Identification of Factors that Influence Implantation Time of an Upper Airway Stimulation System
- 1. Department of Otolaryngology-Head and Neck Surgery, University of Kansas Medical Center, USA
- 2. Saint Antonius Hospital, Utrecht, Netherlands
- 3. The Ohio State University, Columbus, Ohio, USA
- 4. University Clinic of Schleswig-Holstein, Lubeck, Germany
- 5. on behalf of the ADHERE registry investigators
ABSTRACT
Objective: Leverage ADHERE registry data to identify factors that influenced implantation time, including patient demographics, implanter experience, implanter region, and the version of UAS system hardware.
Study design: Retrospective cohort study
Setting: Data from multiple international surgical centers, including 36 sites in the United States and 8 in the European Union.
Methods: Data was gathered retrospectively from the ADHERE registry, a multi-center, prospective, international study of UAS outcomes from October 2016 to December 2019. The study is registered as NCT02907398 on clinicaltrials.gov, and has been approved by respective IRB or ethics committees. The registry collects baseline demographic information, implant data such as device version, implanter experience, implanter location, operating time, and surgical adverse events, among others. Multiple linear regressions were performed to identify factors that impacted implantation time and control for confounding factors. .
Results: Of the 1,537 patients enrolled in the registry, 1,448 had implant timing data, and were divided into three cohorts- 533 patients with the first generation system, 522 patients with the second-generation IPG and first-generation sensor lead, and 482 patients with both the second-generation IPG and second generation sensor lead. Raw, unadjusted implant times, respectively, were 147 ± 46 minutes, 129 ± 46 minutes, and 110 ± 41 minutes. After adjusting for confounding factors, the regression model identified the multiple influences on implant time: complete second-generation system (-20 minutes, 95% CI: -26.6, -13.8, p<0.001), increasing age (-0.3 minute, 95% CI: -0.5, -0.1, p=0.004), implant location (US vs. EU: -11 minute reduction, 95% CI: -19.1, -3.2, p=0.006), and regional implanter experience (EU -0.49 minutes, 95% CI: -0.6, -0.4, p< 0.001; US 0.13 minutes, 95% CI: 0.01, 0.3, p=0.04). BMI above or below 32 was not associated with changes in implant time. The rate of adverse events was low and similar between cohorts (p=0.73).
Conclusion: Second-generation UAS system had the largest influence on implant time compared to the first-generation system, even after controlling for confounding variables.Demographics, region, and implanter experience had a smaller impact. Implant time reduction was not associated with increased adverse event rates.
KEYWORDS
Obstructive Sleep Apnea; Upper Airway Stimulation; Sleep Surgery; Implant
CITATION
Hamacher S, Copper M, Chio E, Steffen A, Larsen C (2022) Identification of Factors that Influence Implantation Time of an Upper Airway Stimulation System. Ann Otolaryngol Rhinol 9(3): 1292.
INTRODUCTION
Upper Airway Stimulation (UAS), has proven to be an effective, safe means of reducing upper airway obstructive events during sleep and improving overall quality of life for patients with severe Obstructive Sleep Apnea (OSA), who meet certain criteria for implantation [1-3]. These criteria include a minimum age of 18, an Apnea-Hypopnea Index (AHI), between 15-65, suggested Body Mass Index (BMI), of less than 35, non-concentric soft palate collapse during drug-induced sleep endoscopy, and intolerance of Continuous Positive Airway Pressure (CPAP), device use [4]. There are three primary implantable components which make up the UAS system: an Implantable Pulse Generator (IPG), a stimulation lead, and a respiratory sensor. The IPG provides the battery and logic for timing and adjustment of stimulation. The stimulation lead is a cuff electrode wrapped around branches of one of the hypoglossal nerves, delivering electrical stimulation to this nerve which in turn leads to activation of the genioglossus muscle of the tongue. The respiratory sensor detects chest wall movement associated with breathing. When activation of the genioglossus muscle is coordinated with inspiration during sleep, the tongue protrudes and the associated dilation of the upper airway helps to relieve collapse of much of the soft tissue implicated with obstruction. The technology included in these components has improved over time, reducing their physical size and simplifying the implantation process. The first-generation system with a 24 cubic centimeter (cc), volume IPG was first approved by the FDA in 2014. In 2017, an upgraded IPG (15 cc) was approved. It was being implanted with the first-generation respiratory sensor until a newer version of the sensor was released in 2018 with a shorter sensor and fewer suture anchors. In 2018, the complete second-generation system was being implanted in patients in the European Union. After FDA approval in 2019, this system was then also being implanted in patients in the United States (Figure 1).
Figure 1: Illustration of IPG and respiratory sensor versions.
The goal of this study was to identify which factors would influence implantation time, including patient demographics, implanter site and experience, and version of the UAS system hardware. It is hypothesized that improvements in UAS system size, when controlled for confounding factors such as demographics and implanter experience, would positively impact implantation time. It is further hypothesized that faster surgical time does not equate to an increase in the rate of adverse events
METHODS
The ADHERE registry is a multi-center, international, prospective registry study of post-market outcomes of the Upper Airway Stimulation System (Inspire Medical Systems, Golden Valley, MN). The study is registered as NCT02907398 on clinicaltrials.gov, and has been approved by the relevant IRB or ethics committees (see Appendix). Description of the study, data collection, and adverse events has been previously published [5]. Registry data was extracted from patients enrolled from October 2016 through December 2019. Specifically, data collected includes patient demographics (age, gender, race, BMI, and AHI), surgical factors (date of surgery, implantation time defined as first incision to first close, procedural adverse events), and implanted device specifics (IPG and sensor lead version and serial number). Surgeon experience was measured by the number of cases performed per implanting site.
Using data collected from the ADHERE registry, a comparison of time in surgery was conducted based upon different permutations of first- and second-generation UAS components using the original three-incision implantation technique used prior to March 2021. The baseline system against which differences were compared was the complete first-generation system, which includes the first-generation IPG coupled with the first-generation respiratory sensor. The second system used for comparison was the second-generation IPG coupled with the original, first-generation respiratory sensor. The final system for comparison was a complete second-generation system, including the second-generation IPG coupled with the second-generation respiratory sensor. In order to account for possible differences in surgical time due to confounding factors, multiple linear regression analysis was conducted with other data collected from the ADHERE registry, including patient demographic information, region where the operation was conducted, and surgeon experience.
For statistical analysis, R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria), was used. ANOVA was used to compare baseline numeric variables between the three groups of IPG and sensor lead permutations. Chi-square tests were used for categorical variables, and Fisher’s exact test was used for comparing adverse event rates. Multiple Linear Regression was used to evaluate the outcome of surgery time based on the various factors of baseline demographics, implanting site and experience, and UAS device/sensor permutations. A full model, including demographics, region, implanter experience defined by the number of implants performed, and UAS system version was created and backward selection was used to find the reduced model. P values less than 0.05 were considered statistically significant.
RESULTS
A total of 1,537 enrollments were analyzed from the ADHERE registry for purposes of this comparative study (Table 1).
Table 1: Group Demographics.
|
Variable |
1st Generation IPG + 1st Generation Sensor (n=533)
|
2nd Generation IPG + 1st Generation Sensor (n=522)
|
2nd Generation IPG + 2nd Generation Sensor (n=482)
|
p – value
|
|
% Male |
79% |
65% |
69% |
<0.001 |
|
% White |
96% |
95% |
95% |
0.7 |
|
Mean BMI (kg/m2) |
29.1 |
29.0 |
29.6 |
0.1 |
|
Mean AHI(events/hour) |
35.6 |
35.6 |
36.0 |
0.4 |
|
Mean Age (years) |
58.8 |
61.7 |
58.7 |
<0.001 |
A majority of the patients included in this study were overweight, middle-aged males with very similar pre-operative AHI scores; however, age and gender were different between groups, with newer-generation device cohorts including more women. Clinical sites typically had significant pre-existing implant experience, averaging 43.84 ± 39.2 implants per site.
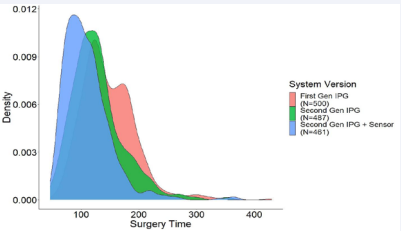
Implant time was available for 1,448 patients. There were 500 patients with the complete first-generation system, 487 patients with the second-generation IPG and first-generation sensor lead, and 461 patients with both the second-generation IPG and second-generation sensor lead. The first-generation system implantation time was 147 ± 46 minutes. Analyzing only the raw, unadjusted surgical times for each cohort compared to the first-generation system, implantation of the secondgeneration IPG and first-generation sensor resulted in a reduction in surgical time of 18 minutes (129 ± 46 minutes, p < 0.001 vs. first-generation). Implantation of the second-generation IPG and the second-generation respiratory sensor reduced surgical time by 37 minutes (110 ± 41 minutes, p < 0.001 vs. first-generation, Figure 2).
Figure 2: Surgical time probability density curve, by system type.
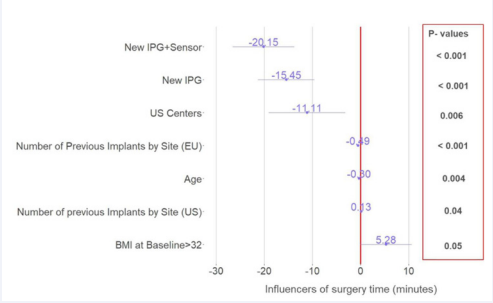
After adjusting for confounding factors such as patient demographics and implanter experience using multiple linear regression, the regression model demonstrated the following factors influencing implantation time compared to the firstgeneration system. Implantation of the second-generation IPG with the first-generation respiratory sensor was associated with 15 minute reduction of surgical time (95% CI: -21.3, -9.6,p<0.001), and similarly the complete second-generation system was associated with 20 minute reduction (95% CI: -26.6, -13.8, p<0.001) (Figure 3).
Figure 3: Factors from regression demonstrating impact on surgical time. New IPG and New IPG+Sensor are compared to the first-generation system.
From the patient demographic factors, age was the only statistically significant factor affecting implantation time, regardless of the system being implanted. Increasing age was associated with a 0.3 minute decrease in surgical time (95% CI: -0.5, -0.1, p=0.004), and baseline BMI of more than 32 had a non-significant trend of a 5 minute increase (95% CI: -0.1, 10.6, p=0.05). There was, however, no interaction between age and BMI on implant time. Another confounding factor was region, and an interaction between implant experience and surgical sites in the United States versus a center in the European Union. US sites was associated with an 11 minute faster surgical time than EU sites (95% CI: -19.1, -3.2, p=0.006). Implanter experience had a small impact on implant time; EU sites had a small reduction of 0.49 minutes (95% CI: -0.6, -0.4, p < 0.001), and US sites had a small increase of 0.13 minutes (95% CI: 0.01, 0.3, p=0.04)
Despite decreased implant times, peri-operative severe adverse event (SAE), rates were similar amongst the three cohorts. SAE were defined using standard criteria and have been previously published, and have been historically primarily related to hematoma, infections, and pneumothorax5 . The SAE rates in the three cohorts were low, and there were no significant differences in SAE rates between cohorts (p=0.73) [Table 2].
Table 2: Severe Adverse Events rates, by IPG & sensor version.
|
|
1st Generation IPG and 1st generation sensor (n=533) |
2nd generation IPG and 1st generation sensor (n=522) |
2nd generation IPG and 2nd generation sensor (n=482) |
|
Serious Adverse Event (n, %) |
3 (0.5%) |
7 (1.3%) |
3 (0.6%) |
|
Non-Serious Adverse Events (n, %) |
17 (3.1%) |
22 (4.2%) |
15 (3.1%) |
|
P value=0.73 (Fisher’s exact test) |
|||
DISCUSSION
Implantation of the newer-generation UAS components was associated with the largest reduction in operative time when compared with implantation of the older-generation components, even when adjusting for confounding factors such as implanter experience, region, and patient demographics. Decreases in surgical time were potentially due to technology improvements and/or implantation technique improvements between generations. The second-generation IPG offers a smaller volume than the first-generation IPG (15 cc vs. 24 cc, respectively). This 63% volume difference likely reduces the dissection required to create a pocket for device insertion. Although the pocket is usually created between tissue planes in the body by means of blunt finger dissection—meaning it takes only seconds longer to create the approximate 9cc volume difference- the smaller pocket also lends itself to closure with a reduced number of sutures, logically decreasing the closure time.
Design enhancements between generations likely make a difference as well. The newer IPG requires two set screws to hold the respiratory sensing leads in place, whereas the older IPG required four. The newer IPG also incorporates a torquelimiting wrench used to turn the set screws, which expedites the process of securing the stimulation and sensor leads in place. The older version relied on subjective feel by the surgeon to provide the correct amount of torque on the screws to hold the leads in place. A surgeon tightening the set screws on the new system simply twists the wrench until an audible “click” is heard and felt, indicating the proper torque has been achieved for lead securement. Similar changes are seen in the newer-generation respiratory sensor element as well. The newer sensor requires five sutures to secure it in place, versus eight for the older version. Finally, the distal element of the newer respiratory sensing lead is stiffer and shorter, allowing greater ease of insertion into intercostal muscle plane between ribs. All these design factors in concert likely result in the significant reduction in operative time associated with implantation of the newer UAS components.
Several demographic factors were correlated with significant, albeit small, differences in surgical time. Increased patient age was associated with slightly reduced surgical time, whereas increased BMI was associated with borderline-significantly increased surgical time, with a p-value of exactly 0.05. Increased BMI logically implies larger necks and thicker chest walls, with more dissection of soft tissue necessary to arrive in the proper surgical plane. Why increased age is associated with slightly decreased surgical time is unclear, however. Extrapolating the logic described above for BMI, an increase in age could be surmised to indicate a decrease in BMI, with a corresponding reduction in surgical time, but when age and BMI were analyzed against each another, there was no significant interaction discernible. Perhaps increased ease of tissue manipulation with the more aged patient accounts for the difference, but this conjecture is unsubstantiated.
There was a small but statistically significant reduction in surgical time noted when comparing surgical centers in the United States versus those in the European Union. This difference is not fully understood. Perhaps differences in the methods used in each region to record surgical time, or operating room/ anesthesia setup time may account for this disparity.
Of course, there are other factors which affect total operative time. As this relates to surgery involving medical device implantation in general, these factors are many and varied. They include surgeon experience, proficiency, and familiarity with the device being installed. The effect of increasing implanter experience leading to shorter surgical time for UAS implantation has been previously demonstrated to plateau after 10-15 implants6,7. In this work, surprisingly, implanter experience had a smaller than expected impact on surgical time compared to previous work. One possible explanation is that study sites were already experienced with the therapy, as the average site had treated approximately >40 cases. New information from this study includes patient-specific details including body habitus, age, and comorbidities. A newer two-incision technique introduced after the time period of this analysis has further reduced implantation time to 87 minutes [8].
Surgery in general is a very dynamic activity involving a multitude of both controllable and uncontrollable factors.
Unforeseen procedural adverse events, equipment problems, availability of supplies, and a significant number of general human factors also play a role in surgical time. All other factors being equal, longer operative case time has been correlated with greater negative outcomes. Prolonged operative time has been associated with an increased risk of surgical site infections [9]. In other implantable electronic medical devices, such as cardiac pacemakers, defibrillators, and neurostimulators, longer implantation time has also been associated with increased infections [10,11].
Additionally, reduced surgical time can have an impact on more favorable operating room economics. Operating room time is valuable, especially in hospitals that have a large volume of surgery booked into the future and insufficient capacity to prevent backlogs. Estimates of the value of OR time range from $40-60 per minute, and a decrease in implantation time without a tradeoff on complication rate could translate to improved OR utilization [12,13].
This analysis has a number of limitations. These findings were derived from the ADHERE registry, which includes data from a mix of private and academic sites, but tends to include more higher-volume centers. These findings may therefore not be applicable to all centers. Additionally, while the manufacturer provides training on the proper implantation of the devices, training techniques may have improved over the years; this could potentially explain some of the decrease in implantation times. Unfortunately, the registry did not collect information about training that could be included in the regression model, and instead used the total number of implants per clinical site as an approximation for experience. Furthermore, the regression assumes that the effect of implanter experience on implantation time is linear, but this may in fact be a non-linear relationship.
CONCLUSION
Implantation of newer-generation UAS components had the largest influence on reducing surgical implantation time versus the first-generation system, without increasing the rate of perioperative severe adverse events rates, even after controlling for other confounding factors. Further work can determine how these efficiency improvements may impact post-operative outcomes and operating room utilization efficiency.
AUTHORS’ CONTRIBUTIONS
Shane Hamacher, MD: topic research, data analysis, interpretation of data, manuscript author
Marcel Copper, MD PhD: topic research, interpretation of data, manuscript review and approval
Eugene Chio, MD: topic research, interpretation of data,manuscript review and approval
Armin Steffen, MD: topic research, interpretation of data, manuscript review and approval
Christopher Larsen, MD: topic development, research design, interpretation of data, manuscript review and approval
REFERENCES
- Strollo PJ, Soose RJ, Maurer JT, de Vries N, Cornelius J, Padhya TA, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014; 370:139-49.
- Woodson BT, Strohl KP, Soose RJ, Gillespe MB, Maurer JT, de Vries N, et al. Upper Airway Stimulation for Obstructive Sleep Apnea: 5-Year Outcomes. Otolaryngol Head Neck Surg. 2018; 159: 194-202.
- Thaler E, Schwab R, Maurer J, Soose R, Larsen C, Stevens S, et al. Results of the ADHERE upper airway stimulation registry and predictors of therapy efficacy. Laryngoscope. 2020;130:1333-1338.
- Mann EA. FDA PMA Supplement P130008/S39 (April 2020).
- Suurna MV, Steffen A, Boon M, Chio E, Copper M, Patil RD, et al. Impact of Body Mass Index and Discomfort on Upper Airway Stimulation: ADHERE Registry 2020 Update. Laryngoscope. 2021; 131: 2616-2624.
- Murphey AW, Baker AB, Soose RJ, Padhya TA, Nguyen SA, Xiao CC, et al. Upper airway stimulation for obstructive sleep apnea: The surgical learning curve. Laryngoscope. 2016; 126: 501-506.
- Larsen C, Boyd C, Villwock M, Steffen A, Heiser C, Boon M, et al. Evaluation of Surgical Learning Curve Effect on Obstructive Sleep Apnea Outcomes in Upper Airway Stimulation. Ann Otol Rhinol Laryngol. 2021; 130: 467-474.
- Kent DT, Chio EG, Weiner JS, Heiser C, Suurna MV, Weidenbecher M. A Noninferiority Analysis of 3- vs 2-Incision Techniques for Hypoglossal Nerve Stimulator Implantation. Otolaryngol Head Neck Surg. 2021.
- Cheng H, Chen BP, Soleas IM, Ferko NC, Cameron CG, Hinoul P. Prolonged Operative Duration Increases Risk of Surgical Site Infections: A Systematic Review. Surg Infect (Larchmt). 2017; 18: 722-735.
- Guralnick M, Benouni S, Corey O’Connor R, Edmiston C. Characteristics of Infections in Patients Undergoing Staged Implantation for Sacral Nerve Stimulation. Urol. 2007; 69: 1073-1076.
- Olsen T, Jørgensen OD, Nielsen JC, Thøgersen AM, Philbert BT, Johansen JB. Incidence of device-related infection in 97,750 patients: clinical data from the complete Danish device-cohort (1982-2018). Eur Heart J. 2019; 40: 1862-1869.
- Girotto JA, Koltz PF, Drugas G. Optimizing your operating room: or, why large, traditional hospitals don’t work. Int J Surg. 2010; 8: 359- 367.
- Childers CP, Maggard-Gibbons M. Understanding Costs of Care in the Operating Room. JAMA Surg. 2018; 153: e176233.