Current Knowledge on No Microenvironment of Head & Neck Squamous Cell Carcinoma: An Evidence-Based Review
- 1. Department of Ear, Nose and Throat, Rennes University, France
ABSTRACT
Head and neck squamous cell carcinomas (HNSCC) are common tumors with a poor prognosis. Their highly lymphophilic nature lead to frequent metastatic adenopathy at diagnosis, which significantly affects the patient’s prognosis. Although lymph nodes play a key role in the evolution of the disease, the mechanisms of tumor dissemination within them are currently poorly understood. Furthermore, lymph nodes have an ambivalent role, since it is both the route of metastatic dissemination of these cancers and the focal point of the anti-tumor immune response. Some authors suggests that some modifications occurs in lymph node microenvironment before the onset of metastases in different types of solid cancer. These changes would be due to the drainage of tumor-associated molecules through lymphatics and would provide the basis for lymph node metastases and lead to the failure of the immune system to fight the tumor. These changes in lymph nodes had been define as the « pre-metastatic niche » by some authors.
Before looking at changes in the non-involved node (N0), this raises the question of how to determine this status. The accuracy of this determination has an impact on the management of the patient as well as on the results of research conducted on this subject.
For this purpose, we performed first a literature review of the current techniques to identify tumor lymph node metastasis and secondly a review of the current knowledge about the N0 node microenvironment of HNSCC.
KEYWORDS
Head and neck squamous cell carcinoma; Lymph node; Microenvironment
CITATION
Mazoué V, Lambert C, Coudert P, Creff G, Jegoux F (2022) Current Knowledge on No Microenvironment of Head & Neck Squamous Cell Carcinoma: An Evidence-Based Review. Ann Otolaryngol Rhinol 9(3): 1293.
ABBREVIATIONS
Ag: Antigen; APC: Antigen Presenting Cells; DC: Dendritic cells; cN+: patient presenting clinical and/or radiological neck lymph node metastases; cN0: patient non- presenting clinical and/or radiological neck lymph node metastases; EpCAM: Epithelial Cell Adhesion Molecule; HES: Haematoxylin Eosin Saffron; HNSCC: Head and Neck Squamous Cell Carcinoma; HPV: Human Papilloma Virus; IFN: Interferon; IHC: Immunohistochemistry; ITC: Isolated Tumor Cells; MHC-I: Major Histocompatibility Complex-I; MHC-II: Major Histocompatibility Complex-II; MRI: Magnetic Resonance Imaging; NK: Natural Killer; OSNA: One Step Nucleic Acid Amplification; PVA: Pemphigus Vulgaris Antigen; RT-LAMP: Retro- transcriptal- Loop isothermal Amplification; SCC: Squamous Cell Carcinoma; SLNB: Sentinel Lymph Node Biopsy; SN: Sentinel Node; TACSTD1: Tumor-Associated Calcium Signal Transducer 1; TDM: Tomodensitometry; PET-TDM: Positon Emission Tomography – Tomodensitometry.
INTRODUCTION
Head and neck squamous cell carcinoma (HNSCC), are common tumours, ranked 6th in the world in 2018 [1]. These cancers continue to have a poor prognosis, with an overall 5-year survival, all stages combined, of between 40 and 60%, despite aggressive multimodal treatment strategies combining surgery and concomitant radio-chemotherapy [2]. This prognosis is related to their high lymphophilia, with a metastatic adenopathy rate at diagnosis of 54% [3]. Metastatic lymph node involvement is the main prognostic factor for these cancers, with an overall survival rate that drops by 50% in the presence of lymph node metastases [4]. More recently, De Juan et al. showed a 5-year survival of 85.5% in patients classified as pN0 compared with 62.5% in pN+ patients without lymph node extracpsular spread and 29.9% in pN+ patients with extracapsular spread [5].
While for cN+ stages or advanced tumours (cT>2), lymph node treatment is systematic, for cN0 patients, the attitude is not currently standardised. Radical neck dissection, modified neck dissection, selective neck dissection, sentinel lymph node biopsy, techniques ranging from the most invasive to the most minimalist. In recent decades, lymph node management in localized stage cN0 patients (T1-T2), has been de-escalated because only 20- 30% of these patients are at risk of occult cervical lymph node metastases [6-9]. Systematic lymph node dissection is therefore inappropriate for 70-80% of these patients who derive no survival benefit from this procedure.
Despite the lymphophilic nature of these tumours, few is currently known about the mechanisms of lymph node invasion in HNSCC. Among these mechanisms, several authors suggest the establishment of a “pre-metastatic niche” within the lymph nodes for different types of solid tumours [10,11]. This would involve changes in the lymph node environment even before the tumour has invaded the lymph node. These modifications would be related to the influence of the primary tumour and its microenvironment via the lymphatic flow. The paucity of data concerning these phenomena in patients with HNSCC leads to a lack of knowledge of potential therapeutic targets.
The aim of this work is to synthesise the current knowledge and in particular the biological (molecular and cellular) knowledge of N0 necks of patients with HNSCC.
Lymph node, immunity and cancer
The lymph node is the focal point where the main cells involved in anti-tumour immunity are located. It is the place where our immune systems initiate the adaptive response against the tumour.
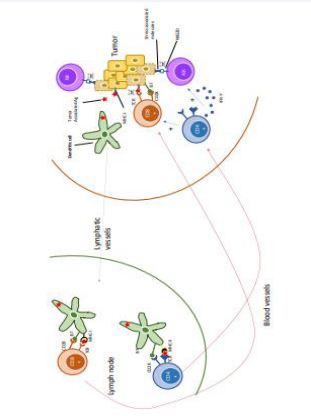
In HNSCC and most solid cancers, the anti-tumour immune response is mainly based on cellular immunity, mediated by T cells and natural killers (Figure 1).
Figure 1: Representation of the main immune cells involved in the anti-tumour response.
The tumour is identified by different immune cells in the mucosa. Natural killers, recognise tumour cells via the interaction between their NKG2D receptor and “stress-associated molecules” present on the surface of tumour cells. This contact leads to the activation of the NK which destroy the tumour cell. This destruction can take place by several mechanisms: through the release of cytotoxic granules (granzymes and perforins) or through the expression of the death receptor ligand present on the tumour cell (Fas-Ligand) [12]. Natural killer action is one of the first reactions of the immune system to the tumour and is part of the innate immune response.
Dendritic cells (DCs) are the APCs that are predominantly present in the mucosa of the upper aerodigestive tract. They pick up tumour antigens and migrate in the lymph to the regional lymph node that drains this area of mucosa. These antigens can also circulate freely in the lymph to the lymph node. They are then taken up by cells such as subcapsular macrophages, follicular dendritic cells or B lymphocytes present in the lymph node. These cells will then be able to present this Ag on their surface and activate LTs in the paracortex [10,13]. Tumourrelated Ag are mainly derived from tumour cells in a state of cell death, which are released during their destruction. DCs present the Ag on their surface via the MHC-I molecule on the one hand and MHC-II on the other. They will then meet all the naive T lymphocytes of the “repertoire” in the lymph nodes. The T cells whose TCR corresponds to this antigen are then activated. However, this interaction alone is not sufficient to achieve full T cell activation for cytotoxic action. A second joint interaction between the two cells is necessary and this is between the CD28 receptor of the T lymphocyte and the B7 protein of the APC [14].
Depending on the type of LT activated by the interaction with the dendritic cell (DC), different immune mechanisms will be put in place:
- DC and CD8+ T cells: The DC presents the tumourassociated Ag to CD8+ T cells TCR through its MHC-I molecule.
The lymphocyte have a direct cytotoxic action on the targeted tumour cells releasing perforins and granzymes.
- DC and CD4+ T cells: The DC presents the the tumourassociated Ag to the TCR of the CD4+ T cells through its MHC-II molecule. Once activated, the lymphocyte releases cytokines such as IFN-gamma which allow the recruitment of more NK against the tumour. They also facilitate the activation of LT-CD8+ by DCs, their clonal expansion through releasing interleukin-2. Finally, they allow the establishment and maintenance of CD8+ T cells memory immunity [15].
Once activated in the lymph node, T cells will enter the bloodstream through the high endothelial venules and then the primary tumour site. The role of the lymphatic system and Ag presentation in tumour control has been well demonstrated in a mouse model. Mutated mice with severe lymphatic dysfunction had increased primary tumour growth compared to the same non-mutated mice [16].
Due to the anti-tumour immune response, HNSCC should never be able to grow as they are eliminated as they appear. However, tumour cells progressively acquire different mechanisms of escape from the immune system through the accumulation of mutations. All these phenomena are part of the concept of immunoediting, which describes the interactions between tumour cells and the immune system [17]. This concept is divided into 3 phases:
- The elimination phase: The immune system identifies abnormal cells and eliminates them before a tumour can form.
- The equilibrium phase: Tumour cells and immune system are in dynamic equilibrium. Immune system is not able to completely eliminate abnormal cells. The tumour remains sub-clinical.
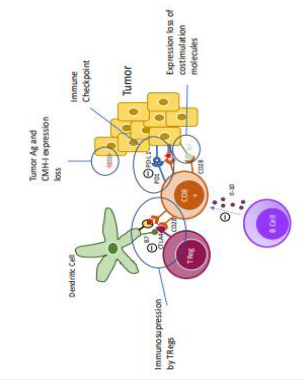
- The escape phase: Tumour cells acquire various modifications (Figure 2),
Figure 2: Representation of the main mechanisms of immune escape. e
that allow them to escape detection by the immune system and even inactivate some anti-tumour immune cells.
One of the first mechanisms of escape is a decrease in the expression of MHC-I molecules, which are necessary for immune cells to recognise tumour-associated Ag. Tumour cells may also lose the expression of tumour-associated Ag. In these two cases, the immune cells no longer recognise the tumour cells, and the tumour proliferates. This is the phenomenon of reduced tumour immunogenicity. This is related by the progressive “selection” of these cells by the immune system. In fact, it is first the most immunogenic tumour cells that are targeted and destroyed by the effector cells. Then cells that no longer express MHC-I due to gene mutations emerge in the tumour and eventually become the majority because they are not recognised by the effector cells.
To be effective, CD8+ T cells must be activated by recognition of the tumour-associated antigen by their TCR but also by costimulation delivered by interaction of the B7 ligand and its CD28 receptor. Tumour cells can progressively lose the expression of B7 and thus render the cytotoxic action of CD8+ T cells ineffective.
Tumour cells can express on their surface PDL-1 which is recognised by PD1 receptor present on CD8+ T cells and whose activation leads to an inhibition of this lymphocyte. This interaction is called an immune checkpoint. A study of HPVrelated oropharyngeal squamous cell carcinomas showed that more than 50% of tumour cells expressed PDL-1[18].
During their development, tumours can attract immune cells with immunosuppressive action such as Tregs lymphocytes or B cells secreting IL-10, a major immune inhibitory interleukin [19]. Tregs through their CTLA-4 molecule can block costimulation between B7 of the dendritic cell and CD28 of CD8+ T cells, inactivating CD8+ T cells.
All of these escape mechanisms, taken separately or in combination, inevitably lead to the failure of the immune system to control the tumour.
Lymph node’s ambivalence
As described above, lymph nodes play an essential role in tumour control. However, the lymphatic system is also the main route of metastatic spread of HNSCC.
The essential step in this dissemination is lymphatic vascular invasion by tumour cells. Several mechanisms of lymphatic vessel invasion by tumour cells have been described and summarised by Fujimoto et al [20]:
- Mechanical rupture of the lymphatic vessel wall
- Immune cell mimicry (using the CCL21 gradient recognised by the CCR7 receptor expressed by the tumour cell)
- Active destabilisation of junctions between lymphatic endothelial cells
- Active creation of entry portals into lymphatic vessel membrane (via chemorepellent signals to lymphatic endothelial cells).
Mechanical rupture of the lymphatic vessel wall is a mechanism that has been demonstrated most specifically in HNSCC by Niimi et al., on tumour samples from patients with SCC of the oral cavity [21].
Invasion by immune cell mimicry also appears to be a mechanism used by HNSCC. CCL19, is a chemokine constitutively expressed in lymphoid tissues. HNSCC tumour cells have been shown to express the chemokine receptor CCR7. The interaction between CCL19 and CCR7 is thought to promote cell migration and adhesion in CEVADS. The activation of CCR7 would then use the intracellular MAPK (Mitogen-Activated Protein Kinase), pathway, notably through the intracellular proteins ERK1/2 and jnK. Once activated, these proteins would induce an increase in the expression of vimentin and a decrease in the expression of E-cadherin, leading to a loss of adhesion between the cells and therefore facilitate their migration [22]. The CCR7-CCL19 pair is not the only one involved in this phenomenon since Tsujikawa et al., demonstrated a role for the CCR4 receptor and its ligand CCL22 in the lymph node invasion. The expression of CCR4 by the primary tumour is significantly associated with the presence of lymph node metastasis and lymphatic invasion. Tumour cells expressing the CCR4 receptor metastasise via an autocrine mechanism mediated by CCL22 and a paracrine mechanism mediated by CCL22 secretion from tumour-associated macrophages [23].
Tumour cells can enter in lymphatic vessels, either as single cells or as clusters of cells [21]. After colonising lymphatic vessels, tumour cells are then carried by the lymphatic flow to the first lymph node relay where they initially localise in the subcapsular sinus [24]. They can then either be destroyed by the immune system, continue through the lymph node to the next, or grow to create a lymph node metastasis that can invade deeper layers of the node [11,25,26]. If a metastasis develops in the subcapsular sinus, it can rapidly lead to extracapsular spread. A study conducted on 63 patients with cN0 HNSCC found that 30% of them had occult metastasis and 19% extracapsular spread. Extracapsular spread was reported in 63% of occult metastases [27]. These data suggest that extracapsular spread is a relatively early event in the natural history of HNSCC’s lymph node metastasis. This fact is probably explained by the primary location of the metastatic cells in the subcapsular sinus, just below the node capsule.
In addition, tumours or some cells in their microenvironment such as macrophages [28], can secrete factors that stimulate lymphangiogenesis. This is the case for molecules of the VEGF family, such as VEGF-C and its receptor VEGFR3, which are widely expressed in HNSCC [29]. Lymphangiogenesis’s stimulation increases the density of peri-tumour lymphatic vessels which correlates with the presence of lymph node metastases in the HNSCC [30-33]. This can be explained by an increased probability of lymphatic vascular invasion. However, molecular mechanisms underlying this lymphangiogenesis in HNSCC are still poorly understood as the majority of studies performed are in mouse models or other cancer sites.
N0 lymph node
By definition, an “N0” lymph node is a lymph node that has no tumour cells in its tissues. This definition raises the question of the completeness and sensitivity of the examinations that are carried out to assess lymph node status at initial management.
N0 lymph node and histology:
Standard analysis:
Currently, routine histological analysis of cervical lymph nodes is performed on a few sections from each lymph node which are then stained with haematoxilin-eosin-safran (HES). Apart from the staining method, which remains universal, the technique for analysing these nodes is not consensual. Indeed, the way in which the lymph node is cut (direction of section) as well as the number of sections performed according to the size of the lymph node varies from one centre to another [34]. The limitation is that the lymph node is not analysed in its entirety: the sections made may miss small macrometastatic foci (size > 2 mm) or micrometastatic foci (size < 2 mm) and isolated tumour cells (ITC; size < 0.2 mm).
Sproll et al., studied lymph nodes from 50 patients with HNSCC classified as pN0 by HES analysis. 30% of these patients had lymph node micrometastases or isolated tumour cells on immunohistochemical analysis. However, no association was found between the presence of these events and patient survival [35]. In their cohort of 23 pN0 patients, Nieuwenhuis et al. found that 20% had lymph node micrometastases not detected by standard histological analysis. RT- PCR analysis of E48, a squamous cell carcinoma’s specific antigen, was used to detect these micrometastases. The presence of these micrometastases in patients initially classified as pN0 was associated with a decrease in cancer-specific survival [36].
These studies highlight the limitation of standard pathology examination, particularly its sensitivity, and suggest that micrometastases may have clinical consequences.
Contribution of immunohistochemistry: The sentinel lymph node biopsy (SLNB), has been validated in multicentre studies for SCC of the oral cavity classified as cT1-T2 N0. This technique can also be performed for certain oropharyngeal locations classified as cT1-T2-N0, provided that the tumour is accessible for tracer injection under local anaesthesia. This technique has a sensitivity of 86 to 92% and a negative predictive value of 95 to 96% in the detection of occult metastases, depending on the study [6,37,38]. The number of nodes analysed is on average 3 per patient and therefore less than for neck dissection [6,39], but it allows for more accurate analysis.
The analysis consists of taking serial sections, with alternating HES staining and immunohistochemical staining. These are mainly antibodies that target cytokeratins (CK19, CK5, CK14, CK17), often in the form of pancytokeratin kits such as the AE1/3 kit [39-42]. Sentinel node involvement is defined according to the 2003 UICC TNM classification based on Hermanek et al.[43], into macrometastasis (size > 2 mm), micrometastasis (size > 2 mm and < 2 mm) and “isolated tumour cells” (size < 0.2 mm). However, there are currently no recommendations regarding the pathology protocol to be performed for these sentinel nodes for HNSCC. Methods differ between laboratories regarding the number of serial sections performed and the interval between them and lead to differences in diagnostic sensitivity [44,45]. A recent study concludes that it is necessary to analyse the whole lymph node with serial sections every 150 micrometres in order not to miss any tumour cells, including isolated ones [44].
These techniques allow the detection of occult lymph node metastases in 23% to 27% of patients initially classified as cN0 when considering a cohort of patients eligible for a sentinel node biopsy [6,37]. Among these occult metastases, the rate of ITC (Isolated Tumor Cell) found varies according to the studies between 13% and 66% and the rate of micrometastases between 30% and 48% [6,46-48].
In case of macro or micrometastatic disease, a selective neck disection is indicated [49]. There is currently no consensus on what to do with ITC. Matsuzuka et al., recently investigated when neck dissection is necessary in cases of sentinel node (SN) involvement. In this study, 57 patients with cT2-T3N0 SCC of the oral cavity underwent SLNB followed by neck dissection (areas I-IV if SLNB positive and areas I-III if SLNB negative). The authors then investigated the rate of metastasis in the nonsentinel nodes (from neck dissections) in the 23 SLNB-positive patients according to the size of the involvement in the GS. For ITC, micrometastases and macrometastases, the rate was 0% (0/3), 14% (1/7) and 23% (3/13) respectively. These results suggest that for patients with positive SN, the risk of secondary relay involvement is only for involvement >0.2mm. These results provide an argument that a modified neck dissection would only be useful for micrometastases and not for ITCs. However, these results need to be confirmed as this study was conducted on a small number of subjects (n=57) and only 10 had micrometastases or ITCs in the SN.
The exhaustive detection of micrometastases and ITCs thus requires serial sectioning techniques and their analysis after IHC. However, these protocols are extremely time-consuming for the anatomopathologist whose workload is considerably increased by the number of slides to be analysed. The alternative to the detection of these lesions is to use amplification and detection techniques for specific molecular markers of tumour cells. Some surgical specialties already use these techniques in routine practice. However, these techniques are currently still in the field of research in the management of CEVADS.
Alternatives for the detection of HNSCC lymph node metastases: There are specific molecular markers for epithelial tumours, and by extension squamous cell carcinomas. The presence of such markers in a lymph node is abnormal and indicates the presence of metastases in this one.
These markers include:
- Cytokeratin mRNAs (CK19, CK18, CK17, CK14, CK7, CK5, …); [50]
- PVA (Pemphigus Vulgaris Antigen) which is a cell adhesion molecule bound to keratinocytes [50-53];
- TACSTD1 (also called EpCAM). It is also a molecule linked to cell adhesion [51-53].
- E48 (Ly-6D): an antigen specifically expressed by squamous cells of normal, tumour and transitional epithelium [36].
- Micro-RNA [54].
Ferris et al., evaluated the use of three molecular markers for the detection of lymph node metastases. TACSTD1, PVA and PTHrP were investigated by RT-qPCR in lymph node lysates. The concordance of N+ or N0 status for each marker ranged from 93 to 98%. TACSTD1 and PVA was the best combination of markers in terms of accuracy. The authors developed a rapid, automated multiplex test using these 2 markers, with a status identification accuracy of 94.2% (on 103 nodes tested) with good reproducibility [53].
Nieuwenhuis et al., investigated the detection of lymph node micrometastases using RT-qPCR amplifying for E-48 (Ly-6D). This is an antigen specifically expressed by squamous cells of normal, tumour and transitional epithelia. Their work seems to confirm that E-48 is a relevant molecular marker for the detection of lymph node metastases of HNSCC [36].
Currently, the detection of certain cytokeratins by RT-PCR is used in current practice in the management of various types of cancers such as breast or colon cancers [55,56]. The use of these molecular biology techniques in current practice is made possible by the automation of the reactions, in particular with the “OSNA” (One Step Nucleic Acid Amplification) technique. This consists of a RT-LAMP which allows the presence of cytoketin-19 (CK-19) mRNA to be detected in the lymph node by amplification of this mRNA. The lymph nodes are crushed and then lysed in their entirety. The lymph node lysates are then processed by the « OSNA technique ». The presence of this CK-19 mRNA above a certain threshold indicates the presence of metastatic cells in the lymph node. The threshold of positivity has been defined at 250 copies/ μL in breast cancer studies. This technique, unlike the usual histological analysis techniques mentioned above, allows for an exhaustive analysis of the lymph node in terms of tissue material. Moreover the result is available in approximately 30 minutes. Depending on the result obtained, the additional treatment by a neck dissection or not can be performed at the same time, thus avoiding a second operation for the patient.
Few studies exist on the use of the OSNA technique in the analysis of N0 nodes of HNSCC. Our team worked on the possibility of transposing this technique in the management of patients with HNSCC. For this purpose, a study was carried out on 157 nodes from 26 patients classified as cN0 who had undergone neck dissection, prospectively collected. Each node was analysed using the standard pathological technique (HES staining) and the OSNA technique. The results of this study gave the OSNA technique a sensitivity of 90% and a specificity of 95.6%, allowing us to conclude that this technique should be considered for improving the analysis of SN in HNSCC [57]. However, this technique has several limitations: 20-61% of tumours do not express CK-19. There are false positives that can be explained by the presence of heterotopic salivary tissue or epithelial inclusion within the lymph node. In addition, complete lysis of the lymph node results in loss of information about possible capsular rupture of the lymph node.
De Carvalho et al., evaluated the usefulness of miRNAs in the detection of lymph node metastases. For this purpose, the expression of miRNAs obtained from deparaffinised lymph nodes was compared between non-invaded (N0) and invaded (N+) nodes of 48 patients. In order to ascertain the invaded or non-invaded nature of the nodes studied, the authors performed serial sections every 50 µm with immunohistochemical labelling of cytokeratins. They identified a pool of 61 miRNAs significantly differentially expressed after RT-PCR (p<0.05), among which 7 miRNAs were 100-fold more expressed in N0 nodes. MiR203 and miR-205 had an NPV and PPV for detection of macro, micrometastases and ITC of 100%. In view of these results, the authors suggest the use of these markers for the detection of cervical lymph node metastases [54]. These results need to be confirmed by other studies looking for the presence of these miRNAs in lymph nodes before knowing their invaded or noninvaded status in IHC. Moreover, these analyses are not currently automated and therefore not exportable to clinical practice.
Together, these techniques optimise the diagnosis of invasion at its microscopic stage and therefore at an early stage of invasion. However, there is currently no molecular or cellular marker that predicts invasion before it occurs.
Lymph node’s pre-métastatic niche - HNSCC and N0 lymph node: Several authors have suggested that there are changes in the molecular and cellular environment within the lymph node before its invasion by cancer cells. These early changes would correspond to the “pre-metastatic niche”. This concept of a premetastatic niche was first formulated in 2005 by N Kaplan et al., in an animal model of lung and melanoma tumour cells [58]. More recently in 2015, Sleeman reviewed the literature regarding the data of this concept of pre-metastatic lymph node in different solids cancers. Pre-metastatic morphological and histological changes could be demonstrated, such as an increase in the volume of the first relay lymph node. This would be linked to an increase in the number of lymphocytes in the paracortical zones, an increase in hyperplastic follicles, distension of the sinuses or hyperplasia of the sinus histiocytes [10]. For these authors, the drainage of tumour Ag into the lymph nodes would initially allow the establishment of an anti-tumour immune response which would prevent the formation of metastases. Secondly, the development of the primary tumour would lead to an increase in the concentration of immunomodulatory factors which drain to the lymph nodes and would lead to an immunosuppression of the microenvironment and a “pre-conditioning” which would allow the development of lymph node metastases [10]. However, these studies have focused on cutaneous melanoma and breast cancer and more anecdotally on gastric and renal cancers but not on HNSCC. As this concept is increasingly studied, in 2020 Gillot et al., conducted a new review of the literature on the subject. This pre-metastatic niche is a complex process recognized as an initial key step in the metastatic cascade. The primary tumor prepares for the remodeling of the draining (sentinel) lymph node by secreting soluble factors or releasing extracellular vesicles that are transported by lymphatic vessels [11]
Regarding scientific data on non-involved (N0) HNSCC lymph nodes, i.e. the pre-metastatic lymph node niche, the literature is extremely poor.
Topf et al., compared the abundance of CD169+ subcapsular macrophages in tumour-invaded and uninvaded nodes of patients with HNSCC. The subcapsular sinuses of invaded nodes had significantly fewer CD169+ macrophages than uninvaded nodes. However, the mechanisms underlying this decrease in the subcapsular lymphocyte population are not known [59]. These macrophages are important in anti-tumour immunity as they induce T cells activation. Their depletion could facilitate tumour development and subsequently the appearance of metastases. High endothelial venules are the portals that allow lymphocytes to pass from the bloodstream to the lymph node. Chung et al., compared the density of lymphatic vessels and high endothelial venules in pN+, pN0 sentinel nodes and non-sentinel nodes (control) of SCC of the oral cavity. They found a significantly higher density of high endothelial venules and lymphatic vessels between sentinel nodes (pN+ or pN0) and control nodes. No difference was found between metastatic and non-metastatic sentinel nodes, possibly due to a lack of power according to the authors (only 12 pN+ versus 120 pN-)[60]. These changes could promote lymph node colonisation by metastatic cells.
These data suggest that there are cellular and vascular changes in the environment of the lymph node draining the tumour. These data lend credence to the existence of a lymph node pre- metastasis niche in the natural history of HNSCC.
DISCUSSION & CONCLUSION
Although lymph node involvement is common and has a major impact on the prognosis and choice of treatment for HNSCC, the mechanisms of lymph node metastasis are currently poorly understood. The lymph node that drains the tumour area plays a key role in the development and maintenance of the antitumour immune response. The concept of a « pre-metastatic niche » in lymph node proposed by some authors has been few explored in HNSCC. The changes in immune cell number and lymphatic density within the N0 lymph nodes are probably not isolated changes. Other early molecular and cellular changes, related to the proximity of the tumour and potentially involved in the failure of its defence role, remain to be discovered. The recent evolution of molecular biology techniques now makes it possible to study changes in the lymph node transcriptome. The study of these changes in the N0 lymph nodes of HNSCC could provide valuable informations on the immune changes that may occur in the lymph nodes prior to the appearance of histological metastases. Knowledge of such changes would provide a better understanding of the lymph node metastasis process and could lead to the discovery of new therapeutic targets.
REFERENCES
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68: 394-424.
- Gatta G, Botta L, Sánchez MJ, Anderson LA, Pierannunzio D, Licitra L, et al. Prognoses and improvement for head and neck cancers diagnosed in Europe in early 2000s: The EUROCARE-5 population-based study. Eur J Cancer Oxf Engl. 1990; 51: 2130-2143.
- Cerezo L, Millán I, Torre A, Aragon G, Otero J. Prognostic factors for survival and tumor control in cervical lymph node metastases from head and neck cancer. A multivariate study of 492 cases. Cancer. 1992; 69: 1224-1234.
- de Juan J, García J, López M. Inclusion of extracapsular spread in the pTNM classification system: a proposal for patients with head and neck carcinoma. JAMA Otolaryngol-- Head Neck Surg. 2013; 139: 483- 488.
- Schilling C, Stoeckli SJ, Haerle SK, Broglie MA, Huber GF, Sorenson JA, et al. Sentinel European Node Trial (SENT): 3-year results of sentinel node biopsy in oral cancer. Eur J Cancer Oxf Engl. 1990; 51: 2777- 2784.
- Shimizu KI, Inoue H, Saitoh M, Ohtsuki N, Ishida H, Makino K, et al. Distribution and impact of lymph node metastases in oropharyngeal cancer. Acta Otolaryngol (Stockh). 2006; 126: 872-877.
- De Zinis LOR, Bolzoni A, Piazza C, Nicolai P. Prevalence and localization of nodal metastases in squamous cell carcinoma of the oral cavity: role and extension of neck dissection. Eur Arch Oto-Rhino-Laryngol. 2006; 263: 1131-1135.
- Bae MR, Roh JL, Kim JS, Choi SH, Nam SY. Prediction of cervical metastasis and survival in cN0 oral cavity cancer using tumour 18F-FDG PET/CT functional parameters. J Cancer Res Clin Oncol. 2020; 146: 3341-3348.