Amelioration of Chronic Kidney Disease (CKD) with Medicinal Mushroom Extract in Rats
- 1. Department of Urology, New York Medical College, Valhalla, NY, USA
Abstract
Background: Oxidative stress (OXS) appears to be a key pathogenic factor for the development of chronic kidney disease (CKD), implying that its diminution with certain antioxidant(s) may prevent CKD. Accordingly, we investigated whether a Poria mushroom extract, PEA with antioxidant activity, might have beneficial effects to ameliorate CKD in the rats.
Materials and methods: Adenine (ADN) is a nephrotoxic agent, which has been often used to chemically induce CKD in a rat model. Twenty rats were divided into four groups: Group 1 (Sham); Group 2 (ADN); Group 3 (ADN with PEA supplement); and Group 4 (PEA only). After ADN and/or PEA were orally given to rats for 2 weeks, they were sacrificed and blood and kidney specimens were collected for histopathological and biochemical analyses.
Results: Compared to the Sham Group, both blood urea nitrogen (BUN) and creatinine (Cr) levels were significantly elevated in the Group 2 with palpable histological alterations. But, the elevated BUN and Cr levels, indicating renal dysfunction, were >30% reduced with PEA supplement (Group 3) with better histology and PEA alone (Group 4) had no effects. Notably, the OXS level was also elevated while activities of antioxidant enzymes were lost in the Group 2. Additionally, those rats in the Group 2 showed the enhanced expressions of three kidney injury biomarkers (neutrophil-gelatinase-associated lipocalin, kidney injury molecule 1, and clusterin), indicating serious renal injury. However, all these adverse effects (caused by ADN) were ameliorated with PEA supplement (Group 3).
Conclusions: OXS appears to play a significant role in ADN-induced CKD in the rats. However, PEA with antioxidant activity could protect renal cells from such OXS-induced adverse effects. Therefore, it is plausible that PEA could be a beneficial renoprotective agent against CKD that is presumably mediated through OXS.
Keywords
Chronic kidney disease; Oxidative stress; Mushroom extract; Rats
Citation
Wagmaister J, Yau R, Choudhury M, Eshghi M, Konno S (2018) Amelioration of Chronic Kidney Disease (CKD) with Medicinal Mushroom Extract in Rats. J Urol Res 5(3): 1107
ABBREVIATIONS
CKD: Chronic Kidney Disease; OXS: Oxidative Stress; ADN: Adenine; PEA: Poria Mushroom Extract; BUN: Blood Urea Nitrogen; Cr: Creatinine; GFR: Glomerular Filtration Rate; ROS: Reactive Oxygen Species; GSH: Reduced Glutathione; TCM: Traditional Chinese Medicine; LPO: Lipid Peroxidation; MDA: Malondialdehyde; TBA: Thiobarbituric Acid; CTL: Catalase; GPX: Glutathione Peroxidase; SDS: Sodium Dodecyl Sulfate; NGAL: Neutrophil-Gelatinase-Associated Lipocalin; Kim-1: Kidney Injury Molecule 1; CLU: Clusterin; ANOVA: Analysis of Variance; SD: Standard Deviation; ESRD: End-Stage Renal Disease; kDa: Kilodalton
INTRODUCTION
Kidney diseases are a serious health problem and particularly chronic kidney disease (CKD) now becomes a major public health problem worldwide, which inflicts tremendous socioeconomic burdens on the patients, families, and societies [1]. The main causes of CKD include diabetes, hypertension, glomerulonephritis, cardiovascular disease and so forth [2,3]. CKD is characterized by reduced glomerular filtration rate (GFR) but increased proteinuria, tubular atrophy, tubulointerstitial fibrosis, glomerulosclerosis, renal vasculopathy, and reduced renal regenerative capability [4,5]. Patients with CKD have a high risk of death from stroke or heart attack, and CKD could progress to permanent renal failure (end-stage renal disease, ESRD) that may require lifelong dialysis or kidney transplantation [1,5]. Unfortunately, CKD is also a progressive, irreversible disease [6,7] and therapeutic options are currently limited and ineffective. Hence, the objective of treatment is to just slow down the disease progression. Meanwhile, the incidence of CKD is steadily increasing and we need to have a better understanding of CKD to reduce or halt this increasing incidence. It is thus urgent and demands for establishing a more effective, suitable modality. There must be something in common and what could be such a common factor for triggering CKD?
Accumulating data now suggest that oxidative stress (OXS), i.e. generation of reactive oxygen species (ROS), is prevalent in CKD patients and may indeed play a significant role in the development of CKD [5-9]. ROS are known as highly reactive and harmful molecules, including oxygen free radicals (superoxide ion, singlet oxygen, hydroxyl radical etc.) and non-radical oxidants (hydrogen peroxide, nitric oxide, ozone etc.) [5]. These ROS can create a state of OXS, attacking, damaging, or even killing all kinds of cells including renal cells. It is thus plausible that OXS could consequently lead to renal dysfunction, renal failure or CKD.
If OXS were the primary cause of CKD, it is theoretically possible that certain antioxidants might be able to effectively reduce the incidence of CKD. In fact, antioxidants have been reported to have beneficial or protective effects on cellular injury/damage associated with OXS [10]. Those include vitamins (C/E), folic acid, β-carotene, reduced glutathione (GSH) etc., but finding the right antioxidant is not an easy task.
We are particularly interested in the bioactive extract from Poria mushroom, “PEA” [11,12]. This is one well-established medicinal mushroom and has been used in Traditional Chinese Medicine (TCM) for 2,000 years [11]. It has been well characterized and its major chemical constituents, such as triterpenes, polysaccharides, and steroids, have been identified [11]. In addition, a number of studies revealed that PEA had antioxidant, renoprotective, anticancer, immunomodulatory, anti-inflammatory, antibacterial, anti-hyperglycemic, diuretic effects etc. [12-18]. We are particularly interested in its antioxidant and renoprotective activities, which may help ameliorate CKD or even reduce its incidence. Moreover, PEA has few side effects (documented in TCM) as it is a natural agent, implying its potential therapeutic utility.
Rat models have been used for studying human CKD and administration of adenine (ADN) to rats has been known to induce kidney damage similar to CKD [19]. ADN given orally to rats will be metabolized to 2,8-dihydroxyadenine (2,8-DHA), which then precipitates and forms tubular crystals leading to palpable kidney injury [20]. Such injury includes extended (70-80%) damage of renal tissue with fibrosis, enlarged granular appearance, apoptotic lesion etc. [21,22], which will eventually result in renal dysfunction with manifestation of CKD. Additionally, those rats may show cardiovascular changes including impaired vascular responses, increased left ventricular stiffness and increased left ventricular mass, which are all characteristics of human CKD [23]. This is an excellent experimental model that has been widely used in CKD studies.
Accordingly, we investigated if PEA might have beneficial effects to ameliorate ADN-induced CKD in a rat model. Additionally, we explored the possible protective mechanism of PEA, focusing on the status of physiological (renal function) and biochemical (renal injury and antioxidant enzymes) parameters associated with CKD. More details of the study are described and the interesting findings are also discussed herein.
MATERIALS AND METHODS
Animal study
Whether PEA might prevent or reduce the incidence of CKD in the rats was examined. Experimentally, CKD was induced by orally giving the rats ADN (40 mg/ml) daily for 2 weeks as described elsewhere [9,12,24]. Twenty male Sprague-Dawley rats (200-250 g), fed with standard chow diet and free access to water, were randomly divided into 4 groups (n=5 per group): Sham Group; ADN Group [Rats received 1 ml of ADN (40 mg/ ml) daily]; ADN/PEA Group [Rats received 1 ml each of ADN (40 mg/ml) and PEA (25 mg/ml) daily]; and PEA Group [Rats received only PEA (25 mg/ml) daily]. At the end of 2 weeks, blood specimens were collected by retro-orbital bleeding and analyzed for blood urea nitrogen (BUN) and creatinine (Cr), while kidney specimens were surgically excised and subjected to histopathologic examination and biochemical analyses.
BUN/Cr tests and histopathologic examination
Blood and kidney specimens were sent to the commercial pathology laboratory for BUN/Cr tests and histopathologic examination, respectively. Histopathologic examination was performed by two independent veterinary pathologists and the pathology reports were sent to us separately. Blood tests for BUN/Cr were also received separately.
Lipid peroxidation (LPO) assay
The severity of OXS (induced by ADN) on rat kidneys was assessed by the LPO assay, measuring the amount of malondialdehyde (MDA) formed in the plasma membrane due to OXS [25] – the more MDA formed, the greater OXS. The detailed procedures were described in the vendor’s protocol (Abcam, Cambridge, MA). Briefly, the reaction was initiated by mixing cell lysates with thiobarbituric acid (TBA) solution and incubated in a boiling water bath (~100 °C) for 1 h. Samples were placed in a 96-well plate and read at A532 on a microplate reader. The amount of MDA formed was determined from the MDA standards and expressed by fold-increase relative to that of Sham (1).
Assays for antioxidant enzymes
Activities of two key antioxidant enzymes, catalase (CTL) and glutathione peroxidase (GPX) [26], were assessed by CTL and GPX Activity Colorimetric Assay Kit (BioVision, Milpitas, CA), respectively, following the manufacturer’s protocols. Activities (mU/ml) of CTL and GPX were expressed by the % relative to the respective Sham reading (100%).
Western blot analysis
Effects of OXS on three kidney injury biomarkers were analyzed using Western blots. Briefly, cell lysates (10 µg) obtained from tissue homogenization was subjected to SDS-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. The blot (membrane) was incubated with primary antibodies against three biomarkers, neutrophil-gelatinase-associated lipocalin (NGAL), kidney injury molecule 1 (Kim-1), and clusterin (CLU) [27-29] (Santa Cruz Biotechnology, Santa Cruz, CA) for 90 minutes, followed by 30-minute incubation with appropriate secondary antibody conjugates. Specific immunoreactive protein bands were detected by chemiluminescence following manufacturer’s protocol (Kirkegaard and Perry Laboratories, Gaithersberg, MD).
Statistical analysis
All data are presented as mean ± SD (standard deviation), and statistical differences between groups are assessed with the unpaired Student’s t test or one-way ANOVA analysis. Values of P <0.05 are considered statistically significant.
RESULTS
Analyses of BUN and Cr in rats
All rats in the four experimental groups were subjected to the evaluation of BUN and Cr in blood specimens at the end of two weeks. As shown in Table 1, the BUN and Cr levels were ~4.1 and ~5.3 times higher with ADN supplement, respectively, than those in the Sham Group. However, those elevated levels with ADN were reduced by ≥33% with PEA supplement. PEA by itself yet had no effects on BUN and Cr. The elevated BUN and Cr levels by ADN indicate renal dysfunction but their partial (≥33%) reduction with PEA implies improved renal function.
Histopathologic examination on kidney specimens
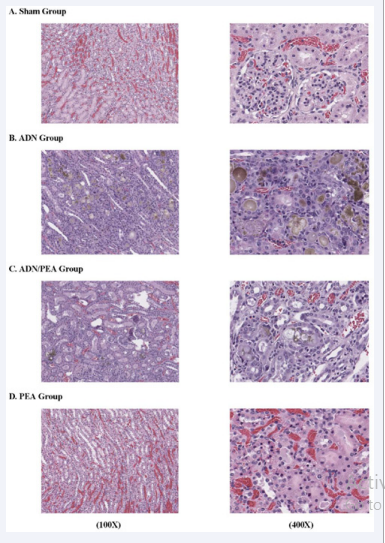
Histopathologic findings on the four groups are displayed in Figure 1.
Figure 1 Histopathology of kidney specimens. Kidney specimens of four experimental conditions were subjected to histopathologic examination. The results shown are: Sham Group (A); ADN Group (B); ADN/PEA Group (C); and PEA Group (D). More details are described in the text.
The Sham Group (A) shows a normal, undamaged kidney while the ADN Group (B) exhibits remarkable histological alterations with renal tubular degeneration, indicating typical kidney damage. However, such palpable kidney damage is less remarkable with PEA supplement in the ADN/PEA Group (C). As expected, histology of the PEA Group (D) looks quite similar to that of the Sham Group with an undamaged, normal kidney. Thus, ADN-induced kidney damage (CKD) could be partially ameliorated with PEA.
OXS induced by ADN
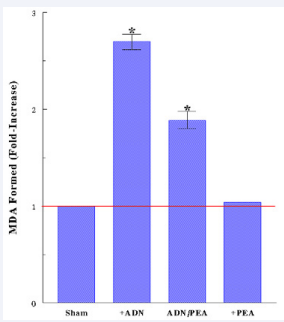
Now, the question is how ADN would induce CKD and how PEA might prevent it. As we hypothesized that OXS (induced by ADN) might play a primary role in ADN-induced CKD, this possibility was tested next. Kidney specimens were subjected to the LPO assay to assess if OXS would be involved in or responsible for CKD. As shown in Figure 2, ADN exerted ~2.7-fold greater OXS than the Sham Group but this elevated OXS level was significantly (~48%) reduced with PEA supplement while PEA alone had no effects. Hence, ADN-mediated toxicity is attributed to OXS but PEA may yet significantly reduce it with its antioxidant activity.
Figure 2 OXS exerted by ADN. LPO assay was performed to assess the severity of OXS by determining the amount of MDA formed. All data are mean ± SD (standard deviation) from three specimens of each group (*P<0.05 compared with Shams).
Up-regulation of kidney injury biomarkers
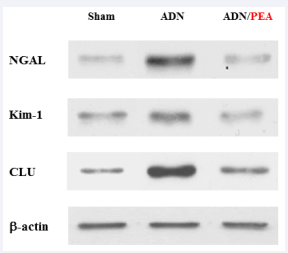
To confirm if extended renal cell injury is indeed induced by OXS, its possible effects on the status of three kidney injury biomarkers, NGAL, Kim-1, and CLU, were examined. Western blots revealed that all three biomarkers were up-regulated or enhanced by ADN, but this was yet apparently prevented with PEA (ADN/PEA) as they remained similar to those in Shams (Figure 3).
Figure 3 Up-regulation of kidney injury biomarkers by ADN. Kidney specimens obtained from different experimental conditions were analyzed for three biomarkers using Western blots. Autoradiographs of NGAL, Kim-1, and CLU, are shown and β-actin was also run as a protein loading control.
It should be noted that the actual intensities of three biomarkers in Sham and ADN/PEA shown here are quantitatively similar but those in ADN are significantly different from those in Sham and ADN/PEA, evidenced by densitometric analysis (data not shown). The up-regulation of these biomarkers is indicative of renal cell injury [27,28,30], but sustaining their natural/basal status with PEA suggests renal cells being somewhat protected (from ADN attack) and remained fairly intact.
Inactivation of antioxidant enzymes in kidneys
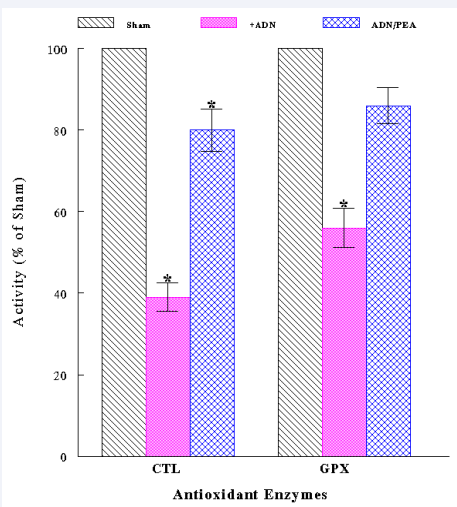
Lastly, we examined if such extended kidney damage/injury could be also due to some adverse effects of OXS on antioxidant enzymes [26], which play a major defense role against OXS [10]. Kidney specimens were assayed for activities of two key antioxidant enzymes, catalase (CTL) and glutathione peroxidase (GPX). The results showed that CTL and GPX activities declined to ~40% and ~55% by OXS, respectively; however, PEA reduced such inactivation, sustaining >80% of their activities (Figure 4).
Figure 4 Inactivation of antioxidant enzymes by ADN. Kidney specimens with different experimental conditions were assayed for activities of two antioxidant enzymes, CTL and GPX. The data are mean ± SD from three specimens of each group (*P<0.05 compared with Shams).
DISCUSSION
To help find a better regimen for CKD, we performed the in vivo study of CKD using a rat model. Our specific aim was to address if PEA might have beneficial effects to ameliorate or improve CKD that was induced by ADN administration to rats.
Our working hypothesis was that OXS could be a primary pathogenic factor for CKD and certain antioxidants might be able to diminish OXS to prevent or mitigate CKD. In fact, antioxidants have been documented to effectively protect (renal) cells from such OXS [10]. PEA has been found to have various pharmacological properties including antioxidant and renoprotective activities [11,12]. Hence, it was a logical approach for testing the possibility of PEA to have any beneficial effects on such an experimental CKD case.
We first found that ADN adversely affected kidney function, indicated by the significantly increased levels of BUN and Cr (Table 1).
Table 1: Effects of ADN on renal function.
|
Table 1: Effects of ADN on renal function. |
||||
|
Analysesa |
|
Experimental Groups
|
|
|
|
Sham |
ADN |
ADN/PEA |
PEA
|
|
|
BUN (mg/dl)
|
17.2 |
70.5b |
47.2b |
17.5 |
|
Cr(mg/dl)
|
0.44
|
2.33c |
1.44c |
0.41 |
|
a On the 14th day of the study, blood specimens were collected from all rats and subjected to analyses for BUN and Cr. bP<0.03 (compared with Sham). cP<0.05 (compared with Sham). Abbreviations: ADN, Adenine; PEA, Poria mushroom extract; BUN, Blood urea nitrogen; Cr, Creatinine. |
||||
However, those elevated BUN and Cr levels have declined with PEA supplement, implying the improvement in renal function. Nevertheless, such renal dysfunction (induced by ADN) is a sign of CKD and more detailed histologic examination also revealed deposition of 2,8-DHA (brown pigmented crystals), tubular degeneration, interstitial mononuclear inflammation, sloughing of renal cells (into the tubular lumen) etc. (Figure 1). These results thus suggest that accumulation of ADN crystals in the rat kidneys may trigger renal cell inflammation, renal tubular degeneration, renal dysfunction, and ultimately leads to CKD. All these histopathological alterations were yet ameliorated with PEA, positively protecting the kidneys from ADN assault.
To address how ADN crystals would induce such severe renal cell damage, we looked into possible OXS exerted by ADN. Indeed, OXS was elevated to ~2.7-fold higher/severer in the ADN group (than that in the Shams) (Figure 2). This finding illustrates that ADN is capable of exerting severe OXS on renal cells, more likely leading to renal cell injury and renal dysfunction. To confirm such OXS-induced renal cell injury, we examined the expressions of three specific kidney injury biomarkers, NGAL, Kim-1 and CLU [27-29], in the rat kidneys. All biomarkers in the ADN group were up-regulated or expressed more intensely (compared to those in the Shams), indicating severe renal cell injury [27,28,30]. Particularly, NGAL is a stable, small molecule (25 kDa) that is excreted and easily detected in urine, so that it has been validated as a useful biomarker for CKD progression [31]. Its production has been also reported as an indicator in response to OXS before kidney dysfunction can be detected by other biomarkers [32]. After all, these findings of biomarkers support the notion that ADN-exerted OXS can lead to extended renal cell damage/injury, resulting in renal dysfunction and ultimate CKD.
Despite severe ADN-induced renal injury through OXS, PEA was capable of significantly (~50%) diminishing OXS (Figure 2) and maintaining the basal status of all three biomarkers (under OXS) (Figure 3), demonstrating its antioxidant activity and protection of renal cells from ADN attack.
Particularly, it would be more interesting to talk about antioxidant activity of PEA. Today, many synthetic antioxidants are available in the market but they have potential hazards to health as well [33]. In contrast, PEA is considered as a natural antioxidant with few side effects and its safety has been well granted by TCM. A number of mushrooms (including Poria) have been shown to exhibit strong activity of scavenging free radicals (ROS) and considered as potential natural antioxidants [34,35]. Strictly speaking, various derivatives (such as PEA) of Poria mushroom can be extracted or isolated with the diverse degrees of biological properties such as antioxidant, anticancer, immunological activities etc. [36]. Especially, antioxidant activity of these derivatives is associated with several structural parameters, such as solubility, molecular weight, monosaccharide composition, glycosidic linkages, and extent of side-chain branching [37]. In other words, actual antioxidant activities of Poria mushroom derivatives could vary to a certain extent. Our PEA appears to have relatively strong antioxidant activity (capable of reducing OXS by ~50%) but it is yet possible that some other derivative(s) may have even stronger activity. This possibility may deserve further exploration.
Another interesting finding was that the two key antioxidant enzymes, CTL and GPX [26], significantly (>40%) lost their enzymatic activities by OXS (Figure 4), implying a further breakdown/collapse of the antioxidant defense system that makes renal cells even more vulnerable to OXS and leads to extended renal cell injury. Besides so-called non-enzymatic or chemical antioxidants (e.g., PEA, vitamins C/E etc.) [10], antioxidant enzymes are also an integral part of the key cellular defense system (against OXS etc.) that must be active and functional. These enzymes are present in our body (since a birth) but will become weaker or inactive as we get older. This may partly account for why old people are less healthy and more vulnerable to illness. Hence, OXS can indeed inactivate antioxidant enzymes in the kidneys as well as other organs, feasibly resulting in CKD and other serious diseases. Nevertheless, PEA may also help ameliorate such OXS-induced adverse conditions.
Moreover, it would be worthwhile mentioning that the amount of ADN crystals formed/deposited in the rat kidneys appears to be significantly less with PEA supplement. This suggests that PEA could somehow interfere with the metabolic pathway of ADN to 2,8-DHA conversion. Generally, as ADN is efficiently salvaged by adenine phosphoribosyl transferase, it is present at the significantly low level in blood and urine [38]. However, when ADN becomes excessive, it acts as a substrate for xanthine dehydrogenase, which could then oxidize ADN to 2,8- DHA [20,39]. Due to the very low solubility of 2,8-DHA, it readily precipitates in renal tubules and forms tubular crystals [22]. Hence, it can be speculated that PEA may disrupt the conversion of ADN to 2,8-DHA by inhibiting or inactivating xanthine dehydrogenase, although other unknown mechanisms cannot be ruled out. This deserves further investigations.
Taken all together, ADN-induced CKD in the rats is primarily attributed to accumulation of ADN crystals in the rat kidneys, which in turn exerts severe OXS on renal cells, causing renal cell damage/injury, accompanied by inactivation of antioxidant enzymes. As a result, such renal cell injury would ultimately lead to CKD. However, all these adverse effects caused by OXS could be apparently reduced with PEA. In addition, this finding is consistent with the early report describing that another extract of Poria mushroom prepared by different procedures could be used for the prevention or treatment of CKD [40].
CONCLUSIONS
This study demonstrates that OXS mediated through ADN appears to eventually induce CKD in the rats; however, PEA with antioxidant activity may have beneficial effects to slow down the progression of ADN-induced CKD. Thus, PEA could be a promising, natural antioxidant for ameliorating CKD. Further studies are warranted.
ACKNOWLEDGEMENT
We thank Ms. Donna Noonan (Mushroom Wisdom, Inc.) for kindly providing us with PEA and financial support in this study.
REFERENCES
- Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat RevNephrol. 2011; 7: 684-696.
- Winearls CG. Chronic kidney disease: definition and staging – an orthodox view.Clin Med (Lond). 2009; 9: 563-564.
- Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013; 382: 260-272.
- Lopez-Novoa JM, Rodriguez-Pena AB, Ortiz A, Martinez-Salgado C, Lopez Hernandez FJ. Etiopathology of chronic tubular, glomerular and renovascular nephropathies: clinical implications.J Transl Med. 2011.
- Small DM, Coombes JS, Bennett N, Johnson DW, Gobe GC. Oxidative stress, anti-oxidant therapies and chronic kidney disease. Nephrology (Carlton). 2012; 17: 311-321.
- Krata N, Zagozdzon R, Foroncewicz B,Mucha K. Oxidative stress in kidney diseases: the cause of the consequence?.Arch ImmunolTherExp (Warsz). 2018; 66: 211-220.
- Turki K, Charradi K, Boukhalfa H, Belhaj M, Limam F, Aouani E. Grape seed powder improves renal failure of chronic kidney disease patients.EXCLI J. 2016; 15: 424-433.
- Ali BH, Al-Husseni I, Beegam S, Al-Shukaili A, Nemmar A, Schierling S, et al. Effect of gum arabic on oxidative stress and inflammation in adenine-induced chronic renal failure in rats.PLoS One. 2013; 8: e55242.
- Chang XY, Cui L, Wang XZ, Zhang L, Zhu D, Zhou XR, et al. Quercetin attenuates vascular calcification through suppressed oxidative stress in adenine-induced chronic renal failure rats. Biomed Res Int. 2017.
- Cutler RG. Antioxidants and aging. Am J ClinNutr. 1991; 53: 373S-379S.
- Rios JL. Chemical constituents and pharmacological properties of Poriacocos. Planta Med. 2011; 77: 681-691.
- Zhou L, Zhang Y, Gapter LA, Ling H, Agarwal R, Ng KY. Cytotoxic and antioxidant activities of lanostane-type triterpenes isolated from Poriacocos. ChemPharm Bull (Tokyo). 2008; 56: 1459-1462.
- Zhou L, Zhang Y, Gapter LA, Ling H, Agarwal R, Ng KY. Cytotoxic and antioxidant activities of lanostane-type triterpenes isolated from Poriacocos. Chem Pharm Bull (Tokyo). 2008;56(10):1459-1462.
- Zhao YY, Lei P, Chen DQ, Feng YL, Bai X. Renal metabolic profiling of early renal injury and renoprotective effects of Poriacocos epidermis using UPLC Q-TOF/HSMS/MSE. J Pharm Biomed Anal. 2013; 81-82: 202-209.
- Chen X, Zhang L, Cheung PC. Immunopotentiation and anti-tumor activity of carboxymethylated-sulfated β-(1→3)-D-glucan from Poriacocos. IntImmunopharmacol. 2010; 10: 398-405.
- Fuchs SM, Heinemann C, Schliemann-Willers S, Hartl H, Fluhr JW, Elsner P. Assessment of anti-inflammatory activity of Poriacocos in sodium lauryl sulphate-induced irritant contact dermatitis. Skin Res Technol. 2006; 12: 223-227.
- Zhang L, Ravipati AS, Koyyalamudi SR, Jeong SC, Reddy N, Bartlett J, et al. Anti-fungal and anti-bacterial activities of ethanol extracts of selected traditional Chinese medicinal herbs. Asian Pac J Trop Med. 2013; 6: 673-681.
- Li TH, Hou CC, Chang CLT, Yang WC. Anti-hyperglycemic properties of crude extract and triterpenes from Poriacocos. Evid Based Complement Alternat Med. 2011; 10: 1-8.
- Feng YL, Lei P, Tian T, Yin L, Chen DQ, Chen H, et al. Diuretic activity of some fractions of the epidermis of Poriacocos. J Ethnopharmacol. 2013; 150: 1114-1118.
- Diwan Y, MistryA, Gobe G, Brown L. Adenine-induced chronic kidney and cardiovascular damage in rats. J PharmacolToxicol Methods. 2013; 68: 197-207.
- Koeda T, Wakaki K, Koizumi F, Yokozawa T,Oura H. Early changes of proximal tubules in the kidney of adenine-ingesting rats, with special reference to biochemical and electron microscopic studies. Nihon Jinzo Gakkai Shi. 1988; 30: 239-246.
- Shuvy M, Nyska A, Beeri R, Abedat S, Gal-Moscovici A, Rajamannan NM, et al. Histopathology and apoptosis in an animal model of reversible renal injury. ExpToxicolPathol. 2011; 63: 303-306.
- Yokozawa T, Zheng PD, Oura H, Koizumi F. Animal model of adenine-induced chronic renal failure in rats. Nephron. 1988; 44: 230-234.
- McQuarrie EP, Patel RK, Mark PB, Delles C, Connell J, Dargie HJ, et al. Association between proteinuria and left ventricular mass index: a cardiac MRI study in patients with chronic kidney disease. Nephrol Dial Transplant. 2011; 26: 933-938.
- Ali BH, Al-Salam S, Al Za’abi M, Waly MI, Ramkumar A, Beegam S, et al. New model for adenine-induced chronic renal failure in mice, and the effect of gum acacia treatment thereon: comparison with rats. J PharmacolToxicol Methods. 2013; 68: 384-393.
- Dargel R. Lipid peroxidation: a common pathogenetic mechanism?. ExpToxicolPathol. 1992; 44: 169-181.
- Wieczorek E, Jablonowski Z, Tomasik B, Gromadzinska J, Jablonska E, Konecki T, et al. Different gene expression and activity pattern of antioxidant enzymes in bladder cancer. Anticancer Res. 2017; 37: 841-848.
- Charlton JR, Portilla D, Okusa MD. A basic science view of acute kidney injury biomarkers. Nephrol Dial Transplant. 2014; 29: 1301-1311.
- Wunnapuk K, Liu X, Peake P, Gobe G, Endre Z, Grice JE, et al. Renal biomarkers predict nephrotoxicity after paraquat.Toxicol Lett. 2013; 222: 280-288.
- Satirapoj B, Nast CC, Adler SG. Novel insights into the relationship between glomerular pathologyand progressive kidney disease. Adv Chronic Kidney Dis. 2012; 19: 93-100.
- Nemmar A, Karaca T, Beegam S, Yuvaraju P, Yasin J, Ali BH. Lung oxidative stress, DNA damage, apoptosis, and fibrosis in adenine-inducedchronic kidney disease in mice. Front Physiol. 2017; 8: 896.
- Malyszko J, Bachorzewska-Gajewska H, Sitniewska E, Malyszko JS, Poniatowski B, Dobrzycki S. Serum neutrophil gelatinase-associated lipocalin as a marker of renal function in non-diabetic patients with stage 2-4 chronic kidney disease. Ren Fail. 2008; 30: 625-628.
- Haase M, Devarajan P, Haase-Fielitz A, Bellomo R, Cruz DN, Wagener G, et al. The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: a multicenter pooled analysis of prospective studies. J Am CollCardiol. 2011; 57: 1752-1761.
- Aug A, Altraja A, Altraja S, Laaniste L, Mahlapuu R, Soomets U, et al. Alterations of bronchial epithelial metabolome by cigarette smoke are reversible by an antioxidant, O-methyl-L-tyrosinyl-g-L-glutamyl-L-cysteinylglycine. Am J Respir Cell Mol Biol. 2014; 51: 586-594.
- Wei XJ, Hu TJ, Chen JR, Wei YY. Inhibitory effect of carboxymethylpachymaran on cyclophosphamide-induced oxidative stress in mice. Int J BiolMacromol. 2011; 49: 801-805.
- Cor D, Knez Z, Hrncic MK. Antitumor, antimicrobial, antioxidant and antiacetylcholinesterase effect of Ganodermalucidum terpenoids and polysaccharides: a review. Molecules. 2018; 23: 649.
- Wei YY, Hu TJ, Su ZJ, Wei XJ, Zhang SX. Immunomodulatory and antioxidant effects of carboxymethylpachymaran on the mice infected with PCV2. Int J BiolMacromol. 2012; 50: 713-719.
- Wang Q, Chen S, Han L, Lian M, Wen Z, Jiayinaguli T, et al. Antioxidant activity of carboxymethyl(1®3)-b-D-glucan (from the sclerotium of Poriacocos) sulfate (in vitro). Int J BiolMacromol. 2014; 69: 229-235.
- Engle SJ, Stockelman MG, Chen J, Boivin G, Yum MN, Daviest PM, et al. Adenine phosphoribosyltransferase-deficient mice develop 2,8-dihydroxyadenine nephrolithiasis. Proc Natl AcadSci USA. 1996; 93: 5307-5312.
- Wyngaarden JB, Dunn TJ. 8-hydroxyadenine as the metabolic intermediate in the oxidation of adenine to 2,8-dihydroxyadenine by xanthine oxidase. Arch BiochemBiophys. 1957; 70: 150-156.
- Zhao YY, Li HT, Feng YL, Bai X, Lin RC. Urinary metabonomic study of the surface layer of Poriacocos as an effective treatment for chronic renal injury in rats. J Ethnopharmacol. 2013; 148: 403-410.