Comparison of Guy’s Stone Score and S.T.O.N.E Nephrolithometry Score for Predicting Outcome of Percutaneous Nephrolithotomy
- 1. Department of Urology and Renal Transplant Surgery, Tribhuvan University Teaching Hospital, Kathmandu, Nepal
Abstract
Objective: To compare Guy’s Stone Score and S.T.O.N.E Nephrolithometry Score for predicting stone-free status and complication after Percutaneous Nephrolithotomy (PCNL).
Materials and methods: A prospectively maintained database of 104 cases, who underwent PCNL from January to October 2016 in Tribhuvan University Teaching Hospital, was analyzed. Guy’s Stone Score and S.T.O.N.E Nephrolithometry Score were calculated based on preoperative computerized tomography images and their correlation with stone clearance and complications was determined statistically using Spearman’s Rank Correlation. Stone clearance was evaluated with X-ray or ultrasound of the kidneys, ureters and bladder up to three months after PCNL and perioperative complications within one month were recorded as modified Clavien-Dindo classification for PCNL.
Result: Mean Guy’s Stone Score in stone-free group and stone-residue group were 1.7 and 2.9 respectively. Similarly, mean S.T.O.N.E Nephrolithometry Score in stone-free group and stone-residue group were 7.2 and 9.8 respectively. Both scores were significantly associated with postoperative stone-free status (p<0.001). In addition, both Guy’s Stone Score and S.T.O.N.E Nephrolithometry Score correlated with complications graded by modified Clavien-Dindo classification for PCNL (p=0.004 and 0.001 respectively) and length of hospital stay(p=0.001 and 0.001 respectively).
Conclusion: Guy’s Stone Score and S.T.O.N.E Nephrolithometry Score equally predicted the stone-free status and complications after PCNL. Both of these scoring systems can be used to stratify the complexity of renal stone preoperatively.
Keywords
Clavien-Dindo classification; Guy’s stone score; S.T.O.N.E Nephrolithometry score; Stone clearance
Citation
Poudyal S, Pradhan M, Chapagain S, Luitel BR, Chalise PR, et al. (2017) Comparison of Guy’s Stone Score and S.T.O.N.E Nephrolithometry Score for Predicting Outcome of Percutaneous Nephrolithotomy. J Urol Res 4(2): 1082.
ABBREVIATIONS
PCNL: Percutaneous Nephrolithotomy; GSS: Guy’s Stone Score; CROES: Clinical Research Office of Endourological Society; AUC: Area Under Curve; ROC: Receiver Operating Curve; KUB: Kidneys Ureters Bladder; CT: Computed Tomography
INTRODUCTION
Percutaneous Nephrolithotomy (PCNL) is the standard of treatment for large renal stones [1,2]. With increased rise of renal stone incidence, there has been a rise in PCNL but still the stone free rate and complications have been the kernel of discussion [3,4]. The outcome of PCNL is measured in terms of stone free rate and complications and the goal of this novel surgery is to provide maximum stone clearance with minimal morbidity. There are different preoperative factors notably related to stone, patient and anatomical characteristics that can determine the outcome of the surgery [5,6]. These factors have been used as different nomograms and scoring systems to bring uniformity for comparison of outcome as well as for proper planning of the surgery and counseling of the patient beforehand [7]. Guy’s Stone score (GSS) developed in 2011 has graded the renal stone in four grades depending upon the stone number, location and abnormalities in renal unit [8].
Similarly, S.T.O.N.E score was developed in 2013 using 5 variables abbreviated as “S.T.O.N.E.” for stone size, tract length (skin-to-stone distance), and degree of obstruction (presence of hydronephrosis), number of involved calices, and stone essence (Hounsfield Unit) [9]. These scores have been validated by multiple studies; nevertheless cross-comparative studies are fewer. This study was carried out to compare Guy’s Stone score and S.T.O.N.E Nephrolithometry Score to predict stone free rate and complications of PCNL.
MATERIAL AND METHODS
A prospectively maintained database of 104 PCNL performed in Tribhuvan University Teaching Hospital from January to October 2016 was analyzed. Approval for the study was taken from Institutional Review Board. Informed consent was obtained from all the patients. Preoperative CT KUB (Computed Tomogram of kidneys, ureters, and bladder) was done in all cases. All PCNL were done by four experienced consultant endourologists of the author’s institute. Patients with age more than 15 were only included. Patient’s characteristics (age, sex, body mass index (BMI), previous history of surgery, comorbidities), stone characteristics (size and density), degree of hydronephrosis, number of involved calices, operative time, fluoroscopy time and length of hospital stay were studied and compared between residual stone and stone-free groups. Chi-square test was used for categorical variables and the Student t-test for continuous data. Stone size was calculated using formula [?(0.785 X lengthmax X widthmax)] as proposed by Tiselius et al [10]. Similarly, postoperative outcomes were defined in terms of stone clearance rate and complications classified by modified Clavien-Dindo classification for PCNL [11]. GSS and S.T.O.N.E Nephrolithometry Score were calculated. Furthermore, S.T.O.N.E score was categorized into three risk groups- low (5-6), moderate (7-8) and high (9-13). Both scoring systems were correlated with stone clearance and complications. Area under the Curve (AUC) was calculated for both scoring systems using Receiver Operating curve. Stone free status was defined as stone residue <4 mm which is asymptomatic, nonobstructive and non-infectious. It was ascertained intraoperatively by fluoroscopy or flexible nephroscope if needed and determined postoperatively by X-Ray KUB for radiopaque stones or ultrasound KUB for radiolucent stones or both immediate postoperatively, at 1 month and at 3 months if residual stones were present. All statistical analysis was done by the Statistical Package for Social Sciences (SPSS) version 21 with statistical significance considered at 0.05.
RESULTS AND DISCUSSION
Results
A total of 104 patients who underwent PCNL and fulfilled the inclusion criteria were included. Stone clearance rate was 87.5% and 27.9% of patients had complications. The patient and stone characteristics between stone-free group and residual stone group are enlisted in Table (1) (Figure 1).
Table1: Baseline Characteristics of Study patients in stone-free and stone-residue group.
|
Table1: Baseline Characteristics of Study patients in stone-free and stone-residue group. |
|||
|
Variable, total (%) |
Stone free(N=91) |
Residual stone(N=13) |
P-value |
|
Age (in years) |
37.37 ± 13.25(18-71) |
37.92 ± 14.17(20-65) |
0.444 |
|
Male, 59(56.7%) Female, 45(43.3%) |
51 (86.4%) 40 (88.89%) |
8(13.6%) 5(11.11%) |
0.474 |
|
Left, 51(49%) Right, 53(51%) |
43 (84.3%) 48 (90.6%) |
8 (15.7%) 5 (9.4%) |
0.253 |
|
BMI (kg/m2) |
23.67 ± 3.05(17-31) |
23.42 ± 2.93(19-28) |
0.764 |
|
Stone size(mm2) |
439.62± 288 (100-1630) |
1001.62± 600(254-1962) |
0.027* |
|
Open renal surgery |
7 |
0 |
0.956 |
|
CKD(GFR<30 ml/min) |
9 |
1 |
0.679 |
|
Total Xray Exposure(sec) |
302.32± 112(115-510) |
416.92± 135(270-600) |
0.09* |
|
Duration of surgery(min) |
74.56± 18.34(44-120) |
98.1± 18.43(60-134) |
0.002* |
|
ASA Class 1 ASA Class 2 ASA Class 3 |
72(86.7%) 18(94.7%) 1(50%) |
11(13.3%) 1(5.3%) 1(50%) |
0.172 |
|
No of Tracts 1 2 |
86(92.5%) 5(45.5%) |
7(7.5%) 6(54.5%) |
<0.001* |
|
Hounsfield unit stone |
1021±269(380-1560) |
1066±268(670-1350) |
0.574 |
|
Complexity of stones Single Multiple Partial staghorn Complete Staghorn |
47(95.9%) 40(88.9%) 4(80%) 0 |
2(4.1%) 5(11.1%) 1(20%) 5(100%) |
<0.001* |
|
Hydronephrosis No Hydronephrosis |
39(79.6%) 52(94.5%) |
10(20.4%) 3(5.5%) |
0.022* |
|
* p value significant BMI: Body Mass Index; CKD:Chronic Kidney Disease |
|||
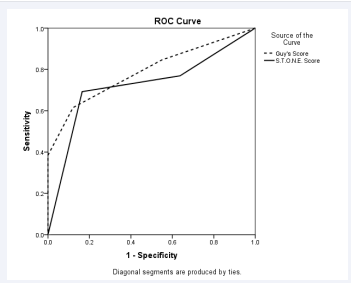
Figure 1 Receiver Operating Characteristic Curve comparing Guy’s stone score and S.T.O.N.E. Nephrolithometry.
Stone size, number of stones and hydronephrosis were statistically different between stonefree and stone-residue group. Total X-Ray exposure, number of tracts to clear stone and duration of surgery were also different between two groups. As the GSS and S.T.O.N.E Nephrolithometry score increased, the stone clearance rate decreased whereas fluoroscopy time, operative time and length of hospital stay all increased as depicted by Table 2.
Table 2: Stone clearance, fluoroscopy time, operative time and length of hospital stay in different Guy’s Stone Score and S.T.O.N.E Score categories.
|
Table 2: Stone clearance, fluoroscopy time, operative time and length of hospital stay in different Guy’s Stone Score and S.T.O.N.E Score categories. |
||||||||||
|
Scoring systems |
Stone Clearance |
Fluoroscopy time (secs) |
Operative time (mins) |
Length of hospital stay(days) |
||||||
|
Stone-free |
Stone-residue |
% free |
p-value |
p-value |
p-value |
p-value |
||||
|
Guy’s 1 |
41 |
1 |
97.6 |
0.001* |
273±127 |
0.001* |
70.7±20 |
0.009* |
2.7±1.6 |
0.001* |
|
2 |
39 |
3 |
92 |
336±88 |
80±18 |
2.4±.6 |
||||
|
3 |
11 |
4 |
73.3 |
364±143 |
80±17 |
3.4±1.7 |
||||
|
4 |
0 |
5 |
0 |
398±138 |
106±10.8 |
7±3.2 |
||||
|
S.T.O.N.E 5-6 |
33 |
3 |
91.6 |
0.001* |
298±12 |
0.018* |
75.6±23 |
0.001* |
2.5±1.3 |
0.002* |
|
7-8 |
43 |
1 |
97 |
299±105 |
72±15 |
2.6±1.4 |
||||
|
9-13 |
15 |
9 |
62 |
378±120 |
91±16 |
4.3±3.3 |
||||
|
*p-value significant |
||||||||||
Mean Guy’s Stone Score in stone-free group and stone-residue group were 1.7 and 2.9 respectively. Similarly, mean S.T.O.N.E Nephrolithometry Score in stone-free group and group with residual stone were 7.2 and 9.8 respectively. Area under the curve for GSS and S.T.O.N.E score were 0.79 and 0.72 respectively. Both scoring systems correlated with the stone clearance (p<.001). In addition, GSS and S.T.O.N.E Nephrolithometry Score correlated with complications graded by modified Clavien-Dindo classification for PCNL (p=.004 and .001 respectively) as shown in Table (3).
Table 3: Stone Scoring systems and complications.
|
Table 3: Stone Scoring systems and complications. |
|||||||
|
Stone scoring systems |
Modified Clavien-Dindo |
p-value |
Spearman correlation |
||||
|
No |
1 |
2 |
3b |
4a |
|||
|
Guy’s Stone Score 1 |
35 |
4 |
2 |
1 |
0 |
0.003* |
0.28(p=0.004*) |
|
2 |
32 |
7 |
3 |
0 |
0 |
||
|
3 |
7 |
3 |
1 |
3 |
1 |
||
|
4 |
1 |
1 |
2 |
1 |
0 |
||
|
S.T.O.N.E Score 5-6 |
30 |
2 |
2 |
1 |
1 |
0.005* |
0.31(p=0.001*) |
|
7-8 |
35 |
5 |
2 |
2 |
0 |
||
|
9-13 |
10 |
8 |
4 |
2 |
0 |
||
|
*p-value significant |
|||||||
The complications of the PCNL are enlisted in Table (4).
|
Table 4: Postoperative Complications- Modified Clavien-Dindo Classification for PCNL. |
||
|
Grade |
Complications |
29(27.9%) |
|
1 |
Fever |
15(14.4%) |
|
2 |
Fever managed with change in antibiotics |
8(7.7%) |
|
3b |
Ureteric stone requiring URSL Pseudoaneurysmrequiring Angioembolisation Clot retention requiring evacuation |
2(1.9%) 2(1.9%) 1(0.96%) |
|
4a |
Abdominal compartment syndrome(required pigtail drainage/ICU monitoring) |
1(0.96%) |
|
URSL: Ureteroscopic Lithotripsy; ICU: Intensive Care Unit |
||
Discussion PCNL is the gold standard treatment for large and/or complex renal stones [1]. Primary goal of the treatment is absolute clearance of stone with minimum complication. PCNL is effective with overall stone free rates between 76-84% and even higher [3]. The Clinical Research Office of Endourological Society Study (CROES) Group has reported complication in 20.5% of the cases with majority of complications being minor [12].
Different scoring systems have been developed to predict stone clearance and complications of PCNL [7]. They stratify the complexity of the stone for proper surgical planning and patient counseling. Stone characteristics like size, number, location and staghorn status are addressed by both GSS and S.T.O.N.E score but renal unit abnormalities and spinal injury/spina bifida are only included in GSS. Similarly, stone density, skin to stone distance and the presence of hydronephrosis are only addressed by S.T.O.N.E score [8,9].
Thomas et al. concluded that GSS was the only factor that significantly and independently predicted the stone free rate whereas stone burden, operating surgeon, patient’s weight, age, comorbidity and urine culture did not correlate statistically significantly with the stone clearance [8]. In addition, GSS did not correlate with the complications. GSS was externally validated by study done by Mandal et al., where GSS was found to be associated with not only stone clearance but also with complications, as shown by our study [13]. Similar conclusion was depicted from the study by Vincenti et al., who validated the GSS with use of CT scan [14].
Similar to the study reported by Okhunov et al., our study showed that S.T.O.N.E. score correlated with the postoperative stone-free status, operative time, and length of hospital stay [9,15]. Noureldin et al. externally validated the usefulness of S.T.O.N.E score where the authors found stone size, number of calyces and S.T.O.N.E score to be significantly associated with stone free status. In our study, not only the stone size and number of calyces but the presence of hydronephrosis also significantly determined the stone clearance [16]. There are few studies comparing GSS and S.T.O.N.E score in predicting the outcome of PCNL. According to the study by Lebadie et al., the mean Guy’s score and S.T.O.N.E Nephrolithometry score were 2.2 vs 2.7 and 8.3 vs 9.5 respectively in stone-free patients vs those with residual stones and both scoring systems equally predicted the stone free status [17]. They reported AUC of GSS and S.T.O.N.E score to be 0.634 and 0.67 respectively. The finding was consistent with our study and the study done by Jaipura et al.[7]. Though our study showed that both stone scores significantly correlated with the complications, one case of diverticular stone in lower calyx falling in GSS 3 but S.T.O.N.E score 5-6 had residual stone as well as grade 4a complication. This highlights the importance of calyceal abnormality which is addressed by GSS but not by S.T.O.N.E score.
In our study, stone clearance rate is higher than other studies as shown in Table (5&6).
Table 5: Comparison of Guy’s Stone Score in different studies.
|
Table 5: Comparison of Guy’s Stone Score in different studies. |
||||||||
|
Guy’s score |
Present study N=104 |
Thomas et al8 N=100 |
Labadie at al17 N=244 |
Mandal et al13 N=221 |
||||
|
% |
% stone free 87.5% |
% |
%stone free 62% |
% |
%stone free 56% |
% |
%stone free 76.1% |
|
|
1 |
40.4 |
97.6 |
28 |
81 |
19 |
70 |
30.8 |
100 |
|
2 |
40.4 |
92 |
34 |
72 |
33 |
65 |
44 |
74 |
|
3 |
14.3 |
73.3 |
21 |
35 |
32 |
48 |
22 |
56 |
|
4 |
4.9 |
0 |
17 |
29 |
16 |
35.9 |
2.2 |
0 |
|
N: Number of cases in the study |
||||||||
Table 6: Comparison of S.T.O.N.E score in different studies.
|
Table 6: Comparison of S.T.O.N.E score in different studies. |
||||||
|
S.T.O.N.E score |
Present Study (Number of case=104) |
Okhunov et al9 (Number of case =117) |
Labadie etal17 (Number of case =244) |
|||
|
% |
% stone free 87.5 % |
% |
% stone free 83.1% |
% |
% stone free 56% |
|
|
5-6 |
34 |
91.6 |
20 |
97 |
14 |
71 |
|
7-8 |
43 |
97 |
46 |
88 |
33 |
66 |
|
9-13 |
23 |
62 |
32 |
44 |
53 |
46 |
The burden of complex stones as determined from GSS and S.T.O.N.E scores is lower than that of other studies. This may be the reason for higher clearance rate in our study. Other probable reasons are use of intraoperative flexible nephroscopy, use of miniaturized instruments creating multiple tracts and use of X-Ray KUB for follow-up of stone clearance [18,19].
Some variables are not clearly defined in both the scoring systems. GSS categorises staghorn to partial and complete; nevertheless these two entities have not been defined clearly. The number of calyces involved in S.T.O.N.E score is not clear, as staghorn status defined by original study of S.T.O.N.E score means only full staghorn and stones involving renal pelvis as well as calyces more than three are not well-defined. Though the interobserver agreement for the GSS was good, the area of disagreement between grade 2 and 3 existed, due to unclear definitions of partial staghorn stone and abnormal anatomy as reported by Thomas et al., and Inmigarsson et al. [8,20]. In addition, GSS was initially developed using abdominal X-Ray whereas S.T.O.N.E score was formulated using CT scan which is the imaging of choice for the renal stone. As our study showed only three parameters of S.T.O.N.E score- stone size, number of calyces and presence of hydronephrosis to be the determining factor for stone clearance, there might not be a much difference between GSS and S.T.O.N.E score when CT scan is used because these are the common parameters in both scoring systems. As both are simple and comprehensible, both can be used for stratification of complexity of renal stone before PCNL. As our study consisted of only one case of horseshoe kidney in which there was failure to make access and there was no case with spinal injury/spina bifida, the effect of these parameters in stone clearance cannot be commented from our study. The other limitation of the study was that CT KUB was not used for determining stone free status after PCNL. The use of CT KUB for follow-up was not practical in our scenario.
CONCLUSION
Both GSS and S.T.O.N.E Nephrolithometry Score can be used to stratify the complexity of renal stone before PCNL to predict the stone clearance and complication. Both can be valuable tools for providing uniformity for comparison of outcome, proper planning of the surgery and preoperative counseling of the patient.
REFERENCES
- Preminger GM, Assimos DG, Lingeman JE, Nakada SY, Pearle MS, Wolf JS, et al. Chapter 1: AUA guideline on management of staghorn calculi: diagnosis and treatment recommendations. J Urol. 2005; 173: 1991-2000.
- Labate G, Modi P, Timoney A, Cormio L, Zhang X, Louie M, et al. The percutaneous nephrolithotomy global study: classification of complications. J Endourol. 2011; 25: 1275-1280.
- de la Rosette J, Assimos D, Desai M, Gutierrez J, Lingeman J, Scarpa R, et al. The Clinical Research Office of the Endourological Society Percutaneous Nephrolithotomy Global Study: indications, complications, and outcomes in 5803 patients. J Endourol. 2011; 25: 11-17.
- Ghani KR, Sammon JD, Bhojani N, Karakiewicz PI, Sun M, Sukumar S, et al. Trends in percutaneous nephrolithotomy use and outcomes in the United States. J Urol. 2013; 190: 558-564.
- Shahrour K, Tomaszewski J, Ortiz T, Scott E, Sternberg KM, Jackman SV, et al. Predictors of immediate postoperative outcome of single- tract percutaneous nephrolithotomy. Urology. 2012; 80: 19-25.
- Zhu Z, Wang S, Xi Q, Bai J, Yu X, Liu J. Logistic regression model for predicting stone-free rate after minimally invasive percutaneous nephrolithotomy. Urology. 2011; 78: 32-36.
- Jaipuria J, Suryavanshi M, Sen T. Comparative testing of reliability and audit utility of ordinal objective calculus complexity scores. Can we make an informed choice yet? BJU Int. 2016; 118: 958–968.
- Thomas K, Smith NC, Hegarty N, Glass JM. The guy’s stone score grading the complexity of percutaneous nephrolithotomy procedures. Urology. 2011; 78: 277–281.
- Okhunov Z, Friedlander JI, George AK, Duty BD, Moreira DM, Srinivasan AK, et al. S.T.O.N.E. nephrolithometry: novel surgical classification system for kidney calculi. Urology. 2013; 81: 1154-1159.
- Tiselius HG, Andersson A. Stone burden in an average Swedish population of stone formers requiring active stone removal: how can the stone size be estimated in the clinical routine? EurUrol. 2003; 43: 275-281.
- de la Rosette JJ, Opondo D, Daels FP, Giusti G, Serrano A, Kandasami SV, et al. Categorisation of complications and validation of the Clavien score for percutaneous nephrolithotomy. EurUrol. 2012; 62: 246–255.
- Labate G, Modi P, Timoney A, Cormio L, Zhang X, Louie M, et al. The percutaneous nephrolithotomy global study: classification of complications. J Endourol. 2011; 25: 1275–1280.
- Mandal S, Goel A, Kathpalia R, Sankhwar S, Singh V, Sinha RJ, et al. Prospective evaluation of complications using the modified Clavien grading system, and of success rates of percutaneous nephrolithotomy using Guy’s Stone Score: a single-center experience. Indian J Urol. 2012; 28: 392-398.
- Vicentini FC, Marchini GS, Mazzucchi E, Claro JFA, Srougi M. Utility of the Guy’s stone score based on computed tomographic scan findings for predicting percutaneous nephrolithotomy outcomes. Urology. 2014; 83: 1248-1253.
- Okhunov Z, Moreira D, George A, Akhavein A, Elsamra S, Duty B, et al. Multicenter validation of S.T.O.N.E. nephrolithometry. J Urol suppl. 2014; 191: e839.
- Noureldin YA, Elkoushy MA, Andonian S. External validation of the S.T.O.N.E. nephrolithometry scoring system. Canadian Urological Association Journal. 2015; 9: 190-195.
- Labadie K, Okhunov Z, Akhavein A, Moreira D, Moreno-Palacios J, Del Junco M, et al. Evaluation and Comparison of Urolithiasis Scoring Systems Used in Percutaneous Kidney Stone Surgery. J Urol. 2015; 193: 154-159.
- Park J, Hong B, Park T, Park HK. Effectiveness of noncontrast computed tomography in evaluation of residual stones after percutaneous nephrolithotomy. J Endourol. 2007; 21: 684-687.
- Pires C, Machet F, Dahmani L, Irani J, Dore B. Sensitivity of abdominal radiography without preparation compared with computed tomography in the assessment of residual fragments after percutaneous nephrolithotomy. Prog Urol. 2003; 13: 581-584.
- Ingimarsson JP, Dagrosa LM, Hyams ES, Pais VM. External validation of a preoperative renal stone grading system: reproducibility and inter-rater concordance of the Guy’s stone score using preoperative computed tomography and rigorous postoperative stone-free criteria. Urology. 2014; 83: 45-49.