Recent Articles
-
June 10, 2015 Research Article
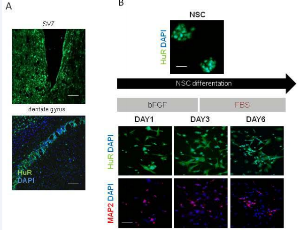 Abstract Lately increasing number of studies is enhancing a functional rule for the long-noncoding-RNAs (lncRNAs) in gene expression regulation at multiple levels: transcriptional, translational and post-translational processes. Interestingly, a key and essen.....
Abstract Lately increasing number of studies is enhancing a functional rule for the long-noncoding-RNAs (lncRNAs) in gene expression regulation at multiple levels: transcriptional, translational and post-translational processes. Interestingly, a key and essen.....




