Vulvar Pain Symptom Improvement and Changes in Resting-State Brain Functional Connectivity Following Mindfulness-Based Stress Reduction for Provoked Vestibulodynia: A Single-Arm Pre-Post Pilot Study
- 1. G. Oppenheimer Center for Neurobiology of Stress and Resilience, University of California, USA
- 2. Brain Research Institute UCLA, Gonda (Goldschmied) Neuroscience and Genetics Research Center, USA
- 3. Department of Obstetrics and Gynecology, University of California, USA
Abstract
Background: Mindfulness-based stress reduction (MBSR) has been shown to improve symptoms in women with provoked vestibulodynia (PVD); however, the mechanism underlying symptom improvement with MBSR remains unknown.
Aim: To investigate the effects of an 8-week MBSR group treatment on PVD symptoms and brain functional connectivity.
Method: In this single-arm pilot study, changes in symptoms were assessed in two groups of women with PVD (n1 =9, n2 =12) who completed an 8-week MBSR group treatment. Resting-state brain imaging was performed pre- and post-MBSR in a subsample of the second group (n=10). Post-treatment effect size changes in symptoms and brain functional connectivity were estimated.
Outcomes: Change in resting state brain connectivity, cognition, vulvar pain and vaginal muscle tenderness, and mood after MBSR
Results: Participants reported reduced vulvar pain, improved mood, decreased rumination, and enhanced mindfulness skills after MBSR group treatment. Changes in functional brain connectivity were observed primarily in default mode and salience/ventral attention networks. Many of these brain connectivity changes were associated with improvements in pain, mindfulness, mood, and rumination.
Clinical Implications: The study provides support for the hypothesis that mindfulness training can improve clinical features of PVD via impact on the brain’s functional connectivity.
Strengths and Limitations: This pilot study identified brain targets and provided empirical estimates for effect sizes related to changes in symptoms and brain connectivity in women with PVD. However, these findings must be interpreted with caution given the small sample size.
Conclusion: The findings from this pilot study provide preliminary support for the hypotheses that 8-week MBSR group treatment results in brain functional connectivity changes associated with clinically relevant improvements in pain symptoms as well as positive shifts in measures of mood and rumination.
Keywords
Brain, Mindfulness-based stress reduction, Pain, Provoked Vestibulodynia, Resting-state functional connectivity
Citation
Tun G, Tillisch K, Rapkin A, Smith S, Stains J, et al. (2022) Vulvar Pain Symptom Improvement and Changes in Resting-State Brain Functional Connectivity Following Mindfulness-Based Stress Reduction for Provoked Vestibulodynia: A Single-Arm Pre-Post Pilot Study. JSM Sexual Med 6(4): 1097.
INTRODUCTION
As the most prevalent subtype of vulvodynia, provoked vestibulodynia (PVD) is a chronic vulvar pain disorder characterized as pain and burning sensations localized to the vulvar vestibule, usually evoked by physical contact (e.g., sex, tampon use, clothing) [1,2]. With a prevalence rate of 8 to 16%, PVD is the leading cause of painful intercourse in reproductiveaged women [3-5], contributing to a cascade of negative effects on intimate relationships and sexual health as well as increased stress, anxiety, and poor cognitive coping skills [6-8].
PVD has been consistently linked to symptom-associated changes in brain structure and function, particularly in brain networks involved in pain processing and cognitive modulation of the pain experience [9-15]. Women with PVD, compared to healthy women, display symptom-associated alterations in the resting-state functional connectivity (RSFC) of default mode, sensorimotor, salience/ventral attention and executive control/ dorsal attention networks. The default mode network is involved in passive self-referential processing, including monitoring internal thoughts and rumination [16]. The sensorimotor network receives sensory input from the periphery and is important for awareness of body sensations and generation of appropriate motor responses [17]. The salience/ventral attention network responds to subjective salience of any interoceptive and exteroceptive stimulus reaching the brain, or to the expectation of such stimulus, and coordinates appropriate attentional, behavioral, affective, and visceral responses to such stimuli.18 The executive control/dorsal attention network coordinates with multiple brain networks to adjust behavior in response to changing demands [18]. As reported in patients with chronic pain conditions, these brain networks are thought to reflect the neurobiological substrates of clinical symptoms, including disordered information processing such as biased threat appraisal, catastrophizing, and symptom-focused attention.
First-line recommended treatments for PVD include psychological treatment and pelvic floor physical therapy, while vestibulectomy surgery is a second- or third-line approach [19]. In particular, mindfulness-based stress reduction (MBSR) treatment alone and combined with cognitive behavioral therapy has been shown to reduce pain in experimental and clinical settings and improve symptoms in chronic pain conditions [20- 23]. Specifically, women with PVD have been shown to benefit from mindfulness interventions with reduced pain vigilance, pain catastrophizing, and genital pain provoked by cotton swab in addition to improved sexual functioning and mood [24,25].
While the mechanisms by which mindfulness impacts chronic pain are likely broad, it appears to engage brain networks responsible for self-referential processing, rumination, cognitive control, and attentional focus. The resulting shift in appraisal of incoming sensory information alters the conscious pain experience [26,27]. Furthermore, MBSR has been shown to change brain structure and function in healthy individuals and patients with chronic pain. These MBSR-related brain changes have been associated with symptom improvement in chronic pain conditions. Specifically, MBSR has changed functioning of brain networks known to be altered in PVD, including the default mode, sensorimotor, salience and executive control/dorsal attention networks [27-31]. To date, the neural mechanisms underlying mindfulness-induced symptom change have yet to be studied in PVD. Identification of these neural substrates can be used to inform the development and optimization of behavioral therapies to specifically target symptoms in PVD.
The goal of this single-arm interventional pilot study was to estimate the effects of an 8-week MBSR group treatment in two areas: 1) changes in clinical measures including vulvar pain and muscle tenderness, mood, and rumination and 2) changes in brain RSFC. We hypothesized that MBSR training would: 1) increase mindfulness, reduce vulvar pain and vaginal muscle tenderness, improve mood, and decrease rumination and 2) alter the RSFC of default mode, sensorimotor, salience, and executive control network regions. We did not have specific hypotheses regarding the direction of change in resting state functional connectivity following MBSR. However, we posited that the observed changes in brain RSFC would correlate with increased mindfulness and improvements in vulvar pain and muscle tenderness, thereby supporting changes in the top-down regulation of pain.
METHODS
Participants
Participants were recruited via advertisement on social media, Craigslist, campus-wide emails via the Registrar’s office at the University of California, Los Angeles (UCLA), onsite recruiting by the study coordinator at the UCLA Division of Digestive Diseases general gastrointestinal clinics and obstetrics/ gynecology (OB/ GYN) clinics, and college newspapers. PVD was confirmed by an OB/GYN physician or specialized Nurse Practitioner, both with expertise in the examination of women with vulvar pain. All participants provided written informed consent to participate and were compensated for completing experimental assessments.
Inclusion/Exclusion Criteria
Inclusion criteria included: 1) Female 18 to 55 years of age. 2) At least 3-month history of pain, burning, or irritation with an intensity of 4 out of 10 or greater of the vulva either localized to the vestibule and precipitated by contact of the vestibule or located in other regions of the vulva. 3) Generally healthy without current neurological, cardiovascular, hepatic, renal, autoimmune diseases, diabetes or cancer. 4) Willingness to use acceptable contraceptive methods (e.g., hormonal, barrier, or sterilization) if sexually active. 5) No prior training in MBSR or other mindfulness or meditation training. 6) Eligibility for magnetic resonance imaging (MRI) assessment was an inclusionary criterion for participants recruited to the second group; however, we made an exception for one subject who refused to undergo imaging and one subject who was MRI ineligible due to a metal rod in her leg.
Exclusion criteria included: 1) Planned major medical intervention or surgery within 3-months of MBSR training start. 2) Presence of a significant and ongoing medical problem that would interfere with participation in the study. 3) Presence of a psychiatric diagnosis. 4) Current use of centrally acting medications that will interfere with the vulva sensory testing. 5) History of drug abuse (including major nicotine dependence). 6) Body mass index greater than 35 (morbid obesity). 7) Pregnancy or planning a pregnancy, post-partum less than 1 year, or currently breastfeeding or post-menopausal women defined as no menses for 12 consecutive months. 8) Past medical history of other chronic pain disorders. 9) Medications or treatments for vulvodynia, including psychological or physical therapy, that have been initiated less than 3-months prior to the study. Topical medication such as gabapentin or estrogens are allowed.
The main goal of this study was parameter estimation and calculation of effect size changes in clinical measure and functional brain imaging. Given budgetary restrictions, which limited sample size and ability to recruit a treatment control group, the study employed a pre-post design in which subjects served as their own control. As such, our goal was to recruit at least 10 subjects in each sequential group which provided adequate power (80%) to detect effect size changes as big as Cohen’s dz =.66 in the study outcome variables within Group 1 and Group 2 [tcrit(9) = 2.78, no centrality parameter = 2.78, 2-sided dependent t-test, alpha = .10] [32]. Of note, Group 1 was recruited primarily to assess for feasibility. After successful implementation in Group 1, an additional group (Group 2) was recruited to include brain imaging pre-, post-MBSR treatment, and an additional ecological valid assessment of vulvovaginal pain.
PROCEDURE
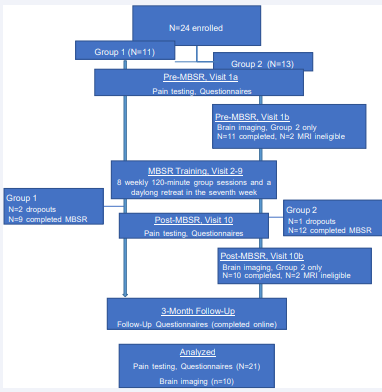
Two sequential groups of patients (n1 = 11, n2 = 13, age range: 18-50 years) were enrolled to examine the effects an 8-week MBSR group treatment on PVD symptoms and RSFC. Figure 1 presents a flow diagram of the study design. Within two weeks prior to the first class, all participants attended a clinical visit to ensure the diagnosis of PVD. Additionally, all participants underwent sensory and pain testing and completed study questionnaires before and after the 8-week MBSR group treatment. When excluding dropouts, a total of 21 participants (n1 = 9, n2 = 12) completed clinical assessments and study questionnaires postMBSR. A subgroup of women in Group 2 (n = 10 of 13) underwent structural and resting-state MRI pre- and post-MBSR. Brain imaging sessions occurred within two weeks before the first class and two weeks after the last class. Participants also provided 3-month follow up reports via the internet.
The diagram depicts the flow of participants throughout the study. A total of 21 out of 24 women with PVD completed MBSR treatment and symptom assessment. Only participants in Group 2 underwent pre- and post-MBSR brain imaging. Out of 13 participants initially enrolled in Group 2, a total of 10 patients completed both imaging sessions; one dropped out of the study, and two were MRI ineligible. Abbreviations: PVD=provoked vulvodynia, MBSR=Mindfulness Based Stress Reduction, MRI= magnetic resonance imaging, N=sample size
MBSR training
An MBSR instructor (S. Smith) with conducted the training at UCLA over a decade of experience. The MBSR intervention was based on the standard MBSR protocol developed and disseminated from the Center for Mindfulness in Medicine, Health Care, and Society at the University of Massachusetts Medical School and has been previously used for irritable bowel syndrome [22]. The MBSR training consisted of eight weekly 120-minute group sessions and a 4-hour retreat in the seventh week, totaling 20 class hours. Each group session included education guided meditations, awareness exercises, yoga, and group discussions and inquiry. The training was designed to foster mindful awareness and a non-judgmental attitude to internal and external stimuli, as well as promote responding versus reacting to stress. Participants were instructed to redirect their attention back to the present moment with a kind attitude when their mind wandered during the practices. Some of the movement practice poses included yoga poses adapted for relaxing the lower part of the body. Additionally, examples of PVD symptoms were used as teaching points to better understand different aspects of mindfulness. Finally, participants were also expected to engage in 20-30 minutes of daily home practice 6 days a week during the 8-week course. They were provided with audio recordings of guided meditations and yoga instructions to support home practice and encourage integration of mindfulness into daily activities. They were instructed to continue the at-home practice until study completion.
MEASURES
Clinical assessment of PVD
During the clinical visit and prior to brain imaging, participants reported detailed information regarding vulvar pain, including pain duration, whether pain arises when provoked and/or spontaneously, and whether the onset of symptoms was primary or secondary (at or after first introital penetration, respectively) [3]. Additionally, a brief neurosensory examination was conducted. Pain testing of the vulva and vestibule was also performed using a cotton swab, which is the main diagnostic tool for PVD [33]. Sensory testing of the sensory dermatomes (T12, L1, S2, S3/4, S5, S1) of the mons pubis and the perineum were examined bilaterally for allodynia (pain with gentle touch with the cotton tip), hyperalgesia (pain with gentle touch with the sharp wooden end of a broken cotton swab), or normal sensation. This examination was to exclude specific neuropathy.
Mapping vulvar vestibular pain and vaginal muscle tenderness
Before and after MBSR, the study OB/GYN mapped patient pain in the vulvar vestibule by touching the vestibule perpendicularly with the cotton end of a swab (enough to indent the mucosa to a depth of less than one-third of the cotton end) for 1 second at 5, 6, 7 (posterior vestibule), 10, 12, and 2 o’clock (peri-urethral). Participants were asked to rate the pain at each site on a scale ranging from 0 (no pain) to 10 (worse pain imaginable). Pain scores across all sites on the cotton swab test were summed for a total vulvar vestibular pain score (0-60). Internal muscle pain was assessed with a single lubricated digit, applying approximately 2 kg of pressure for 2 seconds (the examiner’s finger pressure was calibrated before the exam with an algometer). The right and left levator ani muscles (in the vagina) and the perineal complex (at the vaginal entrance) were assessed. Participants were asked to rate the pain severity at each site on a scale ranging from 0 (no pain) to 10 (worse pain imaginable). Scores were summed across all locations to compute a total vaginal muscle tenderness score (0-30) [34]. Total vulvar pain and total muscle tenderness were used in the analysis. Participants in Group 2 also selfadministered a standardized tampon insertion in the clinic and removal test to measure provoked vulvovaginal pain [35]. Pain was rated on a scale ranging from 0 (no pain) to 10 (worse pain imaginable).
Study questionnaires
Table 1 displays the schedule of assessments by group. In both groups, mindfulness skills were measured at pre-MBSR, post-MBSR, and 3-month follow up using the Mindfulness Awareness Attention Scale (MAAS) and the Five Facets of Mindfulness Questionnaire (FFMQ) subscales [36,37]. The MAAS is a 15-item unidimensional measure of mindfulness that ask participants to report the frequency of their mindful states in daily life. The FFMQ is a validated measure of five specific aspects of mindfulness: 1) non-judgment of experience, 2) non-reactivity to internal experience, 3) describing internal experience, 4) observing internal experience, and 5) acting with awareness. The FFM questionnaire is rated on a 5-point Likert scale ranging from 1 (never or very rarely true) to 5 (very often or always true). Scores on the subscales were summed to obtain a total score. Higher FFM total and MAAS scores reflect greater mindfulness. Scores on the subscales were to obtain a total score.
| Outcome Variable | Pre-MBSR | Post-MBSR | 3-Month Follow Up | ||||
| Group 1 | Group 2 | Group 1 | Group 2 | Group 1 | Group 2 | ||
| Pain | Vulvar pain total | X | X | X | X | ||
| Pain at 6 o'clock | X | X | X | X | |||
| Tampon test | X | X | |||||
| Vaginal muscle tenderness total | X | X | X | X | |||
| Mindfulness | FFM Total Score | X | X | X | X | X | X |
| MAAS Score | X | X | X | X | X | X | |
| Impressions of MBSR training and practice | X | X | |||||
| Hours per week engaged in MBSR practice | X | ||||||
| Psychological | HADS Anxiety | X | X | X | X | X | X |
| HADS Depression | X | X | X | X | X | X | |
| PCS Rumination | X | X | X | X | X | X | |
| Brain Imaging | X | X | |||||
| Note. This table shows the type of outcome variables assessed at each visit (Pre-MBSR, Post-MBSR, and 3-Month Follow Up) by group. Assessments measured at pre-MBSR occurred within two weeks prior to the beginning of MBSR treatment. Assessments measured at post-MBSR and 3-Month Follow Up occurred directly after MBSR treatment and three months after treatment, respectively. Both groups completed MBSR treatment. Only Group 2 underwent brain imaging pre- and post-MBSR. Abbreviations: MBSR=Mindfulness Based Stress Reduction, FFM=Five Facets of Mindfulness, MAAS=Mindful Attention Awareness Scale, HADS=Hospital Anxiety and Depression Scale, PCS=Pain Catastrophizing Scale | |||||||
Both groups also completed the Hospital Anxiety and Depression Scale (HADS) to measure state anxiety and depression over the past two weeks [38]. On both scales, scores from 0-8 are considered a noncase, 8-10 doubtful case, and 11-14 definiteness case. Finally, we administered the Pain Catastrophizing Scale (PCS), the 13-item scale assessing pain-related catastrophic thinking because of helpless, rumination and magnification [39]. From this, we computed scores on the rumination subscale.
Group 2 participants were asked to answer questions regarding their impression of the MBSR training at post-treatment and at 3-month follow up. The assessment questions included: 1) Do you believe the Mindfulness (MBSR) training and practice improved your vulvodynia symptoms and 2) Do you believe the Mindfulness (MBSR) training and practice affected your overall well-being? [21-point numeric scale ranging from -10 (made it worse) to 0 (no effect) to 10 (made it much better)]. Additionally, at the 3-month follow up assessment, they also reported on the total hours per week they continued to engage in the mindfulness practices learned in class and the type of practices (body scan, mindfulness of breathing, yoga, and others).
Brain imaging of functional connectivity during the resting-state
Detailed information regarding specific brain imaging acquisition protocols, quality control, preprocessing, and computation of region-to-region brain connectivity can be found in Supplemental Methods I. Briefly, high resolution structural and a resting state functional MRI data (10-minute 6-seconds eyesclosed) was acquired using a 3.0T MRI scanner (Siemens Trio; Siemens, Erlangen, Germany). This data was used to derive the RSFC between spatially distinct cortical, subcortical and brain stem regions.
Computation of region-to-region functional connectivity during the resting-state
Structural and functional images were entered in CONN 17 toolbox in MATLAB for preprocessing, denoising, and analysis [40,41]. As the first step of calculating RSFC, whole brain images were parceled into brain regions and associated networks. To define cortical regions and networks, we used the Schaefer 400 functional cortical parcellation atlas [42]. This functional connectivity-based atlas parcels the brain into 400 functional, meaningful brain regions that comprise 17 networks, which are then grouped into 8 major networks. These networks include the Somatomotor, Dorsal Attention, Salience/Ventral Attention, Control, Default Mode, Visual (Central, Peripheral), Temporal Parietal (TempPar), and Limbic. The Temporal Parietal network is comprised only of temporal and parietal regions. The Limbic network comprises areas in the orbital frontal cortex and the ventral temporal pole. The Harvard-Oxford subcortical was used to define subcortical regions, including the bilateral thalamus, amygdala, hippocampus, and basal ganglia regions [caudate nucleus, pallidum (global pallidus), putamen, and the nucleus accumbens] [43]. Finally, the Harvard Ascending Arousal Network atlas was used to define brainstem nuclei including the locus coeruleus, mesencephalic reticular formation, parabrachial complex, nucleus reticularis pontis oralis, and the pedunculopontine nucleus bilaterally and the dorsal raphe, median raphe, periaqueductal gray, and the ventral tegmental area [44].
For each participant, RSFC was computed between all brain regions pairwise using bivariate Pearson correlations from the preprocessed and denoised data. RSFC reflects the association between average BOLD time series signals across all voxels in each brain region.
DATA ANALYSES
Estimation of the effects of MBSR on study outcome variables
The main goal of the study was parameter estimation and calculation of effect size changes in symptom and RSFC assessments. To this end, post-treatment effect size changes in pain (total vulvar pain, pain at 6’oclock, pain evoked with the tampon test, and total vaginal muscle tenderness scores), mindfulness, mood (state anxiety, depression), and rumination were computed using Hedges’ g, based on the variable mean differences calculated from –
and the standard deviation of the differences and accounting for the correlation between the paired assessments [45]. Hedges’ g is similar to Cohen’s dz but adjusts for small sample sizes. For interpretation, an effect size of Hedges’ g = .80 is considered large, Hedges’ g = .50 is medium, and Hedges’ g = .20 is small [46].
Since the goal of the study involved estimation of parameters and effect sizes in order to plan for future studies, in lieu of significance testing, we provided 90% confidence intervals (CI) for parameter estimates. A CI that includes zero suggests no effect is present. A 90% CI was chosen to balance type I and type II error control.
The estimates for the effect size change (Hedge’s g) in brain RSFC were derived in CONN using a general linear model specifying the effect for subjects (average effect for subject) and time (Pre, Post). However, 99.9% confidence intervals were calculated for more conservative error control, and brain connectivity changes not containing zero were reported. Associations between observed changes in brain connectivity and changes in pain, mindfulness, anxiety, depression, and rumination at post-MBSR were estimated using Pearson’s correlations, and 90% confidence intervals were computed. As a rule of thumb, r = .10 is interpreted as weak or small association, r = .30 moderate, and r >= .50 strong or large.46 Although we used r >= |.40| as a threshold for reporting associations in the tables, we reported an effect when confidence intervals around the estimate do not contain zero.
RESULTS
As shown in Figure 1, the study enrolled 11 participants in Group 1, and 13 participants in Group 2. In Group 1, 9 out of 11 participants completed MBSR treatment; two were dropped due to not attending enough classes. In Group 2, 12 out of 13 participants completed MBSR treatment; one was dropped due to lack of adherence to the study protocol, including failure to arrive on time to classes and not adhering to home practice. Additionally, a total of 10 participants underwent MRI in Group 2; two participants were MRI ineligible but completed all questionnaires.
Figure 1 Flow diagram of participant progress through the study.
The diagram depicts the flow of participants throughout the study. A total of 21 out of 24 women with PVD completed MBSR treatment and symptom assessment. Only participants in Group 2 underwent pre- and post-MBSR brain imaging. Out of 13 participants initially enrolled in Group 2, a total of 10 patients completed both imaging sessions; one dropped out of the study, and two were MRI ineligible. Abbreviations: PVD=provoked vulvodynia, MBSR=Mindfulness Based Stress Reduction, MRI= magnetic resonance imaging, N=sample size
Characteristics of the Sample
The final sample of participants who completed MBSR comprised a total of 21 women with PVD (n1 = 9, n2 = 12), with a mean age of 31.6 (SD = 7.2, range: 20 – 51 years). There were 13 women with primary onset PVD and 8 with secondary PVD. 2 women also endorsed unprovoked pain, and one of these women also met criteria for provoked and unprovoked generalized vulvodynia. The average reported pain duration was 8.25 years (SD = 7.78). On average, participants reported moderate levels of state anxiety of 9.2 (SD = 3.9). On average participants attended 8.31 (SD = 1.1) of the nine sessions.
Effect of MBSR on pain, mood, and cognitive assessments: Table 2 presents the means for the pain, mindfulness, and psychological variables at each assessment. Table 3 contains the estimated mean differences and effect size changes at post treatment and at 3-month follow up.
| Outcome Variable | Pre-MBSR | Post-MBSR | 3-Month Follow Up | |||||||
| N | Mean | SD | N | Mean | SD | N | Mean | SD | ||
| Pain | Vulvar pain total | 21 | 26.90 | 9.18 | 19 | 21.37 | 8.34 | |||
| Pain at 6 o'clock | 21 | 5.67 | 2.54 | 19 | 5.00 | 2.16 | ||||
| Tampon test | 12 | 6.00 | 3.08 | 12 | 6.00 | 3.08 | ||||
| Vaginal muscle tenderness total | 21 | 9.86 | 7.14 | 18 | 9.67 | 5.55 | ||||
| Mindfulness | FFM Total Score | 20 | 125.85 | 14.00 | 20 | 138.90 | 18.99 | 21 | 143.57 | 18.18 |
| MAAS Score | 21 | 3.62 | 0.72 | 20 | 3.82 | 0.58 | 21 | 4.02 | 0.77 | |
| Psychosocial | HAD Anxiety | 21 | 9.23 | 3.94 | 21 | 8.00 | 3.73 | 21 | 7.71 | 4.20 |
| HAD Depression | 21 | 4.00 | 3.18 | 21 | 2.76 | 2.02 | 21 | 2.81 | 2.80 | |
| PCS Rumination | 20 | 5.85 | 4.40 | 20 | 4.20 | 3.24 | 21 | 4.76 | 3.82 | |
| Note. This table shows the mean of each outcome variable at Pre-MBSR, Post-MBSR, and 3-month Follow Up. Abbreviations: MBSR=Mindfulness Based Stress Reduction, FFM=Five Facets of Mindfulness, MAAS=Mindful Attention Awareness Scale, HAD=Hospital Anxiety and Depression, PCS=Pain Catastrophizing Scale, N=sample size | ||||||||||
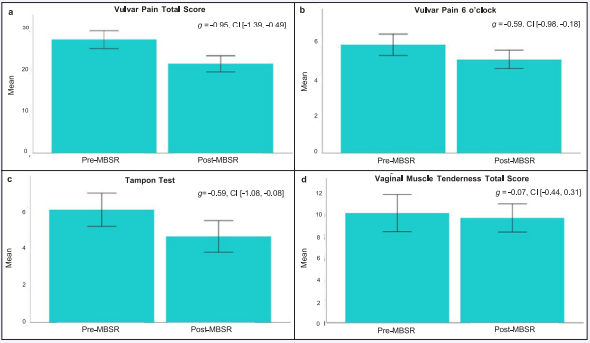
Effects of MBSR on pain: As shown in Figure 2 A-D, improvement in PVD symptoms after MBSR was supported by a large effect size reduction in total vulvar pain (Hedges’ g = -0.95, 90% CI [-1.39, - 0.49]) and moderate effect size reductions in pain at 6 o’clock (Hedges’ g = -0.59, 90% CI [-0.98., -0.18]) and provoked pain measured by the Tampon Test (Hedges’ g = -0.59, 90%, CI [-1.08, -0.08]). No change in total vaginal muscle tenderness was indicated (Hedges’ g = -0.07, 90% CI [-0.44, 0.31]). Further inspection of this variable showed that 50% of the participants reported no change or increased muscle tenderness, while the other 50% reported reductions in muscle tenderness.
Figure 2: Change in vulvar pain and vaginal muscle tenderness following MBSR.
The bar plots show the estimated mean and standard error at Pre- and Post-MBSR for (A) vulvar pain total score, (B) vulvar pain at 6 o’clock, (C) pain evoked during tampon test, and (D) vaginal muscle tenderness. For each outcome variable, the estimated effect size (Hedges’ g) change and 90% confidence interval are included. Hedges’ g index the pre- to post-MBSR effect size change. Abbreviations: MBSR=Mindfulness Based Stress Reduction, g=effect size Hedges’ g, CI=90% confidence interval.
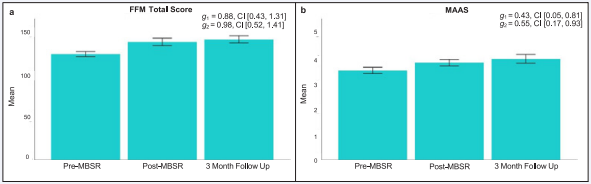
Figure 3: Changes in mindfulness following MBSR.
The bar plots present the estimated mean and standard error at Pre-MBSR, Post-MBSR, and 3-Month Follow-Up for (A) FFM Total Score and (B) MAAS. For each outcome variable, the estimated effect size (Hedges’ g) change and 90% confidence interval are included. Hedges’ g1 index the preto post-MBSR effect size change, and Hedges’ g2 index pre-MBSR to 3-month follow up effect size change. Abbreviations: MBSR=Mindfulness Based Stress Reduction, FFM=Five Facets of Mindfulness, MAAS=Mindful Attention Awareness Scale, g=effect size Hedges’ g, CI=90% confidence interval
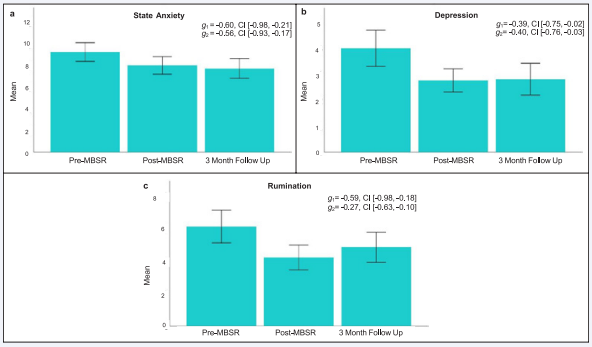
Effects of MBSR on mindfulness, mood and rumination: As expected, and depicted in Figure 3 A-C, mindfulness measured by the FFM total score exhibited a large effect size increase at post-MBSR (Hedges’ g = 0.88, 90% CI [0.43,1.31]) and at 3-month follow up (Hedges’ g = 0.98, 90% CI [0.52,1.41]). In addition, when measured using the MAAS, mindfulness showed moderate increases post-MBSR (Hedges’ g = 0.43, 90% CI [0.05, 0.81]), and at 3-month follow up (Hedges’ g = 0.55, 90% CI [0.17, 0.93]). Figure 4 A-C presents changes in mood and rumination. Moderate effect size improvements in state anxiety (Hedges’ g = -0.60, 90% CI [-0.98, -0.21]; Hedges’ g = -0.56, 90% CI [-0.93, -0.17]) and depression (Hedges’ g = -0.39, 90% CI [-0.75, -0.02]; Hedges’ g = -.40, 90% CI [-0.76, -0.03]) were observed at postMBSR and 3-month follow up. Finally, rumination showed moderate effect size reductions post-MBSR (Hedges’ g = -0.59, 90% CI [-0.98, -0.18]) but not at 3-month follow up (Hedges’ g = -0.27, 90% CI [-0.63, 0.10]).
Figure 4: Changes in mood and rumination following MBSR.
The bar plots depict the estimated mean and standard error at Pre-MBSR, Post-MBSR, and 3-Month Follow-Up for (A) State Anxiety, (B) Depression, and (C) Rumination. For each outcome variable, the estimated effect size (Hedges’ g) change and 90% confidence interval are included. Hedges’ g1 index the pre- to post-MBSR effect size change, and Hedges’ g2 index pre-MBSR to 3-month follow up effect size change. Abbreviations: MBSR=Mindfulness Based Stress Reduction, g=effect size Hedges’ g, CI=90% confidence interval.
Patient impressions of MBSR training and practice: Participants reported that MBSR training and practice improved their PVD symptoms post-treatment (n = 9, M = 5.78, SD = 2.95, range: 2 - 10) and at 3-month follow up (n = 12, M = 6.00, SD = 3.13, range: 1 - 10). They also indicated that MBSR training and practice improved their overall well-being post-MBSR (n = 11, M = 8.18, SD = 2.32, range: 4 - 10) and at 3-month follow up (n = 12, M = 7.50, SD = 2.78, range: 2 - 10). At 3-month follow up, participants reported engaging in MBSR practice on average 3.57 hours per week (n = 12, SD = 1.44, range: 1 - 6).
| Outcome Variable | Post-MBSR | 3-Month Follow Up | |||||||||||
| Mean Difference | SD | N | Hedges’ g | 90% CI | Mean Difference | SD | N | Hedges’ g | 90% CI | ||||
| lower | upper | lower | upper | ||||||||||
| Pain | Vulvar pain total | -5.79 | 5.99 | 19 | -0.95 | -1.39 | -0.49 | ||||||
| Pain at 6 o'clock | -0.79 | 1.32 | 19 | -0.59 | -0.98 | -0.18 | |||||||
| Tampon test | -1.42 | 2.31 | 12 | -0.59 | -1.08 | -0.08 | |||||||
| Vaginal muscle tenderness total | -0.44 | 6.51 | 18 | -0.07 | -0.44 | 0.31 | |||||||
| Mindfulness | FFM Total Score | 14.37 | 16.05 | 19 | 0.88 | 0.43 | 1.31 | 16.60 | 16.63 | 20 | 0.98 | 0.52 | 1.41 |
| MAAS Score | 0.31 | 0.69 | 20 | 0.43 | 0.05 | 0.81 | 0.40 | 0.71 | 21 | 0.05 | 0.17 | 0.93 | |
| Psychosocial | HAD Anxiety | -1.23 | 2.00 | 21 | -0.06 | -0.98 | -0.21 | -1.52 | 2.68 | 21 | -0.56 | -0.93 | -0.17 |
| HAD Depression | -1.24 | 3.15 | 21 | -0.39 | -0.75 | -0.02 | -1.19 | 2.93 | 21 | -0.40 | -0.76 | -0.03 | |
| PCS Rumination | -1.90 | 3.16 | 19 | -0.59 | -0.98 | -0.18 | -1.05 | 3.87 | 20 | -0.27 | -0.63 | 0.10 | |
| Note. This table depicts the mean difference and effect size (Hedges’ g) change of each outcome variable from Pre- to Post-MBSR and from Pre-MBSR to 3-Month Follow Up. Hedges’ g index the effect size change from Pre- to Post-MBSR and Pre-MBSR to 3-Month Follow Up. Positive (+) effect sizes indicate an increase in the value of the outcome variable from Pre-MBSR to Post-MBSR/3-Month Follow Up, and negative (-) effect sizes indicate a decrease. Bolded numbers reflect effect size changes whose 90% confidence interval does not contain zero, thereby suggesting an effect. Abbreviations: MBSR=Mindfulness Based Stress Reduction, FFM=Five Facets of Mindfulness, MAAS=Mindful Attention Awareness Scale, HADS=Hospital Anxiety and Depression, PCS=Pain Catastrophizing Scale, SD=standard deviation, N=sample size, CI=confidence interval | |||||||||||||
Changes in Brain RSFC Following MBSR
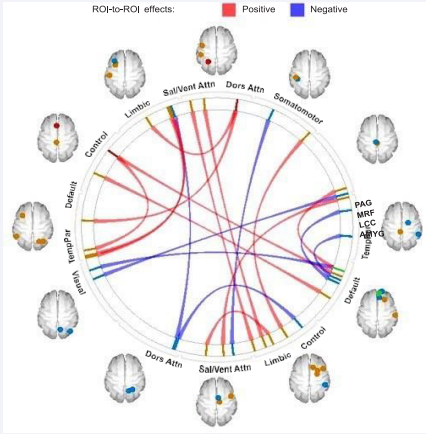
We observed several significant changes in RSFC after MBSR. As shown in Table 4 and Figure 5, these changes were primarily in default mode and salience network connectivity (15 of 20 pairwise connections) and consisted predominantly of increases in connectivity. Below we describe these specific changes organized by functional networks.
Figure 5 Changes in brain resting-state functional connectivity following MBSR.
| Conn No. | Network | Atlas ROI | Anatomical Region | Network | Atlas ROI | Region | Hedges’ g | 99.9% CI | |
| lower | upper | ||||||||
| 1 | Default | L_DefaultB_ PFCv_1 | lateral orbitofrontal cortex | DorsAttn | L_ DorsAttnA_ SPL_5 | superior parietal cortex | 1.79 | 0.92 | 2.61 |
| 2 | Default | R_DefaultB_ Temp_1 | middle temporal cortex | Control | L_ ControlB_ PFCmp_3 | superior frontal cortex | 1.65 | 0.82 | 2.43 |
| 3 | Default | R_DefaultB_ PFCd_1 | dorsomedial prefrontal cortex | Brainstem | R_Brainstem_PAG | Periaqueductal gray | 1.56 | 0.76 | 2.31 |
| 4 | Default | R_DefaultA_ pCunPCC_4 | dorsomedial prefrontal cortex | Default | L_ DefaultA_PFCd_1 | dorsal posterior cingulate cortex | 1.70 | 0.85 | 2.49 |
| 5 | Default | R_DefaultA_ PFCm_5 | prefrontal cortex | TempPar | R_ TempPar_6 | middle temporal cortex | 1.59 | -2.35 | -0.78 |
| 6 | Default | R_DefaultB_ PFCd_1 | dorsomedial prefrontal cortex | Visual | R_ VisCent_ExStr_9 | middle occipital cortex | -1.64 | -2.41 | -0.81 |
| 7 | Default | R_DefaultA_ PFCm_3 | pregenual anterior cingulate cortex | Subcortical | R_ Subcortical_ Amygdala | Amygdala | -1.68 | -2.47 | -0.84 |
| 8 | SalVentAttn | L_SalVentAttnA_ FrOper_1 | frontal operculum | Limbic | R_ Limbic_OFC_1 | medial orbitofrontal cortex | 1.57 | 0.76 | 2.32 |
| 9 | SalVentAttn | L_SalVentAttnA_ ParOper_1 | parietal operculum | Limbic | R_ Limbic_ TempPole_1 | inferior temporal cortex | 1.61 | 0.79 | 2.38 |
| 10 | SalVentAttn | R_SalVentAttnA_ PrC_1 | supplemental motor area | Limbic | R_ Limbic_ TempPole_5 | temporal pole | 1.66 | 0.83 | 2.44 |
| 11 | SalVentAttn | L_SalVentAttnB_ Ins_1 | anterior insula | Visual | R_ VisCent_ExStr_2 | lateral occipitaltemporal cortex | 1.76 | 0.89 | 2.44 |
| 12 | SalVentAttn | L_SalVentAttnB_ Ins_2 | anterior insula | Visual | R_ VisCent_ExStr_3 | medial occipitaltemporal cortex | 1.60 | 0.78 | 2.36 |
|
13 |
SalVentAttn | R_SalVentAttnA_ ParMed_1 | posterior midcingulate cortex | Brainstem | L_Brainstem_ LC.cluster001 | locus coeruleus complex | 1.79 | 0.92 | 2.61 |
| 14 | SalVentAttn | R_SalVentAttnA_ FrMed_3 | supplemental motor area | SomMot | L_ SomMotB_Aud_4 | posterior insula | -1.77 | -2.58 | -0.90 |
| 15 | SalVentAttn | L_SalVentAttnB_ PFCl_3 | middle frontal cortex | DorsAttn | R_ DorsAttnA_ SPL_7 | superior parietal cortex | -1.58 | -2.33 | -0.77 |
| 16 | DorsAttn | R_DorsAttnA_ SPL_8 | superior parietal cortex | Control | R_ ControlB_IPL_3 | inferior parietal/ somatosensory association cortex | -1.60 | -2.36 | -1.79 |
| 17 | DorsAttn | L_DorsAttnA_ SPL_5 | superior parietal cortex | Limbic | L_ Limbic_ TempPole_4 | inferior temporal cortex | 1.57 | 0.77 | 2.33 |
| 18 | Control | R_ControlA_ PFCl_2 | lateral prefrontal cortex | SomMot | L_ SomMotA_4 | primary motor cortex | 1.86 | 0.96 | 2.70 |
| 19 | Control | L_ControlB_ PFCmp_2 | superior parietal cortex | TempPar | L_TempPar_5 | superior temporal cortex | 1.60 | 0.79 | 2.36 |
| 20 | Visual | R_VisPeri_ ExStrInf_3 | lingual/medial occipito-temporal cortex | Brain Stem | L_ Brain Stem_MRF | mesencephalic reticular formation | -1.58 | -2.33 | -0.77 |
| Note. This table shows the result Pre- to Post-MBSR changes in bidirectional region-to-region pairwise functional connectivity, organized by networks. Hedges’ g index the Pre- to Post-MBSR effect size change. Positive (+) effect sizes reflect increased connectivity from Pre- to Post-MBSR, and negative (-) effect sizes reflect decreased connectivity. Conn No. is a number assigned to each listed pairwise connectivity based on order of presentation in the table for reference in subsequent tables and figures. Abbreviations: DorsAttn=Dorsal Attention, SalVentAttn=Salience/Ventral Attention, SomMot=Somatomotor, TempPar=Temporal Parietal, CI=confidence intervals, hemi=hemisphere, L=left, R=right. | |||||||||
The diagram depicts region-to-region changes from pre- to post MBSR treatment in resting-state functional connectivity changes organized by functional brain networks. The resting state connectivity reflects correlations between the average time series signal across all voxels in each brain region. The colored vertical bars reflect individual regions within the networks. The dots projected onto the brain templates correspond to the colored bars and reflect the spatial location of the regions. Change was calculated as Post-MBSR – Pre-MBSR. Red lines connecting two regions reflect increased connectivity after MBSR. Blue lines reflect decreased connectivity after MBSR.
| CONN No | Network | Network | Pre- to Post-MBSR Connectivity Change | Vulvar Pain Total | Pain at 6 o’clock | Tampon Test | ||||||
| r | 90% CI | r | 90% CI | r | 90% CI | |||||||
| lower | upper | lower | upper | lower | upper | |||||||
| 1 | Default | DorsAttn | Increase | -0.69 | -0.90 | -0.22 | -0.69 | 0.90 | -0.22 | |||
| 2 | Default | Control | Increase | -0.51 | -0.83 | 0.06 | -0.40 | -0.78 | 0.19 | |||
| 4 | Default | Default | Increase | -0.46 | -0.81 | 0.12 | ||||||
| 5 | Default | TempPar | Decrease | 0.68 | 0.20 | 0.90 | 0.59 | 0.05 | 0.86 | |||
| 6 | Default | Visual | Decrease | 0.46 | -0.12 | 0.81 | ||||||
| 8 | SalVentAttn | Limbic | Increase | -0.62 | -0.87 | -0.10 | -0.56 | -0.85 | 0.01 | |||
| 9 | SalVentAttn | Limbic | Increase | -0.41 | -0.78 | 0.19 | ||||||
| 10 | SalVentAttn | Limbic | Increase | -0.41 | -0.79 | 0.18 | ||||||
| 12 | SalVentAttn | Visual | Increase | -0.45 | -0.80 | 0.14 | -0.76 | -0.92 | -0.36 | |||
| 13 | SalVentAttn | Brainstem | Increase | -0.71 | -0.91 | -0.25 | ||||||
| 14 | SalVentAttn | SomMot | Decrease | 0.56 | 0.01 | 0.85 | 0.48 | -0.10 | 0.81 | |||
| 15 | SalVentAttn | DorsAttn | Decrease | 0.54 | -0.01 | 0.84 | 0.48 | -0.10 | 0.82 | |||
| 17 | DorsAttn | Limbic | Increase | -0.62 | -0.87 | -0.11 | ||||||
| 19 | Control | TempPar | Increase | -0.66 | -0.89 | -0.17 | -0.51 | -0.83 | 0.05 | |||
| Note. This table presents the correlations between change in pain measures and change in brain functional connectivity from Pre- to Post-MBSR. Change scores in connectivity and pain measures were calculated as Post-MBSR – Pre-MBSR. The number under the column Conn No. provides a reference to Table 4 which contains detailed information on the specific regions and magnitude of the brain changes. The direction of the brain connectivity change is provided. Threshold for reporting a correlation was r=|.40|. Bolded numbers reflect correlations whose 90% confidence interval does not contain zero, thereby suggesting an effect. Conn No.= references the pairwise connectivity showing changes in Table 4.Abbreviations: DorsAttn=Dorsal Attention, SalVentAttn=Salience/Ventral Attention, SomMot=Somatomotor, TempPar=Temporal Parietal, CI=confidence interval, r=Pearson’s correlation statistic | ||||||||||||
Abbreviations: MBSR=Mindfulness Based Stress Reduction, TempPar=Temporal Parietal, Dors Attn=Dorsal Attention, Sal/Vent Attn=Salience/ Ventral Attention, PAG= periaqueductal gray, MRF= mesencephalic reticular formation, LCC=locus coeruleus complex, AMYG=amygdala
Default network: Increased connectivity was observed with the dorsal attention network (lateral orbitofrontal cortex – superior parietal cortex), control network (middle temporal cortex – superior frontal cortex), and the brainstem (dorsomedial prefrontal cortex (dmPFC) – periaqueductal gray). Increased connectivity was also found within the default mode (dmPFC – dorsal posterior cingulate cortex). Decreased connectivity was found with the visual (dmPFC – occipital cortex) and temporal parietal (prefrontal cortex – middle temporal cortex) networks. Decreased connectivity was also seen between the amygdala and pregenual anterior cingulate cortex of the default mode network.
Salience/Ventral Attention Network: The salience network exhibited multiple increased connectivities with the limbic network (frontal operculum – medial orbitofrontal cortex, parietal operculum – inferior temporal cortex, and supplemental motor area (SMA) – temporal pole) and the visual network (anterior insula – lateral occipital-temporal cortex, anterior insula – medial occipital-temporal cortex). Increased connectivity was also observed with a key noradrenergic arousal region of the brainstem (posterior midcingulate cortex – locus coeruleus complex). Decreased connectivity was found with the dorsal attention (middle frontal cortex – superior parietal cortex) and somatomotor networks (SMA – posterior insula).
Dorsal Attention Network: In addition to the changes in connectivity observed between the dorsal attention’s superior parietal cortex and default mode network and salience network (noted above), this region also exhibited increased connectivity with the limbic network (inferior parietal cortex) and reduced connectivity with the control network (inferior parietal cortex)
Control Network: In addition to the previously described alterations with the default mode and dorsal attention networks, the control network also showed increased connectivity with the temporal parietal network (superior parietal cortex – superior temporal cortex) and the somatomotor network (lateral prefrontal cortex – precentral primary motor cortex).
Visual Network: In addition to the previously mentioned changes in visual network region connectivity, the lingual/medial occipito-temporal cortex exhibited decreased connectivity with the midbrain reticular formation, a brainstem region.
Correlations between changes in RSFC and changes in clinical variables following MBSR
As depicted in Table 5-6, several of the changes, in default mode and salience ventral attention brain connectivity at postMBSR showed large effect size associations with reduced pain (Supplementary Figure S1 - S3) and increased mindfulness (Supplementary Figure S4 - S5). In particular, increased connectivity between the default mode and dorsal attention networks (lateral orbitofrontal cortex – superior parietal cortex) exhibited large effect size associations with reduced vulvar pain and increased mindfulness.
| CONN No. | Network | Network | Pre- to PostMBSR Connectivity Change | FFM total | MAAS | Anxiety | Depression | Rumination | ||||||||||
| r | 90% CI | r | 90% CI | r | 90% CI | r | 90% CI | r | 90% CI | |||||||||
| lower | upper | lower | upper | lower | upper | lower | upper | lower | upper | |||||||||
| 7 | Default | Subcortical | Decrease | -0.45 | -0.82 | 0.19 | 0.50 | -0.07 | 0.82 | |||||||||
| 3 | Default | Brainstem | Increase | 0.46 | -0.13 | 0.81 | ||||||||||||
| 2 | Default | Control | Increase | 0.74 | 0.27 | 0.92 | 0.63 | 0.06 | 0.89 | -0.47 | -0.83 | 0.16 | ||||||
| 4 | Default | Default | Increase | 0.43 | -0.21 | 0.81 | -0.47 | -0.81 | 0.11 | -0.73 | -0.91 | -0.29 | ||||||
| 1 | Default | DorsAttn | Increase | 0.79 | 0.38 | 0.94 | 0.65 | 0.11 | 0.90 | -0.49 | -0.84 | 0.13 | ||||||
| 9 | SalVenAtt | Limbic | Increase | -0.47 | -0.83 | 0.16 | ||||||||||||
| 12 | SalVenAtt | Visual | Increase | 0.51 | -0.11 | 0.84 | 0.61 | 0.03 | 0.88 | -0.75 | -0.93 | -0.29 | ||||||
| 11 | SalVenAtt | DorsAttn | Decrease | -0.68 | -0.91 | -0.16 | ||||||||||||
| 14 | SalVenAtt | SomMot | Decrease | -0.41 | -0.80 | 0.23 | ||||||||||||
| 8 | SalVenAtt | Limbic | Increase | 0.47 | -0.17 | 0.83 | 0.56 | -0.04 | 0.86 | -0.69 | -0.91 | -0.17 | ||||||
| 16 | Control | DorsAttn | Decrease | 0.52 | -0.04 | 0.83 | ||||||||||||
| 19 | Control | TempPar | Increase | 0.60 | 0.02 | 0.88 | ||||||||||||
| 20 | Visual | Brainstem | Decrease | 0.48 | -0.15 | 0.83 | ||||||||||||
| Note. This table depicts the correlations between change in brain function connectivity and change in cognitive and mood measures from Pre- to Post-MBSR. Change scores in connectivity and the outcome variables were calculated as Post-MBSR – Pre-MBSR. The number under the column Conn No. provides a reference to Table 4 which contains detailed information on the specific regions and magnitude of the brain changes. The direction of the brain connectivity change is provided. Threshold for reporting a correlation was r=|.40|. Bolded number reflect correlations whose 90% confidence interval does not contain zero, thereby suggesting an effect. Conn No.= references the pairwise connectivity showing changes in Table 4. Abbreviations: FFM=Five Facets of Mindfulness, MAAS=Mindful Attention Awareness Scale, DorsAttn=Dorsal Attention, SalVentAttn=Salience/Ventral Attention, SomMot=Somatomotor, TempPar=Temporal Parietal, CI=confidence interval, r=Pearson’s correlation statistic. | ||||||||||||||||||
Reduction in rumination was associated with increased salience/ventral attention connectivity (Supplementary Figure S6 A, B). Specifically, reduced rumination and increased mindfulness both exhibited a large effect size association with increased connectivity between the salience/ventral attention (anterior insula) and visual networks. We also observed an association between decreased depression and increased connectivity within the default mode (dPCC – dmPFC). However, as seen in Supplementary Figure S6 C, the scatterplot revealed an outlier. Removal of the outlier attenuated the magnitude of the correlation, r = -0.57, 90% CI [0.85, -0.02]. We did not observed association between changes in brain connectivity and changes in anxiety post-MBSR.
DISCUSSION
This exploratory single-arm interventional pilot study provides tentative support for the hypotheses that mindfulness training not only reduces symptoms but may also remodels brain connectivity in women with PVD. Participants reported reduced vulvar pain, improved mood, decreased rumination, and enhanced mindfulness skills, consistent with prior studies on mindfulness for PVD [23-25,47-49]. The changes in functional brain connectivity were observed primarily in default mode and salience/ventral attention network regions known to be altered in chronic pain conditions [50-52]. Furthermore, many changes in RSFC showed large effect size associations with improvements in pain, mindfulness, mood, and rumination. The study provides some evidence that mindfulness training may improve clinical features of PVD via impact on the brain’s RSFC; however, these findings must be interpreted with caution given the small sample size and lack of a control group.
As expected, moderate to large effect size increases in mindfulness were observed after MBSR, which continued onto the 3-month follow up period. Similarly, effect size reductions were observed in the pain evoked by the tampon test and the cotton swab. The improvements in provoked pain were mirrored in self-report measures of decreased anxiety, depression, and rumination, suggesting a global shift in wellbeing.
The most prominent brain changes identified in this study included those in the default mode and the salience/ventral attention networks. The default mode is a widely distributed brain network active during internally focused, non-task directed thought. It has been associated with sense of self, rumination, future planning, and introspective thought [50,53,54]. Function of the default mode network in isolation may even be considered antithetical to the principles of mindfulness, which promotes active awareness and acceptance of the present moment, thereby minimizing future predictions or self-referential judgement. Consistent with this, mindfulness-trained participants in this study showed increased connectivity of the default mode network with other brain networks, suggesting a shift from a predominantly inward, retrospective, ruminative focus to one characterized by more reciprocal connectivity with externally focused attentional, salience, or sensory networks. This is supported clinically by the parallel increase in mindfulness scores and reduction in pain, anxiety, and rumination. These changes in default mode connectivity align with research linking mindfulness treatment to the rewiring of a dysfunctional default mode network in other chronic pain conditions and mood disorders [53,55-58].
Specific increases in default mode network connectivity with the dorsal attention and control networks were observed and correlated with increased mindfulness scores. This is consistent with findings in the literature, which reports that trait mindfulness is related to increase RSFC in regions associated with attentional control and executive function [31]. Additionally, increased connectivity between the default mode network and the dorsal attention network correlated with improved vulvar pain scores. MBSR-induced increased connectivity between these networks may explain a patient’s ability to intentionally direct attention towards the present moment, thereby exerting control over the way one responds to symptom onset or environmental triggers rather than depending on pre-existing patterns of response. By consciously turning attention away from patterns created by maladaptive coping skills, such as catastrophization and rumination, women with PVD may reduce the amplification and impact of painful peripheral sensations.
The salience/ventral attention network orients attentional resources toward unattended stimuli based on sensory and limbic inputs, thus making it highly relevant to both pain disorders and mindfulness [18,59]. After MBSR, the salience network exhibited decreased connectivity with the dorsal attention and somatomotor networks, and increased connectivity with the limbic and visual networks and the brainstem. Many of these changes were associated with reduction in evoked vulvar pain. We have previously shown alterations in the intrinsic connectivity of the supplementary motor area in vulvodynia [10]. In this study, decrease connectivity between the supplementary motor area (salience/ventral attention) and posterior insula (somatomotor) correlated with a large effect size decrease in total vulvar pain of particular interest, patients exhibited increased RSFC between the posterior mid-cingulate cortex (salience/ventral attention) and the locus coeruleus complex (brainstem) following MBSR treatment, and this was associated with decreases in pain evoked by the tampon test. The posterior mid-cingulate cortex has been suggested to participate in the detection and allocation of attention to painful stimuli [60]. The locus coeruleus complex, a primary source of noradrenergic innervation for multiple brain regions, receives projections from cortical and subcortical regions and projects excitatory output to regions of the salience, emotional arousal, and sensorimotor networks [61,62].
Finally, increase in the salience/ventral attention network with visual network was associated with decreased rumination. This finding is consistent with previous studies showing alterations in the visual network in individuals with chronic pain and inflammation [63-66]. Although the exact role of alterations in the visual network in chronic pain remains to be determined, this network contains convergence zones, receiving and processing multisensory input, and has been shown to be involved in descending pain modulation [67]. Together, these finding suggest that mindfulness may induce changes in brain regions involved in the maintenance of hypervigilance to pain.
MBSR was also associated with decreased connectivity between the pregenual anterior cingulate cortex and amygdala. Part of the default mode network, this region of the cingulate cortex plays a role in modulating affective response to pain and is most likely engaged when inferring the emotional meaning of a given situation [68,69]. As part of the flight-or-flight reaction, the amygdala attaches emotional value to sensory input and serves as a warning system in response to threat [70,71]. Implicated in the amplification and cornification of pain [52,72,73], RSFC between the cingulate cortex and amygdala is thought to be associated with mediating fear learning and emotional processing [74- 76]. Therefore, reduced connectivity between the amygdala – pregenual anterior cingulate cortex in PVD may reflect a potential mechanism by which MBSR disrupts the acquisition and maintenance of learned fear. Overall, the brain connectivity and other measured behavioral changes noted in this patient group after MBSR suggests promotion of improved emotional reappraisal and regulation, helping patients develop a healthier approach to perceiving and coping with pain.
LIMITATIONS AND FUTURE RESEARCH
This pilot study identified brain targets and provided empirical estimates for effect sizes related to changes in symptoms and RSFC. However, estimates based on small sample size are often inflated, which needs to be considered when calculating power for a larger study [77]. While there was patient interest and clear feasibility to perform a larger scale trial, the findings were limited by the small sample size, absence of a control group, and lack of longitudinal assessments—mainly due to budgetary restrictions.
Although there was evidence that improvements in mindfulness and anxiety persisted at 3-month follow up, evoked pain at 3-month follow up was not assessed. Furthermore, the interpretation of 3-month follow up outcomes is limited, as we were unable to evaluate the stability of the brain changes 3-months after MBSR. The limited number of assessment points (pre- and post-treatment) and small sample size constrained our ability to determine whether MBSR-induced changes in the brain caused changes in symptoms or vice versa. Additional assessment points (e.g., mid-treatment and follow up) for brain and symptoms should be incorporated in future research design and analysis plans.
While the findings provide important framework for designing a more rigorous study, the lack of a control group limited our ability to discriminate MBSR-induced outcomes from outcomes related to other factors, such as the natural course of PVD and participant or researcher expectations. Although the findings suggest an association between the changes in the brain and symptoms post-MBSR, the cross-sectional correlation does not provide evidence of causation. In addition to including a control group, future studies will need to consider mediators and moderators of response to MBSR and incorporate prospective time-lagged statistical models.
CONCLUSION
The findings from this pilot study provide preliminary support for the hypotheses that an 8-week MBSR group treatment may result in brain RSFC changes and associated clinically relevant improvements in pain symptoms as well as positive shifts in measures of mood and rumination. Overall, the findings support the decision to conduct and inform the design for a larger confirmatory hypothesis-driven randomized control trial. Further longitudinal studies should also target the time course of symptoms and RSFC changes to better define the process through which individuals achieve improvements in this difficult-to-treat disorder.
ETHICS APPROVAL
The study was approved by the University of California, Los Angeles (UCLA) Institutional Review Board (IRB#13- 001113) and was conducted in accordance with the institutional guidelines regulating human subject research. Informed consent was obtained from all individual participants included in the study.
AUTHOR CONTRIBUTIONS
GT: assisted with data analysis, manuscript preparation and critical revisions
KT: study conceptualization and design, data interpretation, manuscript preparation and critical revisions.
AR: was the study physician responsible for final diagnosis and mapping vulvar pain and muscle tenderness. AR was also involved obtaining funding for the study, study conceptualization, design and execution, data interpretation, manuscript preparation and critical revisions.
SS: provide MBSR training and assisted with preparation of the manuscript
JS: was the study nurse responsible for recruitment, clinical assessments, data acquisition, and manuscript preparation
CL: was involved in the execution of the study, data collection, and management
*JSL: obtained funding for the study and was involved in study conceptualization and design, data analysis and interpretation, manuscript preparation and critical revisions.
All authors read and approved the final manuscript.
Each author made the following contributions:
1. Funding (AR, JSL).
2. Study conceptualization and design (AR, JSL, KT).
3. Data acquisition (AR, CL, JS, JSL, SS).
4. Data analysis (GT, JSL).
5. Data interpretation (AR, GT, JSL, KT).
6. Manuscript preparation and critical revisions (AR, GT, JSL, KT).
FUNDING
R01 NICHD076756 (JSL/AR), R21 NICHD086737 (JSL/AR).
REFERENCES
1. Jacob Bornstein, Mario Preti, James A Simon, Sawsan As-Sanie, Colleen K Stockdale, Amy Stein, et al. Descriptors of Vulvodynia: A Multisocietal Definition Consensus (International Society for the Study of Vulvovaginal Disease, the International Society for the Study of Women Sexual Health, and the International Pelvic Pain Society). J Low Genit Tract Dis. 2019; 23: 161-163.
2. Barbara Diane Reed, Siobán Denise Harlow, Ananda Sen, Laurie Jo Legocki, Rayna Monique Edwards, Nora Arato, et al. Prevalence and demographic characteristics of vulvodynia in a population-based sample. Am J Obstet Gynecol. 2012; 206: 171-179.
3. Jacob Bornstein, Andrew T Goldstein, Colleen K Stockdale, Sophie Bergeron, Caroline Pukall, Denniz Zolnoun, et al. 2015 ISSVD, ISSWSH and IPPS Consensus Terminology and Classification of Persistent Vulvar Pain and Vulvodynia. Obstet Gynecol. 2016; 127: 745-751.
4. Brotto LA, Nelson M, Barry L, Maher C. #ItsNotInYourHead: A Social Media Campaign to Disseminate Information on Provoked Vestibulodynia. Arch Sex Behav. 2021; 50: 57-68.
5. Lester RA, Brotto LA, Sadownik LA. Provoked Vestibulodynia and the Health Care Implications of Comorbid Pain Conditions. J Obstet Gynaecol Can. 2015; 37: 995-1005.
6. Arnold LD, Bachmann GA, Rosen R, Kelly S, Rhoads GG. Vulvodynia: characteristics and associations with comorbidities and quality of life. Obstet Gynecol. 2006; 107: 617-624.
7. Henzell H, Berzins K, Langford JP. Provoked vestibulodynia: current perspectives. Int J Womens Health. 2017; 9: 631-642.
8. Claudia Kraus Piper , Laurie J Legocki, Molly B Moravek, Katie Lavin, Hope K Haefner, Kathleen Wade, et al. Experience of symptoms, sexual function, and attitudes toward counseling of women newly diagnosed with vulvodynia. J Low Genit Tract Dis. 2012; 16: 447-453.
9. Ravi R Bhatt, Arpana Gupta, Andrea Rapkin, Lisa A Kilpatrick, Kareem Hamadani, Els Pazmany , et al. Altered Gray Matter Volume in Sensorimotor and Thalamic Regions associated with Pain in Localized Provoked Vulvodynia: A Voxel-based Morphometry Study. Pain. 2019; 160: 1529-1540.
10.Arpana Gupta, Andrea J Rapkin, Zafar Gill, Lisa Kilpatrick, Connor Fling, Jean Stains, et al. Disease-related differences in resting-state networks: a comparison between localized provoked vulvodynia, irritable bowel syndrome, and healthy control subjects. Pain. 2015; 156: 809-819.
11.Arpana Gupta, Davis C Woodworth, Benjamin M Ellingson, Andrea J Rapkin, Bruce Naliboff, Lisa A Kilpatrick, et al. Disease-Related Microstructural Differences in the Brain in Women With Provoked Vestibulodynia. J Pain. 2018; 19: 528.e1-528.e15.
12.Johnson P Hampson, Barbara D Reed, Daniel J Clauw, Rupal Bhavsar, Richard H Gracely, Hope K Haefner, et al. Augmented central pain processing in vulvodynia. J Pain. 2013; 14: 579-589.
13.Els Pazmany, Huynh Giao Ly, Leen Aerts, Michiko Kano, Sophie Bergeron, Johan Verhaeghe, Ronald Peeters , et al. Brain responses to vestibular pain and its anticipation in women with Genito-Pelvic Pain/ Penetration Disorder. Neuroimage Clin. 2017; 16: 477-490.
14.Caroline F Pukall, Irina A Strigo, Yitzchak M Binik, Rhonda Amsel, Samir Khalifé, M Catherine Bushnell, et al. Neural correlates of painful genital touch in women with vulvar vestibulitis syndrome. Pain. 2005; 115: 118-127.
15.Schweinhardt P, Kuchinad A, Pukall CF, Bushnell MC. Increased gray matter density in young women with chronic vulvar pain. Pain. 2008; 140: 411-419.
16.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network - Anatomy, function, and relevance to disease. Ann Ny Acad Sci. 2008; 1124: 1-38.
17.Emeran A Mayer, Jennifer Labus, Qasim Aziz, Irene Tracey, Lisa Kilpatrick, Sigrid Elsenbruch, et al. Role of brain imaging in disorders of brain-gut interaction: a Rome Working Team Report. Gut. 2019; 68: 1701-1715.
18.Vossel S, Geng JJ, Fink GR. Dorsal and ventral attention systems: distinct neural circuits but collaborative roles. Neuroscientist. 2014; 20: 150-159.
19.Andrew T Goldstein, Caroline F Pukall, Candace Brown, Sophie Bergeron, Amy Stein, Susan Kellogg-Spadt. Vulvodynia: Assessment and Treatment. J Sex Med. 2016: 13: 572-590.
20.Elizabeth Cash, Paul Salmon, Inka Weissbecker, Whitney N Rebholz, René Bayley-Veloso, Lauren A Zimmaro, et al. Mindfulness meditation alleviates fibromyalgia symptoms in women: results of a randomized clinical trial. Ann Behav Med. 2015: 49: 319-330.
21.Natalia E Morone, Carol M Greco, Charity G Moore, Bruce L Rollman, Bridget Lane, Lisa A Morrow, et al. A Mind-Body Program for Older Adults with Chronic Low Back Pain: A Randomized Clinical Trial. JAMA Intern Med. 2016: 176: 329-337.
22.Bruce D. Naliboff, Suzanne R. Smith, John G. Serpa, Kelsey Laird, Jean Stains, Jennifer Labus, et al. Mindfulness-based stress reduction improves irritable bowel syndrome (IBS) symptoms via specific aspects of mindfulness. Neurogastroenterol Motil. 2020: e13828.
23.Dunkley CR, Brotto LA. Psychological Treatments for Provoked Vestibulodynia: Integration of Mindfulness-Based and Cognitive Behavioral Therapies. J Clin Psychol. 2016; 72: 637-650
24.Brotto LA, Basson R, Smith KB, Driscoll M, Sadownik L. Mindfulnessbased Group Therapy for Women with Provoked Vestibulodynia. Mindfulness. 2015; 6: 417-432.
25.Lori A Brotto, Sophie Bergeron, Bozena Zdaniuk, Miriam Driscoll, Andrea Grabovac, Leslie A Sadownik, et al. A Comparison of Mindfulness-Based Cognitive Therapy Vs Cognitive Behavioral Therapy for the Treatment of Provoked Vestibulodynia in a Hospital Clinic Setting. J Sex Med. 2019; 16: 909-923.
26.Zeidan F, Vago DR. Mindfulness meditation-based pain relief: a mechanistic account. Ann N Y Acad Sci. 2016; 1373: 114-127.
27.Fadel Zeidan, Katherine T Martucci, Robert A Kraft, Nakia S Gordon, John G McHaffie, Robert C Coghill. Brain mechanisms supporting the modulation of pain by mindfulness meditation. J Neurosci. 2011; 31: 5540-5548.
28.Bilevicius E, Kolesar TA, Kornelsen J. Altered Neural Activity Associated with Mindfulness during Nociception: A Systematic Review of Functional MRI. Brain Sci. 2016; 6: 14.
29.Gotink RA, Meijboom R, Vernooij MW, Smits M, Hunink MG. 8-week Mindfulness Based Stress Reduction induces brain changes similar to traditional long-term meditation practice - A systematic review. Brain Cogn. 2016; 108: 32-41.
30.Nascimento SS, Oliveira LR, DeSantana JM. Correlations between brain changes and pain management after cognitive and meditative therapies: A systematic review of neuroimaging studies. Complement Ther Med. 2018; 39: 137-145.
31.Parkinson TD, Kornelsen J, Smith SD. Trait Mindfulness and Functional Connectivity in Cognitive and Attentional Resting State Networks. Front Hum Neurosci. 2019; 13: 112.
32.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007; 39: 175-191.
33.Bohm-Starke N, Johannesson U, Hilliges M, Rylander E, Torebjork E. Decreased mechanical pain threshold in the vestibu’lair mucosa of women using oral contraceptives - A contributing factor in vulvar vestibulitis? J Reprod Med. 2004; 49: 888-892.
34.Alappattu M, Robinson M, Lamvu G. Vulvodynia is not created equally: pain-related distress subgroups in vulvodynia. Journal of Pain. 2016; 17: S21-S21.
35.David C Foster, Merrill Beth Kotok, Li-Shan Huang, Arthur Watts, David Oakes, Fred M Howard, et al. The tampon test for vulvodynia treatment outcomes research: reliability, construct validity, and responsiveness. Obstet Gynecol. 2009; 113: 825-832.
36.Carlson LE, Brown KW. Validation of the Mindful Attention Awareness Scale in a cancer population. J Psychosom Res. 2005; 58: 29-33.
37.Ruth A Baer, Gregory T Smith, Emily Lykins, Daniel Button, Jennifer Krietemeyer, Shannon Sauer, et al. Construct validity of the five facet mindfulness questionnaire in meditating and nonmeditating samples. Assessment. 2008; 15: 329-342.
38.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 2083; 67: 361-370.
39.Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: Development and validation. Psychol Assessment. 1995; 7: 524-532.
40.Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012; 2: 125-141.
41.Nieto-Castanon, A. Handbook of functional connectivity Magnetic Resonance Imaging methods in CONN. Hilbert Press. 2020.
42.Alexander Schaefer, Ru Kong, Evan M Gordon, Timothy O Laumann, Xi-Nian Zuo , Avram J Holmes, et al. Local-Global Parcellation of the Human Cerebral Cortex from Intrinsic Functional Connectivity MRI. Cereb Cortex. 2018; 28: 3095-3114.
43.Dale AM, Fischl B, Sereno M I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999; 9: 179- 194.
44.Brian L Edlow, Emi Takahashi, Ona Wu, Thomas Benner, Guangping Dai, Lihong Bu, et al. Neuroanatomic connectivity of the human ascending arousal system critical to consciousness and its disorders. J Neuropathol Exp Neurol. 2012; 71: 531-546.
45.Hedges LV. Distribution theory for Glass’s estimator of effect size and related estimators. J Educ Stat. 1981; 6: 107-128.
46.Cohen JA. power primer. Psychol Bull. 1992; 112: 155-159.
47.Brotto LA, Basson R, Luria M. A mindfulness-based group psychoeducational intervention targeting sexual arousal disorder in women. J Sex Med. 2008; 5: 1646-1659.
48.Brotto LA, Bergeron S, Zdaniuk B, Basson R. Mindfulness and cognitive behavior therapy for provoked vestibulodynia: Mediators of treatment outcome and long-term effects. J Consult Clin Psychol. 2020; 88: 48-64.
49.Guillet AD, Cirino NH, Hart KD, Leclair CM. Mindfulness-Based Group Cognitive Behavior Therapy for Provoked Localized Vulvodynia: A Randomized Controlled Trial. J Low Genit Tract Dis. 2019; 23: 170- 175.
50.Alshelh Z, Marciszewski K K, Akhter R, Di Pietro F, Mills E P, Vickers E R, et al. Disruption of default mode network dynamics in acute and chronic pain states. Neuroimage Clin. 2018; 17: 222-231.
51.Ying Jiang, Desmond Oathes, Julia Hush, Beth Darnall, Mylea Charvat , Sean Mackey, et al. Perturbed connectivity of the amygdala and its subregions with the central executive and default mode networks in chronic pain. Pain. 2016; 157: 1970-1978.
52.Ong W Y, Stohler C S, Herr, D. R. Role of the Prefrontal Cortex in Pain Processing. Mol Neurobiol. 2019; 56: 1137-1166.
53.Harrison R, Zeidan F, Kitsaras G, Ozcelik D, Salomons TV. Trait Mindfulness Is Associated With Lower Pain Reactivity and Connectivity of the Default Mode Network. J Pain. 2019; 20: 645-654.
54.Simon R, Engstrom M. The default mode network as a biomarker for monitoring the therapeutic effects of meditation. Front Psychol. 2015; 6: 776.
55.Baliki MN, Geha PY, Apkarian AV, Chialvo DR. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J Neurosci. 2008; 28: 1398-1403.
56.Baliki MN, Mansour AR, Baria AT, Apkarian AV. Functional reorganization of the default mode network across chronic pain conditions. PLoS One. 2014; 9: e106133.
57.Letzen JE, Craggs JG, Perlstein WM, Price DD, Robinson ME. Functional connectivity of the default mode network and its association with pain networks in irritable bowel patients assessed via lidocaine treatment. J Pain. 2013; 14: 1077-1087.
58.Napadow V, Kim J, Clauw DJ, Harris RE. Decreased intrinsic brain connectivity is associated with reduced clinical pain in fibromyalgia. Arthritis Rheum. 2012; 64: 2398-2403.
59.Doll A, Holzel BK, Boucard CC, Wohlschlager AM, Sorg C. Mindfulness is associated with intrinsic functional connectivity between default mode and salience networks. Front Hum Neurosci. 2015; 9: 461.
60.Frot M, Mauguiere F, Magnin M, Garcia-Larrea L. Parallel processing of nociceptive A-delta inputs in SII and midcingulate cortex in humans. J Neurosci. 2008; 28: 944-952.
61.Cedarbaum JM, Aghajanian GK. Afferent projections to the rat locus coeruleus as determined by a retrograde tracing technique. J Comp Neurol. 1978; 178: 1-16.
62.Luppi PH, Aston-Jones G, Akaoka H, Chouvet G, Jouvet M. Afferent projections to the rat locus coeruleus demonstrated by retrograde and anterograde tracing with cholera-toxin B subunit and Phaseolus vulgaris leucoagglutinin. Neuroscience. 1995; 65: 119-160.
63.Jian Kong, Marco L Loggia, Carolyn Zyloney, Peichi Tu, Peter LaViolette, Randy L Gollub. Exploring the brain in pain: activations, deactivations and their relation. Pain. 2010; 148: 257-267.
64.Yohei Matsuo, Jiro Kurata, Miho Sekiguchi, Katsuhiro Yoshida, Takuya Nikaido, Shin-Ichi Konno. Attenuation of cortical activity triggering descending pain inhibition in chronic low back pain patients: a functional magnetic resonance imaging study. J Anesth. 2017; 31: 523-530.
65.Wei Shen, Yiheng Tu, Randy L Gollub, Ana Ortiz, Vitaly Napadow, Siyi Yu, et al. Visual network alterations in brain functional connectivity in chronic low back pain: A resting state functional connectivity and machine learning study. Neuroimage Clin. 2019; 22: 101775.
66.Hoameng Ung, Justin E Brown, Kevin A Johnson, Jarred Younger, Julia Hush, Sean Mackey. Multivariate classification of structural MRI data detects chronic low back pain. Cereb Cortex. 2014; 24: 1037-1044.
67.Glaucia Melo Reis, Quintino Moura Dias, João Walter S Silveira, Flavio Del Vecchio, Norberto Garcia-Cairasco, Wiliam A Prado. Antinociceptive effect of stimulating the occipital or retrosplenial cortex in rats. J Pain. 2010; 11: 1015-1026.
68.Vogt B A. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005; 6: 533-544.
69.Smith R, Ahern GL, Lane RD. The role of anterior and midcingulate cortex in emotional awareness: A domain-general processing perspective. Handb Clin Neurol. 2019; 166: 89-101.
70.Kolb B, Whishaw IQ. Fundamentals of human neuropsychology. 6th edn. Worth Publishers. 2009.
71.Craigmyle NA. The beneficial effects of meditation: contribution of the anterior cingulate and locus coeruleus. Front Psychol. 2013; 4: 731.
72.Javeria A Hashmi, Marwan N Baliki, Lejian Huang, Alex T Baria, Souraya Torbey, Kristina M Hermann, et al. Shape shifting pain: chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain. 2013; 136: 2751-2768.
73.Aaron Kucyi, Massieh Moayedi, Irit Weissman-Fogel, Michael B Goldberg, Bruce V Freeman, Howard C Tenenbaum, et al. Enhanced medial prefrontal-default mode network functional connectivity in chronic pain and its association with pain rumination. J Neurosci. 2014; 34: 3969-3975.
74.Jennifer S Labus, Arpana Gupta, Kristen Coveleskie, Kirsten Tillisch, Lisa Kilpatrick, Johanna Jarcho, et al. Sex differences in emotionrelated cognitive processes in irritable bowel syndrome and healthy control subjects. Pain. 2013; 154: 2088-2099.
75.Labus JS, Mayer EA, Jarcho J, Kilpatrick LA, Kilkens TOC, Evers EAT, et al. Acute tryptophan depletion alters the effective connectivity of emotional arousal circuitry during visceral stimuli in healthy women. Gut. 2011; 60: 1196-1203.
76.Hiroki Toyoda, Xiang-Yao Li, Long-Jun Wu, Ming-Gao Zhao, Giannina Descalzi, Tao Chen, et al. Interplay of amygdala and cingulate plasticity in emotional fear. Neural Plast. 2011; 2011: 813749.
77.Leon AC, Davis LL, Kraemer HC. The role and interpretation of pilot studies in clinical research. J Psychiatr Res. 2011; 45: 626-629.