JSM Regenerative Medicine and Bioengineering
Volume 1 - Issue 2 Articles
-
ArticleDecember 18, 2013 | Pages : 205 - 450Abstract It is often difficult to facilitate the translation of an advanced therapy medicinal product (ATMP) slated for use in regenerative medicine from the laboratory bench into the clinic [1]. In order to allow clinical trials for such ATMPs to commence, r.....
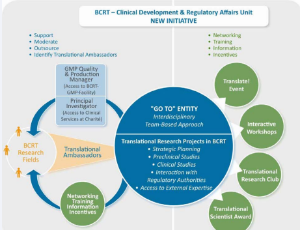
-
ArticleDecember 13, 2013 | Pages : 205 - 450Abstract Traumatic brain injury (TBI) is a serious public health and socioeconomic problem throughout the world. Each year, TBI contributes to a substantial number of cases of permanent disability and even death. According to CDC data, about 1.7 million TBI o.....
-
ArticleNovember 29, 2013 | Pages : 205 - 450Abstract The largest organ in the body, the skin conducts a wide range of functions to support and maintain human health [1,2]. The skin epidermis and its appendages (e.g., hair follicle, sebaceous and sweat glands) provide a protective barrier against physic.....
-
ArticleDecember 06, 2013 | Pages : 205 - 450Abstract The repairing of injured skin tissue is a fundamental biological process essential to the continuity of life. Wound repair is a complex and dynamic process which consists of inflammation, angiogenesis, and tissue formation and remodeling [1,2].
Volume 1 - Issue 1 Articles
-
ArticleDecember 21, 2013 | Pages : 205 - 450Abstract Given the limited capacity of the central nervous system (CNS) and the heart for self-repair or renewal, cell-based therapy represents a promising therapeutic approach closest to provide a cure to restore normal tissue and function for neurological a.....
-
ArticleNovember 06, 2013 | Pages : 205 - 450Abstract Mesenchymal stem cells (MSCs) have been identified as ideal source for regenerative purposes. Over the past decade their potential to migrate, proliferate, differentiate and modulate has shown significant promise.
-
ArticleNovember 06, 2013 | Pages : 205 - 450Abstract The field of tissue engineering has expanded at a staggering rate. In terms of bone tissue engineering research alone this has been represented by a dozen or so manuscripts being published in 1990, increasing to almost 18,000 at the time of writing.
-
ArticleNovember 02, 2013 | Pages : 205 - 450Abstract Heart failure is a major contributor to mortality in the United States. Many cardiomyocytes (CM) die following myocardial insult, and the post-natal mammalian heart has very limited regenerative capacity.
-
ArticleSeptember 21, 2013 | Pages : 205 - 450Abstract Diabetes mellitus represents a growing burden both on health-care expenditures and the quality of life of the afflicted individuals. Current estimates for the prevalence of diabetes indicate a global prevalence of about 285 million people [1].
Browse by Year
Author Information
X
Subscribe to Newsletters
And stay informed about our news and events