Peptides: A Novel Approach to Enhance Eyelash and Eyebrow Growth
- 1. Jesselton Medical Centre, Kota Kinabalu, Malaysia
- 2. European Wellness Academy, Germany
- 3. European Wellness Academy, Germany FCTI Research & Development GmbH, Germany
Abstract
Purpose: Evaluate the safety and efficacy of topical peptides as a treatment to enhance eyelash and eyebrow appearance.
Subjects and methods: An open label cohort study over a period of 12 weeks was performed on healthy adult female subjects. All subjects daily applied a peptide extract solution on their eyelids and eyebrow. They also attended a fortnightly microneedle session to apply the peptide extract solution on the eyelash and eyebrow. The length of the eyelash was determined using an eyelash ruler while the overall eyelash appearance was assessed using the GEA scale. The eyebrows appearance was evaluated using digital photography and assessed by two independent dermatologist while individual eyebrow follicle thickness and the presence of new eyebrow hair follicles were evaluated using the Micro skin and hair diagnostic device (EW-EWB-1007-307, Germany).
Results: The peptide extract solution significantly increased the length of the eyelash by 3.7± 1.4mm (p = 0.014). There was a 100% improvement in the GEA scale evaluation, where 83% showed a one-grade improvement while the remaining 17% demonstrated a 17% improvement. Eyebrow analyser scans showed an increased thickness in individual eyebrow thickness and there were the presence of many new eyebrow hair follicles at the end of the 12 weeks.
Conclusion: Peptide extract solution is a safe and efficient treatment to enhance eyelash and eyebrow appearance without causing any major adverse reactions.
Keywords
Peptides; Eyelash elongation; Eyebrow thickness
Citation
Alvin G, Chernykh V, Chan M (2020) Peptides: A Novel Approach to Enhance Eyelash and Eyebrow Growth. J Dermatolog Clin Res 8(2): 1137.
INTRODUCTION
he eyelash and eyebrows are anatomical structures of the face that are closely associated with beauty but also central to all perceived human interaction [1]. The eyebrow protects the eye and its movements bequeaths the bearers emotion and an art of communication [1,2].
Aesthetics has intensified the craving for better technique and methods to improve the female facial appearance. Long and thick eyelash with equally beautiful eyebrows have long been the envy of all women [3,4]. The introduction of Iantropost to treat glaucoma resulted in eyelash hypertrichosis being reported as a regular phenomenon amongst its users [5]. 0.03% Bimatoprost solution was approved by the United States (US) Food and Drug Administration (FDA) for the indication of increasing the eyelash length, thickness and darkness [6]. However, these ophthalmic prostaglandins analogs have been associated with iris pigmentation, conjunctival hyperemia and anterior uveitis [7,8]. An invasive and permanent method to increase eyelash prominence is transplantation, which involves the transfer of scalp hair follicles onto the margins of the eyelid [9]. Herbbased formulations like the Jarilla-coffea extract and green tea extract have proven to be capable of inducing hair growth and increasing hair thickness and length without the need for invasive procedures nor the undesired ocular effects of the synthetic analogs of prostaglandin [10,11].
Peptide therapeutics commenced in medical practice since the advent of insulin therapy in the 1920’s [12]. A peptide is a chain of less than 50 amino acids linked by peptide bonds21. They are very specific in activity, stable, efficacious and are generally safe [13]. Peptides have a broad therapeutic spectrum, reflecting the potential utility across a wide range of indications. Peptides may be synthetic or a chain of amino acids naturally synthesized by the human body [14]. Some of the peptides identified in the skin like cathelicidins, copper peptides (GHK-Cu) and transforming growth factor (TGF-β) promote growth and regulation of hair follicles [15]. Oesser reported a study in Germany that collagen peptides significantly increased hair thickness in adult women after 16 weeks of treatment [16]. A study by Klopcic et al proved that peptide like TGF-β is essential for the development of hair as well as its proliferation and differentiation in adults [17].
The purpose of this study is to clinically evaluate the efficacy and safety of topical peptides in enhancing eyelashes and eyebrow growth. We sought to assess the clinical response objectively by measuring the length of individual eyelash growth and the density of the eyelashes using the Global Eyelash Assessment (GEA) and photographic assessment of eyebrow thickness by dermatologist. A micro skin and hair analyzer was used to scan the eyebrows to assess an improvement in hair growth in relation to thickness, presence of new hair follicles and an increase in individual eyebrow length. Assessment on adverse effects were conducted via questionnaires filled by subjects and examination conducted by a dermatologist.
MATERIALS AND METHODS
This is an open label cohort study over a period of 12 weeks from November 2019 to January 2020, conducted on healthy adult female subjects who voluntarily joined the study at the European Wellness Academy (Asia Pacific Hub) in Kota Kinabalu, Sabah, Malaysia
The protocol was approved by the institutional ethics review board and the study was conducted in compliance with Good Clinical Practice guidelines. Volunteers were recruited during a period of 3 months prior to the commencement of the clinical trial. The clinical investigators screened all potential recruits and briefed them on the objectives of the study procedures, the duration of the clinical trial and the potential benefits and risks of participation. Subjects were free to withdraw from the study at any time. A clinical specialist would appropriately treat adverse effects endured anytime during the duration of the trial free.
All recruited subjects were healthy adult females of ages ranging between 20-50 years old with a clean bill of health and not on any form of medication or treatment in the last 6 months and not pregnant at the commencement of the clinical study. They had normal eyelash and eyebrow hair density and were free from skin and hair related diseases. The subjects must be willing to sign the informed consent and agreed to observe and comply with the study protocol as advised by the clinical investigators. The exclusion criteria includes the presence of any medical including psychiatric conditions or the usage of any medications, especially steroids, immunosuppressant’s, anticancer medication or topical eye medication in the last 3 months. Females with a history of skin or eye diseases and those with allergic skin reactions to cosmeceuticals and stem cells and their derivate were refrained from participating in the clinical study. Finally, women with eyelash extensions, eyebrow or eyelash extension treatment or modification in the last 6 months cannot take part in this clinical study.
The active ingredient used in the study are tissue peptides extracts procured and manufactured at a laboratory of a private hospital in Germany. Fresh tissue specimens of skin fragments were removed from day-12 rabbit embryos of Specific Pathogen Free (SPF)/ Virus Antigen Free (VAF) certified closed colony, laboratory rabbits from a USA FDA approved laboratory in the United States of America (USA). The procedure was performed under a biological hood and all specimens collected were kept in sterile conditions. The tissue specimen was then washed in phosphate-buffered saline (PBS) containing antibiotics (100 IU/ ml penicillin and 100 μg/ml streptomycin) and fragmented in a culture plate. The minced tissue was then placed in a 50ml tube and centrifuged at 500rpm for 1 minute. It was then repeatedly rinsed in PBS until there was no blood visible. Next, the tissue was incubated for 1 hour at 37?C in 10mls of PBS containing collagenase, DNAase and papain solution. The tissue was then subjected to mechanical forces and the remainder fluid which was now free of any visible tissue fragments was collected via a pipette. The solution then undergoes centrifugation at 500rpm for 5 minutes. Finally, the solution is filtered and placed in a 2.5ml vial containing mainly PBS with no other preservatives.
Microneedles are a minimally invasive but an effective skin permeation enhancement method. It facilitates an effective and sustained delivery of chemicals, hence better results [18]. Tiny microneedles with lengths in micron can increase skin permeability with negligible pain by breaching the epidermis when administered by trained personnel. The microneedle technique is associated with a minimal risk of infection. Li et al concluded that microneedles are a safe and effective method to deliver minerals or peptides through skin [19]. Studies have shown that pretreatment of the skin with the microneedle technique can enhance the delivery of topically applied solutions, including peptides [19].
Six adult females were included in the study. The subjects selected underwent baseline testing where they answered a questionnaire regarding symptoms and other anomalies they are experiencing prior to the initiation of the study. The subject’s eyebrow follicle thickness, density and the presence of new follicle were measured at baseline and during every clinical review at a predetermined site of the eyebrows using a Micro skin and hair diagnostic device (EW-EWB-1007-307, EW Biomedical, and Switzerland) which is a video microscope with a magnification of 200x. The dermatologist-rated GEA eyelash scale which was invented by Friborg et al., is a scale for clinically assessing eyelash prominence of an individual [20]. The scale assesses eyelash prominence using the validated 4-point GEA scale with a photonumeric guide (1=minimal; 2=moderate; 3 =marked; 4=very marked)[20]. The eyelash length was measured using an eyelash ruler which has a calibrated micrometer scale of 0.01mm. Digital photographs of the eyelashes and eyebrows were taken at baseline investigations and then monthly until the final clinical review using standardized equipment. The subjects were questioned regarding the presence of any form of discomfort, which was recorded by the clinical investigators in to the adverse effects questionnaire. Ophthalmic screening and vital signs examination were performed at baseline examination and upon completion of the clinical study. Patient reported outcome (PRO) measures are used to assess treatment benefit are increasingly important in clinical research [16]. In this study, the subjects views were sought to assess the safety of the treatment and the subjects’ satisfaction with the eyelash and eyebrows prominence.
All subjects underwent a microneedle session where the peptides extract solution was inseminated into the subjects’ eyelid margins and eyebrow using a 0.16mm hypodermic needle. Topical anesthesia in the form of 10% Lidocaine cream was applied to the subject’s eyelid margin and eyebrows for 30 minutes prior to the micro needling procedure. The subjects were also provided with a small bottle containing a clear liquid, which is a fetal stem cell extract solution to be applied using a disposable sterile applicator to the upper eyelid margin at the base of the eyelashes and eyebrows every night, excess solution should be absorbed using a facial tissue and left on overnight. All subjects were forbidden to apply any other products over their eyes and eyebrows during the entire duration of the study. They were advised to avoid severe sunlight and skin tanning products and treatment.
The subjects were reviewed every 2 weeks, where they were presented with a questionnaire regarding any discomfort or adverse effects experienced in the last 2 weeks. Their eyelashes were then measured and their eyelashes and eyebrows were assessed using the Micro skin and hair analyzer. The micro needle session was then conducted. Photographs were taken monthly. This exercise was carried out every fortnightly until 12 weeks of assessment was completed.
The 2 main parameters assessed are:
EFFICACY: Qualitative measurement of hair growth defined as hair growth in length and thickness and appearance of new hair follicles using the Micro skin and hair analyzer and the physician-rated GEA score of prominence.
Safety and Tolerability: Presence of pain, itchiness or tolerability based on the Eyelash Satisfaction Questionnaire (ESQ)
Statistical Analysis
The key efficacy outcome is GEA score of prominence, a rating scale ranges from 1 (minimal) to 4 (very marked) based on anterior and superior eyelash observations. Further analyses were based on the eyelash length growth throughout the treatment period. Comparison between eyelashes length beforeand-after treatment were performed using a Wilcoxon signedrank test. Correlation of change in GEA scores with age of the subjects were conducted using the Spearman rank test. Statistical significance is reflected by a p-value that is less than or equal to 0.05.
RESULTS
A total of six healthy women enrolled in this study. Their ages ranged from a minimum age of 21 years to a maximum of 46 years old. All 6 subjects completed the study. Table 1 describes the subject’s age and baseline characteristics. The mean age of
Table 1: Subject age and baseline characteristics.
| N =6 | |
| Mean age (range), y | 32 (21-46) |
| Baseline eyelash length (range), mm | 4 (3-5) |
| Baseline GEA score, n (%) | |
| 2 (Moderate) | 3 (50.0) |
| 3 (Marked) | 3 (50.0) |
he study population was 32 years old. The baseline eyelash length of the majority of the subjects were between 3-5mm, with a mean baseline eyelash length of 4mm. Based on the GEA Scale at baseline evaluation, 3 subjects had a GEA scale 2 while the remaining 3 subjects had a baseline GEA scale of 3.
At the end of the 12-week treatment period, all 100% of the subjects (6/6) experienced at least a ≥ 1-grade increase in the GEA scale score. Furthermore, 17% of the subjects (1/6) experienced a 2-grade while the remainder 83% had a1-grade improvement based on the GEA scale (Figure 1).
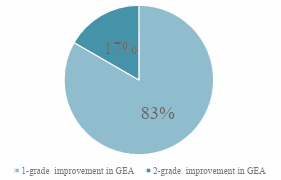
Figure 1: Percentage of subjects according to grade enhancement in GEA score from baseline.
Figure 2 shows the mean change in the length of the eyelashes throughout the 12 weeks study period.
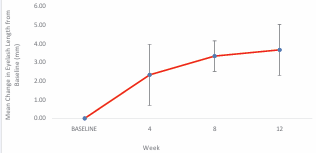
Figure 2 Mean change in eyelash length from baseline, with standard deviation bars, throughout the 12 weeks treatment period.

Figure a Figure A demonstrates the subjects GEA scale evaluation at baseline (1) and at the end of week-12 (2). The baseline GEA scale evaluation for this patient was GEA scale: 3 at baseline and it then further improved at week-12 to GEA scale:

Figure b Figure B shows the changes in eyebrow density based on digital photography. There was a clinical improvement in the density of the eyebrow thickness from baseline to week-12 of the clinical study.

Figure c Figure C shows the hair analyzer magnification of the eyebrow at baseline (1) and at week-12 (2) for the subject. Compared to the baseline scan, the scan at week=12 shows a significant increase in the number of new hair follicles. There was also an increase in the length of the individual hair and its overall thickness based on the photograph from the scan.
The mean change in the eyelashes length was found to be 2.3 ± 1.6mm for the initial 4 weeks (p = 0.021). While the eyelash continued to grow with a mean elongation length of 1mm between week-4 to week-8, while the change in the mean eyelash length from baseline to week-8 was 3.3± 0.82mm (p = 0.013). The eyelash still grew from week-8 of the study to week-12 with a mean eyelash elongation of 0.4mm, while the total change in the mean eyelash length from baseline to the end of the clinical study was 3.7± 1.4mm (p = 0.014). Longer eyelash length was noticeable starting from week 4 and continued through the study at week 8 and at week 12. Table 2 presents the summary of change in eyelash length from baseline. Meanwhile, Spearman rank test indicates no significant correlation between change in GEA scores with the subject age (p = 0.158).
Table 2: Summary of change in eyelash length from baseline.
| Length growth from baseline (mm) | Treatment period (week) | ||
| 4 | 8 | 12 | |
| Mean ± Std. Dev | 2.3 ± 1.6 | 3.3 ± 0.82 | 3.7 ± 1.4 |
| Minimum | 0 | 2 | 2 |
| Maximum | 4 | 4 | 6 |
There were no reports of any serious adverse effects from the subjects. However, 1 subject did complain of a slight discomfort at her right eyelid margin at week-12 of the clinical study. A slit lamp examination by an ophthalmologist showed the discomfort was due to minor trauma, most probably due to the applicator instrument used by the subject during the application of the eye drops at home. There was no ocular adverse reaction suffered by any of the participant.
DISCUSSION
Physical beauty is associated with social advantages and positive psychological effect on women [21]. The eyelash function beyond aesthetic includes protection of the eye against debris and triggering the blinking reflex [21]. There are between 100-150 eyelashes emanating from each upper eyelid, which are arranged in 2-3 rows while the lower eyelid has half of the upper lids lashes. The eyelashes on the upper eyelids were significantly longer than those on the lower eyelids [21,22]. The eyelashes are terminal hairs and they do suffer from greying with age [21,23]. In general, East Asian individuals tend to have shorter eyelashes of a lower density relative to those in Caucasian or West Asian populations [18].
The eyebrow is simple to regard but complicated to understand. It plays a major role in facial appearance, expression and non-verbal communication as seen in the TV character ‘Mr. Bean’ [24,25]. Males have eyebrows that sits lower than females and the male eyebrows has a flatter contour3 . The brow tends to descend with age due to the loss of periorbital bone and fat, giving a tired and sometimes depressed look [24,25]. The fashionable trends of the eyebrows revolve around its thickness, hue and the shape of its arch [24].
The global cosmetics and toiletries market totaled more than 155 billion dollars [7]. Eye make-up including eye shadow, eyebrow line and mascara represents a fast growing market with a growth rate of more than 6% annually [15]. Artificial eyelashes are held in place by adhesives that may trigger an allergic contact dermatitis. Similarly, its removal using solvents may also trigger an allergic reaction [15]. Ocular disorders reportedly caused by eyelash extensions includes keratoconjunctivitis, allergic blepharitis, conjunctival erosions and subconjunctival hemorrhage [8]. Eyelash transplantations in the absence of congenital eyelash defects or the need for reconstruction is controversial and not without their complications postoperatively [19]. Interestingly, the transferred hair follicles mainly being from the scalp, requires regular trimming and curl [19].
The effects of make-up is temporary and subject to smudging. Women have yearned for long-lasting or even permanent options to improve their eyelashes beside eyelash extensions and eyebrow embroidery [19]. Patients treated with topical bimatoprost for eyelash growth reported feeling satisfied and confident in their more attractive and professional look. However, topical bimatoprost is associated with adverse effects including conjunctival hyperemia, eye dryness and pruritus, a burning or foreign-body sensation, eyelid pigmentation and the more serious complaints including iris pigmentation and visual disturbances [19].
The mean age of our study population consisting of healthy adult women was 32 years old. The baseline length of the eyelash of the majority of our subjects were between 3-5mm. All subjects showed an improvement in the length of their eyelash based on the GEA scale, where 83% showed a one-grade improvement while the remaining 17% showed a two-grade improvement. The mean length of eyelash elongation from baseline to week12 was 3.7± 1.4mm (p = 0.014). Longer eyelash was clinically noticeable at week-4 and it progressively continued until week12. The eyebrow density and thickness showed an improvement based on digital photography while hair analyser scans showed evidence of an increase in the overall eyebrow follicle thickness and the presence of new hair follicles. There were no serious adverse effects experienced by any of the subjects.
In a study conducted in Japan by Harii K et al using 0.03% Bimatoprost there was a 77.3% showing at least one grade improvement in the GEA score at week-16 of the study compared to 100% of subjects showing at least a one grade improvement at week-12 in this study using protein peptides [25]. Furthermore, 17% of the subjects showed a two grade improvement at week-12 in this study using protein peptides. The mean changes in length at week-16 from baseline for the 0.03% Bimatoprost study was 1.75mm while the mean changes in length from baseline to week12 for the protein peptide study was 3.7 ± 1.4. There were reports of conjunctival hyperaemia and a decline in visual acuity amongst the subjects in the Japanese 0.03% Bimatoprost studies [25]. Another study using 0.03% Bimatoprost which was reported by Fagien S showed that almost 80% of the subject population achieved at least a one grade improvement in the GEA score at week-16 of the study [24]. The mean change in the eyelash length from baseline to week-16 was noted to be 1.4mm in this study using 0.03% Bimatoprost. Some of the adverse effects reported in this 0.03% Bimatoprost study included eye pruritus, conjunctival hyperaemia, ocular irritation, dry eye symptoms and erythema of the eyelid [24].
Another excellent study on eyelash and eyebrow growth conducted by Alonso et al conducted a clinical study using an herbal, Jarilla-Coffea extract in Argentina [23]. The subjects in this study had a mean increase in eyelash length from baseline to week-12 of ±1.5mm or a 3.59% increase in eyelash length over a period of 12 weeks [23]. There was also a 19.44% change in eyelash thickness in individual eyelashes during the study period. The eyebrow thickness increased in 20% of the subjects with a variation percent of 9.16% observed. 60% of the subjects experienced an increase in the number of new follicles at the eyebrows and there were no adverse effects reported during the entire duration of the 12 week study [23].
CONCLUSION
The results from this study indicate that the daily topical application of the peptides extract solution was able to elongate the eyelash and increased the density of the eyebrows and stimulate the growth of new eyebrow follicles. This is a pioneer study on the efficacy and safety of peptide extract solution as a treatment to enhance the appearance of eyelash and eyebrow. A larger clinical trial with a longer duration will draw definitive conclusions obtained from this study.
REFERENCES
5. Amano Y, Sugimoto Y, Sugita M. Ocular disorders due to eyelash extensions. Cornea. 2012; 31: 121-25.
7. Law SK. Bimatoprost in the treatment of eyelash hypotrichosis. Clin Ophthal. 2010; 4: 349-58.
16.Friborg, et al. Clinical assessment scales and methods. US Pat Appl Pub. 2010.
19.Dabagh BA, Woodward J. Eyelash-Enhancing Products: A Review. Cosmet Dermatol. 2012; 25: 134-143.
23.Preet P. Peptides: A New Therapeutic Approach. Int J Curr Pharma Res. 2018; 10: 29-34.







































































































































