosynthetic clay liners (GCLs), and how their hydraulic conductivities are affected by Florida groundwater chemistry. Two groundwater chemistries were used in the study, the chemistry of the groundwater used span the low ionic strength (GW2), and high ionic strength (GW1), of the groundwater data available in literature. Six commercially available GCLs were used in the study. Three of the GCLs were conventional and contained only granular sodium bentonite with different bentonite gradation, while three of the GCLs were polymer-modified and contained granular sodium bentonite that has been dry blended with different quantities of proprietary polymer(s). The polymer modified GCLs contain linear polymer (water-soluble polymer). Our data revealed that fine grained GCL achieved lower water contents than coarse grained GCL. The polymer modified GCLs showed increase in water uptake over conventional bentonite counterparts (with similar bentonite and geotextiles). The polymer modified GCLs with 3.2 % polymer loading reached higher water content than GCLs with 1.6% polymer loading. Prehydration of the GCLs effectively aided the hydraulic performances when prehydrated with tap water, GW2 and GW1 and permeated with municipal solid waste leachate. When permeated with co-disposal landfill leachates and ash monofil leachates after hydration, the hydraulic conductivity of all GCLs increased.
KEYWORDS
Groundwater; Leachate; Prehydration; Bentonite; Polymer modified GCL; Hydraulic performance.
CITATION
BARCLAY C, WIREKO C, ABICHOU T (2022) Effects of Hydration of Geosynthetic Clay Liners (GCLs) with Groundwater on their Hydraulic Performance. Chem Eng Process Tech 7(1): 1066.
INTRODUCTION
Over the past two decades, geosynthetic clay liners (GCLs), have gained popularity as an adequate substitute in cover and composite bottom liner landfill applications. GCLs are also used as protection barriers in transportation facilities, single liners in canals and ponds [1]. GCLs are made of a thin layer of bentonite sandwiched between two geosynthetics which are usually geotextiles. Unlike compacted clay liners which are at least 150 mm thick, GCLs are very thin, about 5-10 mm thick [1,2], and are manufactured by mass production which produces large rolls of GCLs in each batch. The bentonite sandwiched between the top and bottom geotextiles or geomembranes are bonded by adhesives, stitch bonding or needle punching [1,3,4]. GCLs can consist of either granular or powdered bentonite and the bentonites can be classified as sodium or calcium bentonite. For landfill applications, sodium bentonite (Na-B), is used. Sodium bentonite occurs naturally or can be processed from calcium bentonite [5].
GCLs are known to handle differential settlements of underlying soil or waste in the field better than CCL. Due to the easy handling of GCLs, GCLs take less effort to install and cost less to manufacture. Compacted clay liners need in situ field test which is not needed for GCLs [1]. The flexibility and cost effectiveness makes the geosynthetic clay liners a viable replacement for compacted clay liners.
The hydraulic conductivity of GCL is governed by the swelling capacity of the mineral montmorillonite which exist in bentonite [6]. In contact with water or dilute solution, water occupy the interlayer region of montmorillonite particles forming hydration shells around the interlayer Na+ ions. Hydrating bentonite with dilute solution result in the formation of a thick immobile layer of Na+ ion and water molecules around individual montmorillonite particles. This immobile layer is called the diffused double layer (DDL), [7] resulting from osmotic swelling. The diffuse double layer (DDL), is described by the Stern-Guoy model. Based on the Stern-Guoy model, the DDL is comprised of two distinct layers. These two layers are the fixed inner layer known as the Stern layer, consisting of hydrated cations and some water molecules that are bound to the montmorillonite surface and a diffuse outer layer termed as the Guoy layer consisting of hydrated cations and water molecules that balance the electrostatic charge of the montmorillonite mineral [8]. As a result of osmotic swelling, the hydrated particles form the house of cards structure. This structure creates a tortuous path for the permeant solution by reducing the number of pores and pore sizes [9]. Hydraulic conductivity as low as (< 1X10-8 cm/s) [10,11], were reported for GCLs when hydrating fluid is diluted. Sodium bentonite GCLs lose their effect as a barrier material when in contact with high ionic strength leachates from the garbage pile up in landfills. It is believed that high concentration of salts especially solutions with divalent cations like Ca2+ hampers osmotic swelling and results in increase hydraulic conductivity above the maximum threshold of 1X10-7 cm/s for landfill applications [12].
It is assumed that GCLs placed in the field will sufficiently hydrate to water contents which facilitate low permeability. Active hydration involves directly wetting the GCL after installment while hydration from subsoil is seen as passive hydration. The hydration phase after installment is important before the GCL encounters the contaminant fluid [4,13,14]. GCLs are usually covered immediately after installment with an impervious geomembrane hence passive hydration is more favorable and is more important to hydraulic conductivity in the field. Before encountering waste in a landfill, the extent of hydration is generally unknown [4,14]. Although consensually it is agreed that GCLs will take water from the subsoil and the hydraulic performance of GCLs are partially based on the degree of saturation, limited studies deal with hydration of GCLs from subgrade.
Florida, specifically South Florida is a coastal area. A Region like this is prone to saltwater intrusion due to the inward movement of salt water attributed to rising sea levels. The water table in Florida is typically at or below sea level and increase in the salinity of groundwater is a direct consequence of the intruding salt water. It is possible for the bottom liner systems of landfills built in low-lying areas be constructed at or below the water table. In instances like this, GCLs encounter groundwater before leachate. Hydration of GCLs by saline groundwater cannot be treated as GCLs hydrated with groundwater without the influence of cations like Ca2+ and Na+ . It is known that cations affect the performance of GCLs and with the groundwater being more salty, it is important to know how hydration of GCLs by changing groundwater chemistry is affecting GCLs performance when permeated with common landfill leachates. The objective of this study is to simulate GCL being hydrated by the groundwater chemistry of the underlying soil in contact with GCL. The ground water used is representative of Florida ground water chemistry data from the (FDEP). Hydraulic conductivity tests of the hydrated GCLs were performed with synthetic leachate representing leachates from municipal solid waste (MSW), co-disposal (CD), and ash monofil (AM) landfills. The Effect of hydrating selected commercially available GCLs with synthetic groundwater chemistry on their hydraulic performance were observed.
MATERIALS AND METHODS
Geosynthetic clay liners (GCL)
Six commercially available GCLs were used in the study. Three of the GCLs were conventional and contained only granular sodium bentonite with different bentonite gradation, while three of the GCLs were polymer-modified and contained granular sodium bentonite that has been dry blended with different quantities of proprietary polymer(s). The polymer(s), used in the polymer modified GCLs can be classified as linear polymer based on the swelling characteristics of the bentonite extracted from the polymer modified GCLs [15,16]. Linear polymers are water-soluble polymer and form a viscous hydrogel when hydrated with water [17,18]. Table 1
Table 1: Commercial GCL properties used for this study.
|
|
Conventional |
Polymer Modified |
||||
|
Property |
GCL1 |
GCL2 |
GCL3 |
GCL4 |
GCL5 |
GCL6 |
|
Manufacturer |
A |
B |
C |
A |
A |
B |
|
Grainsize |
Coarse |
Medium |
Fine |
Coarse |
Coarse |
Medium |
|
Upper Textile |
NW |
NW |
NW |
NW |
NW |
NW |
|
Carrier Textile |
NW |
SNW |
SNW |
NW |
NW |
SNW |
|
Reinforcement |
NP |
NP |
NP |
NP |
NP |
NP |
|
Tensile Strength (kN/m) |
8.8 |
7.3 |
8.7 |
8.8 |
8.8 |
7.3 |
|
Peel Strength (kN/m) |
0.61 |
0.61 |
0.61 |
0.61 |
0.61 |
0.61 |
|
Mass per unit area (kg/m2) |
5.1 |
3.3 |
4.3 |
4.8 |
5.5 |
4.1 |
|
Initial water content (%) |
17.5 |
7.7 |
12.4 |
19.3 |
17.5 |
7.4 |
|
Effective granule size, D10 (mm) |
0.42 |
0.20 |
0.10 |
0.40 |
0.65 |
0.26 |
|
Polymer Loading (%) |
0 |
0 |
0 |
1.6 |
3.2 |
4.4 |
Note: NW: Nonwoven; SNW: Scrim nonwoven; NP: Needle Punch.
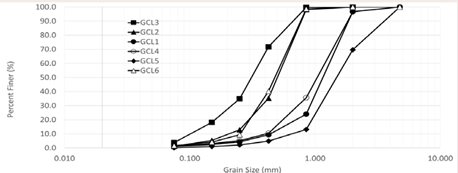
consists of the properties of each GCL. The granule size distribution of bentonite extracted from the GCLs were obtained through mechanical sieve analysis following ASTM C136/C136M. Figure 1
Figure 1: Grain size distribution of bentonite from GCL.
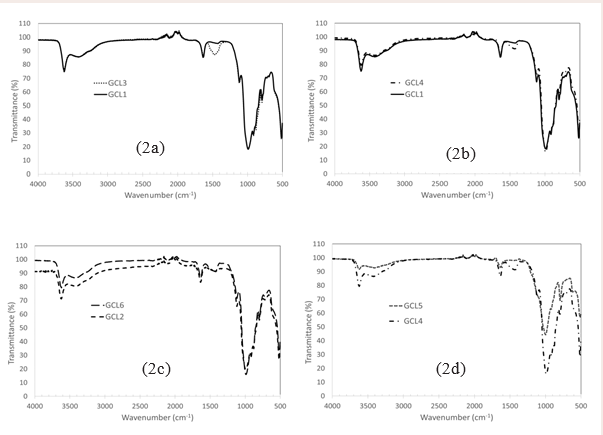
shows the grain size distribution of the GCLs used in this study. GCLs are compared based on manufacturer, grain size, polymer loading and polymer content. FT-IR high resolution data were collected using Agilent Technologies, Cary 630 FT-IR with a resolution of 4 nm in the range 500 cm-1 to 4000 cm-1. FT-IR data confirms and characterize the bentonite of the GCLs used (Figure 2)
Figure 2: Fourier Transform Infrared (FTIR) for the commercial GCLs used for the study.
[19,20]. To make any comparison of the GCLs used, it is important to have an idea of the similarity of the GCLs.
Representative groundwater in Florida
The first extensive review of Florida groundwater geochemistry was done by [21]. Subsequent groundwater data have been collected by Florida Water Management Districts and the FDEP. Calcium bicarbonate is the major compound in Florida groundwater at 53% while sodium chloride accounts for approximately 5% of the salt in groundwater [21,22]. Data from [21], was used to determine the ionic strength and ratio of divalent to monovalent (RMD), cations of Florida groundwater chemistry. Based on retrieved data, two synthetic Florida groundwater were prepared to represent the variability of the collected groundwater database. Groundwater samples were chosen representing the two extremes of the ionic strength of Florida groundwater. Groundwater 1 (GW1) is representative of groundwater with high ionic strength while GW2 represents low ionic strength groundwater in Florida. As can see from Table 2
Table 2: Representative Florida groundwater chemistry used in the study.
|
Hydrating Groundwater |
Na+ (mM) |
Ca2+ (mM) |
Cl- (mM) |
H2O (mM) |
Measured EC (mS/cm) |
Measured pH (mS/cm) |
I (mM) |
RMD (M0.5) |
|
High I (GWI) |
35.42 |
4.63 |
62.79 |
4.17 |
13.40 |
6.70 |
58.37 |
0.52 |
|
Low I(GW2) |
0.79 |
2.73 |
6.03 |
2.45 |
2.54 |
6.40 |
8.87 |
0.02 |
and Table 3,
Table 3: Chemistry of synthetic leachates used in the study.
|
Representative leachate |
Na+ (mM) |
Ca2+ (mM) |
Cl- (mM) |
H2O (mM) |
Measured EC (mS/cm) |
Measured pH (mS/cm) |
I (mM) |
RMD (M0.5) |
|
MSW |
45.26 |
5.45 |
79.40 |
4.90 |
16.40 |
6.80 |
73.23 |
0.61 |
|
CD |
137.76 |
21.81 |
250.56 |
19.60 |
49.00 |
6.10 |
237.78 |
0.93 |
|
AM |
216.87 |
42.25 |
408.98 |
37.98 |
69.00 |
6.00 |
397.43 |
1.06 |
GW1 has similar chemistry to municipal solid waste (MSW), leachate.
Representative landfill leachate in Florida
Hydraulic conductivity and index tests were performed using synthetic leachates representative of municipal solid waste (MSW), landfills and landfills where MSW incineration (MSW-I), ash is either mono-filled or co-disposed with regular MSW. The chemical properties of the synthetic leachates used in this study are summarized in Table 3.
Table 3: Chemistry of synthetic leachates used in the study.
|
Representative leachate |
Na+ (mM) |
Ca2+ (mM) |
Cl- (mM) |
H2O (mM) |
Measured EC (mS/cm) |
Measured pH (mS/cm) |
I (mM) |
RMD (M0.5) |
|
MSW |
45.26 |
5.45 |
79.40 |
4.90 |
16.40 |
6.80 |
73.23 |
0.61 |
|
CD |
137.76 |
21.81 |
250.56 |
19.60 |
49.00 |
6.10 |
237.78 |
0.93 |
|
AM |
216.87 |
42.25 |
408.98 |
37.98 |
69.00 |
6.00 |
397.43 |
1.06 |
The chemical composition of the synthetic leachates were determined based on the composition of real leachates collected from MSW, MSW-I ash monofil (AM), and co-disposal (CD), landfills in Florida, characterized by [23], including leachate data from literature. The composition of synthetic leachates were based on the average salt concentrations of the overall data for either the MSW, AM or CD landfills. For simplicity, K? and Mg2 ? salts where not included in this study because, [11,24], showed that cations species with the same valence have a similar impact on the swelling and hydraulic conductivity of GCLs over a pH range of 2–10. Leachates were prepared by dissolving both reagent grade powdered NaCl (representative of all monovalent salts) and CaCl? ?2H? O (representative of all divalent salts), in DI water.
GCL hydration
GCL cylindrical samples of 4-inch diameter were cut from commercially available GCL roles collected for the study. As a precaution not to lose bentonite, mist of DI water was sprayed at the edges. Specimens were labeled with the respective hydrating groundwater. GCL thickness was measured at four equal quadrants then averaged. The initial mass of each specimen was recorded. Specimens were loaded to a flexible wall permeameter at cell pressure of 34kPa/5psi (3.52m). Precaution must be taken to ensure the membrane and O-rings protect the specimen from water in the permeameter seeping through latex membrane and interacting with GCL specimen. To hydrate the GCLs, the permeameter cell was connected to a custom-made flat board, which enabled the monitoring of water intake of each GCL (Figure 3).
Figure 3: Illustrating the height of hydrating GCL and hydrating board.
The flat board, which consisted of rigid tubes containing the hydrating solution was mounted at the same elevation as the GCL specimen in the permeameter. Hydrating GCL at same level as hydrating board is to simulate hydration of the GCL at zero hydraulic gradient. The GCL was hydrated with synthetic groundwater to simulate the scenario where the GCL is installed in cell constructed at or slightly below the groundwater table and is in contact with groundwater. Water intake under this condition is strictly due to the affinity of bentonite and polymer for the hydrating liquid. While hydrating, one of the effluent/ output valves on the permeameter remain opened to allow air to escape and avoid air pressure buildup. Each GCL specimen was hydrated with one of the two synthetic groundwater for at least seven days. The volume of water absorbed by the GCL was recorded and water content calculated.
Hydraulic conductivity test
After hydration with groundwater the GCLs were permeated with one of three different synthetic leachates using the falling headwater-constant tailwater method in ASTM D6766. Conventional GCLs are permeated to at least two pore volume fractions. Polymer modified GCLs were permeated for at least 10 pore volume fractions, even if chemical and volume equilibria criteria were satisfied before 10 pore volume fractions. Polymer modified GCLs with hydraulic conductivity higher than the prescribed value 1x10-7 cm/s required for landfill applications were terminated once chemical and volume equilibria were reached [12]. The GCLs were permeated using a waterhead pressure difference of 14 kPa (2 psi), with cell pressure of 5 psi, which resulted in an average effective stress of 20.7 kPa (3 psi) and an average hydraulic gradient ranging from 120-200 (depending on the bulk thickness of the GCL). Initial thickness of the GCL specimens ranged between 7 and 10 mm. High hydraulic gradients (ranging from 50 up to 2800), are frequently used in hydraulic conductivity testing of GCLs to shorten the test duration [10,25-29]. After permeation, the wet mass and final average thicknesses of the specimens were recorded immediately. The specimens were put in an oven to dry at 110? C for 24 hours and then the dry mass recorded.
Swell index (SI) testing
Swell index or free swell test, typically measured in accordance with ASTM D5890, and is commonly used to assess how the permeant solution can affect the volume change (swelling), of bentonite component of GCLs during permeation with a potentially incompatible liquid. Several studies have shown that a correlation exists between the swelling and hydraulic conductivity of both conventional and modified bentonites [5,11,24,30-35]. Wireko [15], found a strong correlation between the swell index and hydraulic conductivity of polymer-modified bentonite PMB GCLs (containing bentonite dry blended with linear polymer or crossline polymer), when bentonite extracted from GCL is not subjected to the crushing and sieving routine specified by the ASTM D5890 (which can result in separation of polymer and bentonite).
Thus, in this study, comparable swell index tests were performed on bentonite extracted from the CB and PMB GCLs to assess the effect that the polymer additive has on the swelling and chemical resistance of the bentonite. The swell index tests were performed in accordance with the procedure described in the ASTM D5890 using the permeant solutions except that only specimen of the CB GCLs were prepared following the ASTM D5890 (i.e., crushed and sieved until 100% passed the U.S. No. 100 sieve and at least 65% passed the U.S. No. 200 sieve). First, 90 mL of the hydrating liquid was poured into a 100 mL graduated cylinder. Then, two grams of oven-dried specimen (powdered bentonite for the CB GCLs and granular bentonite for the PMB GCLs) was dusted over the surface of the liquid at increments of 0.1 g over 10 min intervals. After adding the last portion of bentonite, the graduated cylinder was filled to the 100 mL mark with the same liquid, covered and allowed to sit for 24 h. The volume of the swollen bentonite after 24 h was recorded as the swell index (mL/2 g).
RESULTS AND DISCUSSION
Swell index
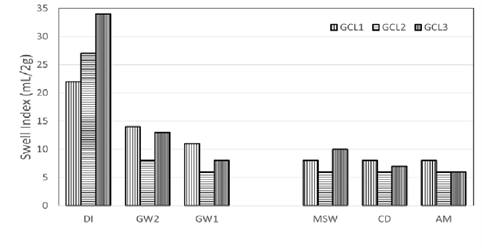
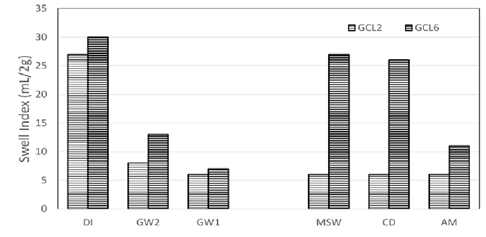
Swell index data of the conventional GCLs used are depicted in Figure 4.
Figure 4: Comparison of swell indices of the conventional bentonite GCLs used in the study.
DI water showed the highest swell volume as is expected. The swelling capacity of the bentonite decrease drastically when swell index was done with groundwater and leachates. Low Ionic strength groundwater GW2 swell index data shows slight improvement of swelling compared to high ionic strength groundwater GW1 and synthetic leachates MSW, CD and AM. This is due to the low ionic strength of GW2. GW1 and MSW are in the same range of ionic strengths. This means that there is hydration of GCL in the field with liquid that is as detrimental to the GCL as leachates produced in landfills. High ionic strength and or divalent cations like calcium are known to collapse the structure of the resulting from cation exchange of calcium for sodium ions. High concentrations of monovalent cations like sodium can also reduce swelling by suppression of the DDL.
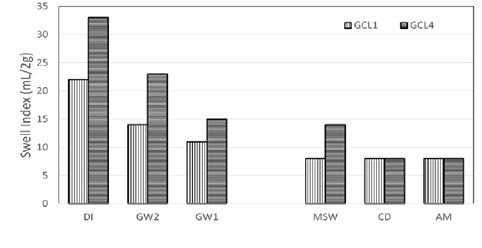
The inclusion of linear polymer to bentonite is known to increase the swelling capacity of bentonite. In Figure 5
Figure 5: Comparison of swell indices of a conventional bentonite GCL and a polymer modified GCL (same manufacturer).
and Figure 6,
Figure 6: Comparison of swell indices of a conventional bentonite GCL and a polymer modified GCL.
the swelling of conventional GCLs is compared to polymer modified GCLs, a similar bentonite is used for the polymer modified GCLs. Swelling for both GCLs decrease with the increase of ionic strength. GCL4 with 1.6% of polymer loading showed higher swelling over GCL1 with no polymer in the bentonite matrix except for CD and AM. CD and AM have ionic strengths of 237.78 mM and 397.43 mM respectively. At these ionic strengths, there is no advantage of the polymer associated with swelling of the GCLs.
Like in Figure 5, polymer modified GCL, GCL6 in Figure 6 swell to a greater volume than GCL2 which is a conventional GCL with similar bentonite as GCL6. GCL2 reached the threshold swell volume at the ionic strength of GW1. The higher ionic strength of CD and AM did not further reduce the swell volume. It is important to note that there was no real trend for the swelling of GCL6 in terms of the ionic strengths of the bulk solutions. The issue with analyzing these commercially available polymers based GCLs is the fact that the specific polymer is unknown.
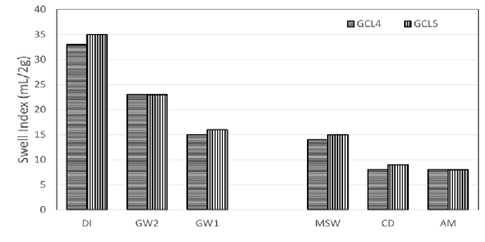
Increase in polymer loading results in increased swelling of bentonite. Each GCL4 and GCL5 with polymer loading of 1.6% and 3.2% respectively, showed reduced swelling as the ionic strength of the solution increase. GCL5 swells more than or at least to the same volume of GCL4 in Figure 7.
Figure 7: Comparison of swell indices of polymer modified bentonite with different polymer loading.
Equal swelling was seen in AM with high ionic strength of 397.43 mM and high RMD of 1.06 M0.5. The concentration of cations rendered the polymer useless. Because the polymer no longer had the effect of aiding the swelling of bentonite in such harsh conditions. At that point, GCL4 and GCL5 have the similar swell capacity of conventional bentonite GCL1 in AM leachate.
Water uptake plots
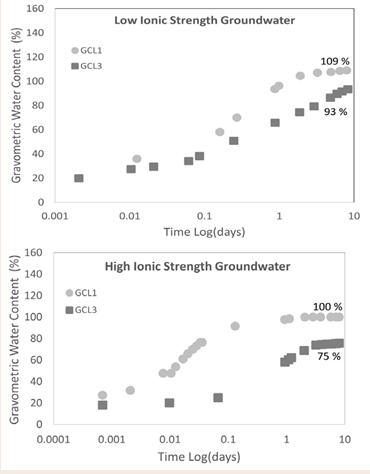
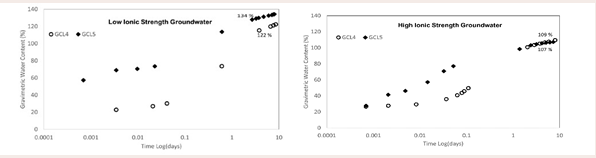
Effect of grainsize on water uptake: The water uptake curves Figure 8
Figure 8: Effect of grainsize on water uptake.
are indicating that the larger grain GCL, GCL1 reached higher water contents during hydration over the period of hydration for both high ionic strength groundwater GW1 (Figure 8b), and low ionic strength groundwater GW2 (Figure 8a). The hydration of a GCL is dependent on the form of the bentonite whether fine or coarse granule bentonite [4,36]. Work done by (Anderson and Rayhani [13,14], showed similar result like in this study for water uptake between coarse granule and fine granule GCLs. The coarse granule GCL reached higher water content than the fine grain GCLs.
The bigger grain size of the bentonite used in GCL1 provided bigger pore spaces for the hydrating fluid to percolate. On the other hand, the smaller granules in GCL3 made the pathway for the hydrating fluid more resistive due to smaller pore sizes. With all experimental conditions being the same and FT-IR data (Figure 2a), showing the close similarity of the bentonite in GCL1 and GCL3, the comparison of the rate of hydrant uptake is purely mechanical, for the same hydrant. Throughout the hydration process the coarse grain GCL achieved maximum achievable water content under the circumstances when hydrated with both groundwater solutions, as is confirmed by the plateau water content at termination. The fine grain GCL, GCL3 was still hydrating at the termination point. Bentonite hydrates in three steps, Cation hydration, capillary action and by way of osmosis. Interlayer cation hydration, which is associated with the initial stages of hydration, is due to the adsorption of up to 4 layers of water molecules on interlayer cations result in increased interlayer spacing [37]. Capillarity is associated with the phase of hydration dominated by pore size distribution; this is the linear phase of the figures and is hydration at the macroscale. Water uptake plot of GW2 (Figure 8a), shows that fine granule GCL3 is still undergoing capillary hydration at the time of termination while GCL1 was at the osmotic phase. Osmosis, the final phase of hydration is associated with the the water uptake plot [37]. At this stage water diffuses across the volume of the GCL and is not due to the further intake of hydrant. It is obvious that water content is constant with time at that point.
Both GCLs reached higher water content when hydrated with GW2 (Figure 8a), than with GW1 (Figure 8b). The high concentration of cations like Na+ and Ca2+ leads to shrinking of the diffused double layer (DDL) which is directly related to the adsorption of water molecules in the interlayer of the montmorillonite.
Comparing Figure 8a and 8b, it is noticeable that the chemistry of the hydrating liquid affected the achieved water content of both GCL1 and GCL3. When hydrated with high ionic strength groundwater GW1 both fine granule GCL3 and coarse granule GCL1 had lower water contents than when respectively hydrated with low ionic strength GW2.
The absorption and adsorption of water into the bentonite matrix is controlled by the ability of the montmorillonite to interact with water molecules in aqueous solution. Cations in solution effects charge screening and reduce the interaction of water with montmorillonite in the presence of cations, especially divalent ones and shrinking the DDL [38]. Divalent or polyvalent cations like Ca2+ and Mg2+ is said to restrict crystalline swelling to an upper limit of 19 Å (1.9 nm), compared to 22 Å observed for monovalent cations like Na+ and Li+ [39]. In relation to this study, GW1 has higher ionic strength and RMD than GW2. Also, GW1 has more Ca2+ than GW2. It is not a surprise that for both GCLs hydrated with a more dilute hydrant GW2 resulted in more water adsorption to montmorillonite surface than with GW1. The lower water content for GW1 is due to the reduced DDL. High ionic strength of bulk solution and the presence of Ca2+ decrease the thickness of the DDL [40]. The increase water absorbed by both GCLs with GW2 is proof that there was less interaction of the montmorillonite surface with water molecules when the GCLs were hydrated with GW1. This is due to more charge screening of clay surface in contact with GW1 hence resulting in reduced DDL thickness which is proportional to water content of the similar GCL hydrated with different concentrations like GW1 and GW2. Ca2+ is more detrimental to the DDL compared to Na+ and other monovalent cations. It requires half the amount of Ca2+ to satisfy the amount of clay interaction than if it was Na+ . The negative charges on the external surfaces and the net positive charges in solution adjacent to external surfaces form the electric diffused double layers [41].
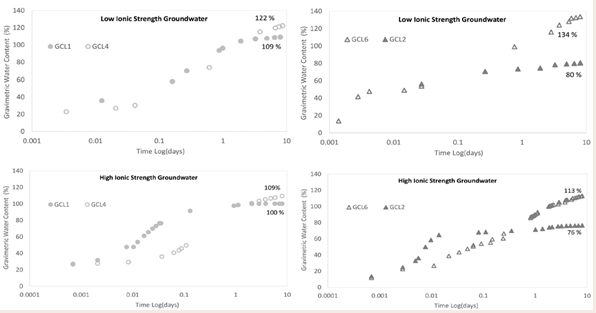
Effect of polymer inclusion on water uptake: The inclusion of linear polymer to conventional bentonite in GCLs increase the water uptake capacity of the GCL. The linear polymers used in commercial GCLs are hydrophilic hence they have high affinity for water. Literature show that the inclusion of polymer in bentonite matrix resulted in increased water content than untreated clay [42]. Figure 9
Figure 9: Effect of polymer inclusion on water uptake.
depicts GCL1 without polymer and GCL4 with 1.6 % polymer based on total mass of bentonite. The polymer modified bentonite, GCL4, achieved higher water content than GCL1 for both GW2 and GW1. GCL1 reached maximum water content while GCL4 was still undergoing water uptake at termination in both scenarios.
The comparison of conventional bentonite GCL2 and polymer modified GCL6 with 4.4% polymer loading (Figure 9c and 9d) is like that of Figure 9a and 9b. For Figure 9c, both GCLs yielded higher water contents when hydrated with the low ionic strength and low RMD groundwater GW2. GCL2 reached equilibrium water content under the given conditions for both hydrating solutions. Polymer based GCL6 was still on a linear path of water uptake at the point of termination.
The increase water uptake in GCL4 and GCL6 exceeding their respective conventional counterparts is due to hydrolysis of the polymers. In the presence of water, linear hydrophilic polymers absorb water and dissolves. The presence of hydrophilic functional groups like hydroxyl, carboxyl and sulfonate groups tends to readily dissolve in water [43,44]. The presence of a liquid creates a high-water concentration gradient between the hydrating liquid and the polymer. As the dry coiled polymer contact the hydrating liquid, it disentangles forming hydrogen bonds between polymer and water molecules which results in relaxation/swelling of the polymer chains [43,45,46]. It has been reported that addition of hydrophilic linear polymer to soil matrix increase the water retained by 65% [47]. The high amount of water uptake in polymer based GCLs compared to the water uptake in conventional GCLs is evidence of the mass/volume of water that water soluble polymer can absorb compared to its dry weight. At the point of termination, GCL1 and GCL2 were at the equilibrium water contents. Polymer modified GCL4 had 13% and 9% higher water contents at termination than GCL1 when hydrated with GW2 and GW1 respectively. GCL6 also indicated that the polymer addition allowed the GCL to absorb more water than GCL2 without polymer in the bentonite matrix. GCL6 reached water content that are 54% and 37% higher than GCL2 at termination when hydrated with GW2 and GW1 respectively. It is important to note that although GCL4 and GCL6 are significantly higher water contents than GCL1 and GCL2 respectively, the polymer modified GCLs were still absorbing water. The linear phase present in GCL4 and GCL6 at the time of termination unlike the plateau at termination seen for GCL1 and GCL2 mean that the polymer modified GCLs can absorb more water with time.
It is already mentioned in the above section that untreated bentonite reached lower water contents when the ionic strength of the hydrating liquid increase (Figure 9b). The same trend is seen for untreated bentonite in GCL2 (Figure 9d). From the plots, it is seen that both polymer treated GCLs, GCL4 and GCL6 water uptake are also affected by the difference in chemistry of the hydrating solutions. The higher ionic strength and specifically calcium concentration is the reason for the lower water uptake of GW1 for both polymer-modified GCL4 and GCL6. Adsorption of water to charged polymer surfaces depends on the concentration of hydrant. The ionic strength of the solution and the type of cation, whether the monovalent or polyvalent affects the water adsorption of polymers [48-50]. This statement is validated by data in the current work. High ionic strength GW1 constitutes more Ca2+, Na+ and higher RMD than that of low ionic strength GW2. This is the reason for the greater water contents achieved for GCL4 and GCL6 when hydrated with GW2 over GW1.
It is not scientifically viable to compare GCL4 with GCL6 although it is evident from the plots in Figure 9 that GCL6 with higher polymer content attained higher water contents. GCL1 and GCL2 are replicas of the bentonite used in GCL4 and GCL6. The only difference is the polymer inclusion. The specific polymers in the GCLs are not known. Important information like functional groups and degree of polymerization would at least be required to make such comparison of GCL4 and GCL6.
Effect of polymer loading on water uptake: The data in Figure 10
Figure 10: Effect of polymer loading on water uptake.
show that the polymer inclusion enhanced the water uptake rate of the GCL. GCL4 and GCL5 are GCLs of different polymer loading. GCL5 with a higher polymer content of 3.2% based on the total mass of bentonite reached higher water content than GCL4 with 1.6% of polymer with both hydrants (Figure 10a and 10b). For the same polymer, the achieved water content with increase polymer loading is expected. The more polymer in the bentonite matrix means there is more active charged sites for water to interact with polymer via hydrogen bonding [42,51]. GCLs hydrated with low ionic strength groundwater GW2 (Figure 10a), had greater water content than the GCLs hydrated with high ionic strength groundwater GW1 (Figure 10b). The plots show that GCL5 hydrates faster than GCL4. The difference in the water contents at termination for GCL4 and GCL5 hydrated with GW2 is more pronounced. In the instance of GW1, GCL4 and GCL5 with polymer loading of 1.6% and 3.2% respectively have very similar water contents, though GCL5 had slightly higher water content. The ionic strength of GW1 diminished the advantage of the higher polymer existing in GCL5.
Hydraulic conductivity
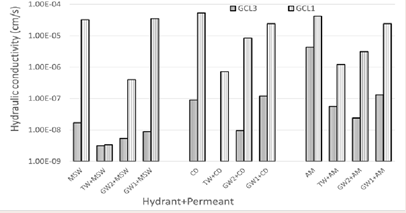
Effect of grainsize on hydraulic conductivity: Figure 11
Figure 11: Effect of grainsize on hydraulic conductivity.
illustrates the hydraulic performance of a coarse grained (GCL1), and a fine grained (GCL3). The bars labeled MSW, CD and AM are for GCLs hydrated and permeated with the same leachate. The bars indicating TW, GW2 or GW1 before the leachates means that the GCLs are hydrated with different solution before leachate permeation.
As can be seen from Figure 11, the fine granule GCL3 showed better hydraulic performance than coarse grain GCL1 across the board. This is expected, for similar bentonite hydrated and permeated with the same liquids it can be said that the swelling is the same; hence the difference in flow paths is attributed to the pore spaces. Granular bentonite contains several macropores, the pores can close or become smaller through hydration of the bentonite hence improving hydraulic conductivity [52,53]. In the presence of water, granular bentonite disintegrates into individual particles because of the DDL [32]. Though the coarse granule GCL1 showed higher water contents, the hydraulic conductivity is not directly proportional to the hydraulic conductivity for different granule size GCLs. The improved hydraulic conductivity noticed for GCL3 is attributed to the effective gel formation that is associated with the greater surface area of the GCL3 being exposed to the hydrating liquid than for coarse grain GCL1 [36]. Granular bentonite is known to allow water to flow through the soil before self-healing or osmotic swelling occurs due to the availability of macropores in the matrix [2]. This is evidence that the fine grain GCL3 essentially undergoes osmotic swelling faster than coarse grain GCL1 for a similar time. Yadav & Tadikonda [54], reported similar findings, which stated that the hydraulic conductivity of the finer bentonite GCL achieved lower hydraulic conductivity than a coarser granule GCL. The higher hydraulic conductivity of the coarse GCL was attributed to the existing macropores. It is possible that osmotic swelling occurs, but the pores are too large for substantially impeding permeants.
Prehydration of GCL3 with TW, GW2 and GW1 before contact with municipal solid waste MSW landfill leachate aided in GCL3 retaining lower k values around 1 x 10-8 cm/s. Hydraulic conductivity of GCL1 hydrated with TW and permeated with MSW leachate has similar hydraulic conductivity to GCL3. It has been reported that coarse grain GCL hydrated with water achieved favorable hydraulic conductivity [54]. When GCL1 is hydrated by water, the lack of cations made it possible for the bentonite to adequately swell and seal macropores, hence making the path for flow much more tortuous. GCL1 had unfavorable hydraulic conductivity values for all other experimental combinations. When GCL1 is hydrated with low ionic strength GW2 and high ionic strength GW1, the DDL is suppressed by the presence of Na+ and Ca2+ [55]. Since the diffused layer is suppressed, the macropores are present during permeation. GW1 with similar chemistry of MSW resulted in comparable k value when hydrated with GW1 then permeation with MSW like when hydrated and permeated with MSW then permeated with MSW.
TW and GW2 hydration before permeation with CD has shown to lower k of GCL1 and GCL3. Permeation with CD after GW1 hydration did not help the GCLs significantly. GCL3 hydrated with TW and GW2 to be permeated by CD can be considered for landfill applications under such conditions. GCL3 hydrated with CD leachate before permeated with the same leachate, has k borderline the acceptance hydraulic conductivity and would not be recommended to be used under these conditions.
Ash monofil leachate is very harsh with an ionic strength of 397.43 mM and RMD of 1.06. Prehydration with solutions less concentrated than the leachate enhanced the hydraulic performance of the GCLs. GCL3 specimens permeated with AM after TW or GW2 hydration had k lower than 1 x 10-7, which is the maximum acceptable k for landfill use.
It is observable from the plot that GCL1 and GCL3 hydrated with GW1 had similar hydraulic conductivities when permeated with both CD and AM. CD has Ionic strength of 237.78 mM and RMD of 0.93. AM has an ionic strength of 397.43 mM and RMD of 1.06. Although AM is much stronger than CD, GCL1 and GCL3 had similar hydraulic conductivity with CD and AM. According to the plot, GCL3 no longer had an advantage over GCL1 at that point. With the permeants CD and AM being such harsh leachates with high concentration of Ca2+. At such concentration of leachates, osmotic swelling is nullified by the Na+ and Ca2+ present in solution.
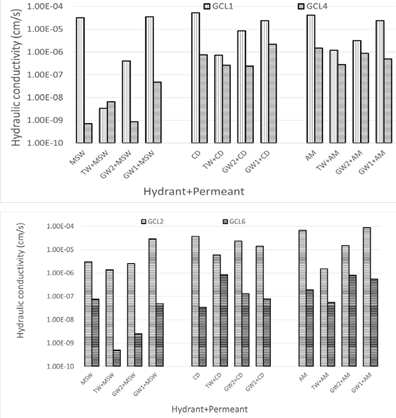
Effect of polymer inclusion on hydraulic conductivity: Polymer modified GCL4 with polymer content of 1.6% showed improved hydraulic performance compared to conventional bentonite GCL1 for all the hydraulic conductivity experiments conducted (Figure 12).
Figure 12: Effect of polymer inclusion on hydraulic conductivity.
Without knowledge of the specific polymer used in the polymer base GCL4 and GCL6, no claims can be made about the specific mechanism that enhances the hydraulic conductivity of polymer modified GCLs enhancing the hydraulic performance. It is known that hydrophilic linear polymers improve the hydraulic performance of expansive soils [15,42]. It is a possibility that the polymer used result in intercalation of polymer between montmorillonite interlayer spacing. Hydrolysis of the polymer in the interlayer spacing leads to increased d spacing. This will cause increased DDL [16,42,51]. It is also hypothesized that the noticed improved hydraulic conductivity of polymer modified GCLs is due to the clogging of macropores by the viscous polymer gel [47,56]. During the present work, it was noticed that polymer eluted the GCLs in all scenarios no matter the concentration of the permeant. If clogging was the governing phenomenon, then while the polymer is eluting, the hydraulic conductivity should increase while the polymer elutes. However, it has been reported that for commercially available GCLs, polymer elution was observed even at equilibrium hydraulic conductivity [16,35]. Wireko & Abichou [16], proposed the possibility of linear polymers scavenging of cations, predominantly Ca2+ ions from leachate/hydrant to make the bulk solution less ionic hence more swelling of bentonite. As stated in the previous section, conventional bentonite GCL1 hydraulic performance was only significantly improved when prehydrated with TW and permeated by MSW. Prehydration did not help the polymer modified GCL4 hydraulic conductivity at all. In all cases the hydraulic conductivity of GCL4 is about the same or higher when the GCL is permeated by the leachate and when the GCL is hydrated with TW, GW2, and GW1 of lower ionic strength then permeated with the leachate. GCL1 and GCL4 are not compatible for landfills which produce CD and AM harsh leachates. The high ionic strengths and abundance of Ca2+ in these leachates affect the swelling potential of both GCLs. Divalent cations like Ca2+ is known to contract polymer chains, Ca2+ causes physical crosslinking or chemical crosslinking of polymer chains [57].
GCL6 with 4.4% polymer loading had lower hydraulic conductivities than conventional bentonite GCL2 like Figure 12b. The data shows that prehydration of GCL2 did not help to achieve improved performance. GCL2 is not compatible with none of the leachates used in this study, not even when the hydrating liquid is water. Prehydration was beneficial to GCL6 only when the hydrating liquids are TW and low ionic strength groundwater GW2 with MSW leachate. The harsher CD and AM leachates are not compatible with polymer modified GCL6.
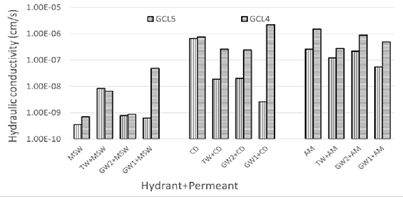
Effect of polymer loading on hydraulic conductivity: Higher polymer loading positively affected the performance of the GCL. GCL4 has polymer loading of 1.6% while GCL5 has a polymer content of 3.2%. Increased polymer loading resulted in reduced k (Figure 13).
Figure 13: Effect of polymer loading on hydraulic conductivity.
GCL4 and GCL5 permeated with MSW leachate meets the required 1 x 10-7 cm/s for landfill applications. Prehydration of GCL5 with TW, GW2 and GW1 before contact with CD leachate aided the performance, making GCL5 viable for landfill use with co-disposal leachates. The lower hydraulic conductivity noticed with higher polymer content is due to the additional functional group sites for water to interact with hence further increasing the viscosity which is beneficial if clogging is the mechanism at play. If the improvement is due to intercalation of polymer into montmorillonite, more polymer to cause more swelling in the clay matrix. If the governing phenomenon is the scavenging of cations from solution, then the increased sites can allow trapping of more cations hence a more dilute solution which can affect swelling.
Final water content relationship with hydraulic conductivity
The general trend in Table 4 shows that for a particular GCL when hydrated with low ionic strength GW2 and permeated with MSW, CD or AM leachate has greater final water contents than a similar GCL hydrated with high ionic strength GW1 and permeated with the same leachates. This is so for the same reason that a particular GCL reached higher water content when hydrated with lower ionic strength GW2, see Figures 8-10. Less concentrated bulk solution affords the bentonite to intake and holds more water resulting in an increase in the diffuse double layer (DDL). Conflicting results were realized for GCL3 and GCL4 permeated with CD after hydration with GW2 and GW1 respectively. Final water content reached for GCL3 and GCL4 were greater when hydrated with GW1 and permeated with CD. GCL3 specimen used for GW2+CD was taken from the edge of the roll due to limited GCL3. Due to this limitation, it is possible that less bentonite was present in that specimen hence less bentonite for water – montmorillonite interaction. GCL4 is a polymer modified GCL. A possible reason for GCL4 hydrated with GW1 have higher water content than GCL4 hydrated with GW2 after permeation with CD are that the polymer bentonite mixtures can be heterogenous caused by transportation of the GCL roll from manufacturing to laboratory. It is assumed in the study that the polymer in the GCLs is evenly distributed.
From the plots of hydraulic conductivity and the table for final water contents of the GCLs after permeation, it can be noticed that there is a direct relationship between the final water content and the hydraulic conductivity. Final water content is indirectly proportional to equilibrium hydraulic conductivity. Conventional GCLs, GCL1, GCL2 and GCL3 showed that the hydraulic conductivity at equilibrium increase with a decrease in the final water content of the GCL. GCLs hydrated with GW2 before permeation have higher water contents and lower k than the similar GCL hydrated with GW1 before permeation with the same leachate. Exceptions are GCL3 permeated with CD after hydration, as explained in the paragraph above where the GCL3 hydrated with GW2 achieved lower water content than when hydrated with GW1 before permeation with CD. However,GW2+CD still recorded lower k than for GCL3 subjected to GW1+CD. One other anomaly observed in the data is for GCL2. GCL2 hydrated with GW2 and permeated with CD had a slightly higher k than when hydrated with GW1 then permeated with CD although GW2+CD had higher final water content than GCL2 with GW1+CD. This should not be considered significant since the k values are of the same order of magnitude (very close).
The indirect correlation of polymer modified GCLs k measurements with final water content is not as defined as seen for the conventional bentonite GCLs. GCL4 show instances of k inversely proportional to final water content and k directly proportional to final water content. In all cases, GCL5 showed higher k for specimen hydrated with GW2 than specimens hydrated with GW1 permeated with the same leachate. Like GCL4, GCL6 did not indicate a clear distinction in the relationship of final water content and k. The inconsistency in the data is proof that a control study is needed to assess the relationship between final water content and hydraulic conductivity. Certain parameters like polymer type, polymerization, functional groups are needed to ascertain the behavior of a specific polymer. From an experimental perspective, parameters like termination pore volume fraction (PVF), and homogeneity of polymer modified GCLs are needed.
CONCLUSIONS
The data indicates that the performance of GCLs, whether conventional bentonite or polymer modified GCL are affected by the hydrating liquid [58]. Lower ionic strength hydrant (GW2), resulted in more water uptake by the GCL because there are more readily available water molecules in solution to interact with the clay particles than when hydrated with high ionic strength (GW1). The observed higher water uptake for the coarse granule GCL1 compared to GCL3 is attributed to the large pores of GCL1 which allows for more water intake at an instant in time, this is due to capillary action.
Polymer addition to bentonite caused increase in the water uptake of GCLs because of the high affinity of water-soluble linear polymer for water during hydrolysis. The polymer increases the number of active sites for water interaction/absorption. The exact functional groups at play in the polymer used for commercially available GCLs are unknown to the user since the specific polymer used is unknown. Increasing the polymer amount in a GCL will result in the further increase in active sites via functional groups. It is expected that GCL5 which has similar bentonite as GCL4, but more polymer in the bentonite matrix should achieve higher water uptake as is seen in this study.
It is generally believed that higher hydrated water content is an indication of better performance of the GCL other another. This is a broad statement which does not consider how the granule sizes can affect hydration and swelling of GCLs. In this study, although coarse granule GCL1 achieved higher water uptake than fine granule GCL3, GCL3 exhibited better hydraulic performance than GCL1. GCL3 show lower hydraulic conductivity because the fine granule only requires minute swelling to block pores. However, with GCL1 even if osmotic swelling occurs the pores are too large to be closed small enough to effect low hydraulic conductivity.
Polymer modified GCL4 and GCL6 have superior hydraulic performance compared to the conventional GCL1 and GCL2 respectively. The exact phenomenon which gives polymer modified GCLs better compatibility is not yet fully understood. Authors have theorized that the mechanism at work is clogging, polymer effecting increase DDL and polymer scavenging cations from bulk solution making it more dilute causing more swelling. With no idea of the specific polymer used in GCLs, it is not viable to say what mechanism is responsible for the hydraulic improvement. Further controlled studies are needed to ascertain the mechanism controlling hydraulic improvement of polymer modified GCLs. Without knowledge of the specific polymer, important information like functional groups and degree of polymerization are unknown.
ACKNOWLEDGEMENT
This material is based on work supported by the Hinkley Center for Solid and Hazardous Waste Management and Environmental Research and Education Foundation (EREF). Any opinions, findings, and conclusions in this material are those of the authors and do not necessarily reflect the views of the Hinkley Center for Solid and Hazardous Waste Management and EREF. Thanks to the Instrumentation laboratory in the Department of Chemistry at FAMU for making the FT-IR equipment available for characterizing our samples. Special thanks to Sabine Beaumont an undergraduate intern in our laboratory.
REFERENCES
- Bouazza A Geosynthetic clay liners. Geotextiles and Geomembranes. 2002: 3-17.
- Acikel AS, Gates WP, Singh RM, Bouazza A, and Rowe RK. Insufficient initial hydration of GCLs from some subgrades: factors and causes. Geotextiles and Geomembranes. 2018; 40: 770-781.
- Koerner RM. Designing with Geosynthetics. 2012; 2.
- Bouazza A, Ali MA, Gates WP, Rowe RK. New insight on geosynthetic clay liner hydration: the key role of subsoils mineralogy. Geosynthetics Int. 2017; 24: 139-150.
- Guyonnet D, Touze-Foltz N, Norotte V, Pothier C, Didier G, Gailhanou H, et al. Performance-based indicators for controlling geosynthetic clay liners in landfill applications. Geotextiles and Geomembranes. 2009; 27: 321-331.
- Norrish K. The swelling of montmorillonite. Discussions of the Faraday society. 1954; 18: 120-134.
- McBride MB. Environmental Chemistry of Soils. 1994; 141580.
- Mitchell JK and Kenichi Soga. Fundamentals of soil behavior. New York: John Wiley & Sons. 2005; 3.
- Olphen H. An introduction to clay colloid chemistry. Interscience Publishers. 1963.
- Shackelford CD, Benson CH, Katsumi T, Tuncer BE, Lin L. Evaluating the hydraulic conductivity of GCLs permeated with non-standard liquids. Geotextiles and Geomembranes. 2000; 18: 133-161.
- Jo HY, Katsumi T, Benson CH, and Tuncer BE. Hydraulic conductivity and swelling of nonprehydrated GCLs permeated with single-species salt solutions. J Geotech Geoenviron Eng. 2001; 127: 557-567.
- Jo HY, Benson CH, Shackelford CD, Lee JM, Tuncer BE. Long-term hydraulic conductivity of a geosynthetic clay liner permeated with inorganic salt solutions. J Geotech Geoenviron Eng. 2005; 131: 405- 417.
- Rayhani MT, Rowe RK, Brachman RWI, Take WA, and SiemensG. Factors affecting GCL hydration under isothermal conditions. Geotextiles and Geomembranes. 2011; 29: 525-533.
- Anderson R, Rayhani MT, Rowe RK. Laboratory investigation of GCL hydration from clayey sand subsoil. Geotextiles and Geomembranes. 2012; 31: 31-38.
- Wireko C, Zainab B, Tian K, Abichou T. Effect of specimen preparation on the swell index of bentonite-polymer GCLs. Geotextiles and Geomembranes. 2020; 48: 875-885.
- Wireko C, Abichou T. Investigating factors influencing polymer elution and the mechanism controlling the chemical compatibility of GCLs containing linear polymers. Geotextiles and Geomembranes. 2021; 49: 1004-1018
- Billmeyer FW. Textbook of polymer science. John Wiley & Sons. 1984.
- Rivas BL, Urbano BF, Sánchez J. Water-soluble and insoluble polymers, nanoparticles, nanocomposites and hybrids with ability to remove hazardous inorganic pollutants in water. Front chem. 2018; 6: 320.
- Kumar A, Lingfa P. Sodium bentonite and kaolin clays: Comparative study on their FT-IR, XRF, and XRD. Materials Today: Proceedings. 2020; 22: 737-742.
- El-Enein SA, Okbah MA, Hussain SG, Soliman NF, Ghounam HH. Adsorption of selected metals ions in solution using nano-bentonite particles: isotherms and kinetics. Environmental Processes. 2020; 7: 463-477.
- Berndt MP, Marian P., and Brian G. Katz. Hydrochemistry of the surficial and intermediate aquifer systems in Florida. Water- Resources Investigations Rep. 1992; 91: 4186.
- Reese RS, Cunningham KJ. Hydrogeology of the gray limestone aquifer in southern Florida. 2000; 4199-4213.
- Li L, Tang Y, Abichou T, Higgs B, Wireko C, Li R, Characterization of leachates from landfills containing MSW-I residues. Journal of
- Kolstad DC, Benson CH, Edil TB, Hydraulic conductivity and swell of nonprehydrated geosynthetic clay liners permeated with multispecies inorganic solutions. J Geotech Geoenviron Eng. 2004; 130: 1236-1249.
- Petrov RJ, Rowe RK, Geosynthetic clay liner (GCL)-chemical compatibility by hydraulic conductivity testing and factors impacting its performance. Canadian Geotech J. 1997; 34: 863-885.
- Ruhl JL, Daniel DE, 1997. Geosynthetic clay liners permeated with chemical solutions and leachates. Journal of Geotechnical and Geoenvironmental Engineering. 1997; 123: 369-381.
- Shackelford CD, Sevick GW, Eykholt GR, Hydraulic conductivity of geosynthetic clay liners to tailings impoundment solutions. Geotextiles and Geomembranes. 2010; 28: 149-162.
- Chen JN, Benson CH, Tuncer BE. Hydraulic conductivity of geosynthetic clay liners with sodium bentonite to coal combustion product leachates. J Geotech Geoenviron Eng. 2018; 144: 04018008.
- Tian K, Likos WJ, Benson CH. Polymer elution and hydraulic conductivity of bentonite-polymer composite geosynthetic clay liners. J Geotech Geoenviron Eng. 2019; 145: 1-12.
- Jo HY, Benson CH, Edil TB, Hydraulic conductivity and cation exchange in non prehydrated and prehydrated bentonite permeated with weak inorganic salt solutions. Clays and Clay minerals. 2004; 52: 661-679.
- Olsta JT, Chung JH, Daniel DE, 2004. Various aspects of sodium bentonite testing, in: Mackey, R.E., von, K.M. (Eds.), Advances in geosynthetic clay Liner technology: 2nd symposium. 2004; 3-10.
- Lee JM, Shackelford CD, Benson CH, Jo H-Y, Tuncer BE. Correlating index properties and hydraulic conductivity of geosynthetic clay liners. J Geotech Geoenviron Eng. 2005; 131: 1319-1329.
- Di Emidio G, Mazzieri F, Van Impe W, Hydraulic conductivity of a dense prehydrated GCL: impact of free swell and swelling pressure. Proceedings of the 4th European Geosynthetics Conference. 2008.
- Katsumi T, Ishimori H, Onikata M, Fukagawa R, Long-term barrier performance of modified bentonite materials against sodium and calcium permeant solutions. Geotextiles and Geomembranes. 2008; 26: 14-30.
- Scalia J, Benson CH, Bohnhoff GL, Edil TB, Shackelford CD. Long-term hydraulic conductivity of a bentonite-polymer composite permeated with aggressive inorganic solutions. J Geotech Geoenviron Eng. 2014; 140: 1-13.
- Vangpaisal T, Bouazza A. Gas permeability of partially hydrated geosynthetic clay liners. J Geotech Geoenviron Eng. 2004; 130: 93-102.
- Wayllace A. Volume change and swelling pressure of expansive clay in the crystalline swelling regime. PhD diss., University of Missouri— Columbia. 2008.
- Slade PG, Quirk JP. The limited crystalline swelling of smectites in CaCl2, MgCl2, and LaCl3 solutions. J Colloid Interface Sci. 1991; 144: 18-26.
- Theng BKG. Formation and properties of clay-polymer complexes. Elsevier. 2012.
- Joerg R, Bartelsen T, Dohrmann R, Siegesmund S. Moisture expansion as a deterioration factor for sandstone used in buildings. Environ Earth Sci. 2011; 63: 1545-1564.
- Laird DA. Influence of layer charge on swelling of smectites. Applied clay sci. 2006; 34: 74-87.
- De Camillis M, Di Emidio G, Bezuijen A, Flores RDV. Wet and dry ageing of polymer treated clays using seawater: swell potential and hydraulic conductivity. 10th International conference on geosynthetics (IGS-
- Hatakeyama H, Hatakeyama T. Interaction between water and hydrophilic polymers. Thermochimica acta. 1998; 308: 3-22.
- Kadajji VG, Betageri GV. Water soluble polymers for pharmaceutical applications. Polymers. 2011; 3: 1972-2009.
- Ghori MU, Conway BR. Hydrophilic matrices for oral control drug delivery. Am J Pharmacol Sci. 2015; 3: 103-109.
- Li CL, Martini LG, Ford JL, Roberts M. The use of hypromellose in oral drug delivery. J pharmacy pharmacol. 2005; 57: 533-546.
- Chen FM. The effect of polymers for soil stabilization and soil nutrient retention. Int J Applied Sci. 2016; 3
- Abraham T, Kumpulainen A, Xu Z, Rutland M, Claesson PM, Masliyah J. Polyelectrolyte-mediated interaction between similarly charged surfaces: role of divalent counter ions in tuning surface forces. Langmuir. 2001; 17: 8321-8327.
- Seantier B, Kasemo B. Influence of mono-and divalent ions on the formation of supported phospholipid bilayers via vesicle adsorption. Langmuir. 2009; 25: 5767-5772.
- Al-Hashmi AR, Luckham PF, Al-Maamari RS, Zaitoun A, Al-Sharji HH. The role of hydration degree of cations and anions on the adsorption of high-molecular-weight nonionic polyacrylamide on glass surfaces. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2012; 415: 91-97.
- Di Emidio G. Hydraulic and Chemico-Osmotic Performance of Polymer Treated Clays. PhD thesis, Ghent University. 2010.
- Rowe RK, Li TK. Self-healing of circular and slit defects in GCLs upon hydration from silty sand under applied stress. Geotextiles and Geomembranes. 2020; 48: 667-683.
- Li TK, Rowe RK. GCL self-healing: fully penetrating hole/slit hydrated with RO water and 10 mM Ca solution. Geosynthetics Int. 2020; 27: 34-47.
- Yadav H, Tadikonda BV. The Influence of Mechanical Granulation Process and Granular Bentonite Plasticity on Self-Sealing and Volume Change Behavior. J Hazardous, Toxic, Radioactive Waste. 2022; 26: 04022003.
- Tadikonda BV, Das P, Buragadda V. Specific ion effects on surrogate compatibility indices of bentonite for hydraulic barrier applications. Int J Geotech Eng. 2019; 13: 360-368.
- Tian K, Benson CH, Likos WJ. Hydraulic conductivity of geosynthetic clay liners to low-level radioactive waste leachate. J Geotech Geoenviron Eng. 2016; 142: 04016037.
- Peng S, Wu C. Light scattering study of the formation and structure of partially hydrolyzed poly (acrylamide)/calcium (II) complexes. Macromolecules. 1999; 32: 585-589.
- Kolstad DC, Benson CH, Edil TB, Jo HY, Hydraulic conductivity of a dense prehydrated GCL permeated with aggressive inorganic. Geosynthetics Int. 2004; 11: 233-241.