Middle Ear Functional Morphology of the Domestic Cat (Felis catus) and the Domestic Sheep (Ovis aries): A Comparative Study with Two Echolocating Mammals (Globicephala macrorhynchus and Tadaridabrasiliensis mexicana
- 1. Faculty of Biological and Environmental Sciences, Molecular and Integrative Biosciences Research Program, University of Helsinki, Finland
- 2. School of Animal and Veterinary Sciences, Charles Sturt University, Australia
ABSTRACT
Sound localization in nature is important especially for those creatures who hunt in the dark. Swimming and flying animals do this three-dimensionally, as opposed to animals living on the ground that mainly need to localize sound in a horizontal plane. Earlier studies have suggested directional asymmetry between the left and right middle ears in two mammals echolocating in three dimensions, the short-finned pilot whale (Globicephala macrorhynchus) and the free-tailed Mexican bat (Tadarida brasiliensis mexicana). In them, the asymmetry is likely to improve localization of incoming sounds, especially in the “zone of acoustic ambiguity”, in the vertical plane in front and behind the head. Here we study whether this kind of asymmetry can be found in the middle ears of two terrestrial mammals, the domestic cat (Felis catus), a carnivore, and the domestic sheep (Ovis aries), an artiodactyl and a close relative of cetaceans. We provide measurements on the masses and lengths of the ossicles, and on the stapes footplate area and applied a t-test to the data. We found that asymmetry was not present between the left and right middle ears in the cat or in the sheep.
We provide qualitative photomicrographs of sheep and cat middle ear dissections, obtained using Leica Microsystems stacked image technology, with accompanying commentary. Structure and function is discussed and the likely contribution of the tensor tympani muscle of these two species in the acoustic reflex, and in transmission of high frequency sound from the middle ear to the perilymph of the vestibule, is highlighted.
CITATION
Tsur I, Christie BA, Ladd L (2022) Middle Ear Functional Morphology of the Domestic Cat (Felis catus) and the Domestic Sheep (Ovis aries): A Comparative Study with Two Echolocating Mammals (Globicephala macrorhynchus and Tadaridabrasiliensis Mexicana). Ann Otolaryngol Rhinol 9(5): 1301.
INTRODUCTION
All terrestrial mammals have a tympanic middle ear apparatus within an air-filled cavity, a tympanic membrane, and three ossicles- malleus, incus and stapes. A theory accepted by many is that all aquatic mammals have their origins in some group of terrestrial mammals, and have thus secondarily adapted to life in water, and during this adaptation process, their body organs, including their sensory organs, may have gone through morphological changes.
The mammalian hearing organ has been anatomically described for a great number of taxa. In the 19th century, Hyrtl [1] and Doran [2], among others, provided detailed descriptions of a wide range of various mammals, living in different habitats in air and water. Later Henson [3] published descriptions and morphometric data on bat ears and ossicles.
Fleischer [4,5] categorized mammalian middle ears into several main groups, with a wide synopsis on the evolution of these different types. He also presented measurements on various parts of the ear, and comparisons between them, with functional interpretations. The main categories in his system are the micro type, the transitional type, and the freely-mobile type. He further distinguishes the ancestral type and some other smaller groups. It is not our intention in this submission to attempt to place the middle ears of the cat, sheep, pilot whale or Mexican bat into any of the Fleischer categories.
The size variation and function of the auditory organ of mammals has been studied thoroughly by Rosowski [6-9], who also focused on fossil forms and the evolution of hearing [10]. Nummela [11] found that the size variation of the mammalian middle ear structures is allometric against animal size and isometric when ossicular masses are compared to each other or to the tympanic membrane area raised to the power 3/2. Her measurements provided data for the functional models of Hemilä et al. [12], showing that in isometric middle ears, the highfrequency hearing limit is inversely proportional to the cubic root of the ossicular mass, and hence predicted by the size of the middle ear. Huang et al. [13], applied a similar approach to felids, and Puria and Steele [14] studied the role of the morphology of the middle ear structures and their rotational axes in efficient high-frequency hearing.
Modern cetaceans are obligatory aquatic mammals with terrestrial ancestors in the Eocene epoch [15]. The ear structure and function are clearly different from land mammals. Never-theless, a major similarity is the functionality of the ossicular chain in sound transmission [5,16-25].
Cetaceans rely largely on their auditory sense in water, and especially odontocetes. When catching prey using echolocation they are crucially dependent on good sound localization ability. One way to improve this ability is to have asymmetry between the left and right side, so-called directional asymmetry. Odontocetes have asymmetrical skulls [26,27] and recently, directional asymmetry has been shown between the middle ears of the shortfinned pilot whale, Globicephala macrorhynchus [28]. Apart from toothed whales, directional asymmetry of the middle ear has also been claimed to exist in another echolocating mammal, the free-flying Mexican bat, Tadarida brasiliensis mexicana [29]. This claim points to a possible common denominator of echolocating mammals that swim or fly in the dark where eyesight cannot account for their performance and survival as hunters.
Given that hearing high frequencies (> 30 kHz) is a common phenomenon among mammals [30,31], it is reasonable to ask whether directional asymmetry can be found in other mammals as well. Different species use different sensory modalities when gathering information from their surroundings. Each species can be said to have its own sensory space, to which a combination of different sensory cues contribute [32,33].
Our comparative study is based mainly on four mammalian species: the short-finned pilot whale, the free-flying Mexican bat, the domestic cat and the domestic sheep. Our main purpose being to compare the two latter species with the two previously studied echo-locators with respect to middle ear symmetry/asymmetry and to set the comparison in the more general context of their different sensory abilities and life styles.
We present quantitative measurements of cat and sheep auditory ossicles to determine if left/right asymmetry is present. We then will compare these two species with the two echolocators and discuss the different sensory abilities of these animals. The domestic cat is a carnivore, and may tell us whether middle-ear asymmetry is a more general feature of animals hunting in the dark, improving their ability to locate prey and survive in that environment. The sheep is a close relative of the artiodactyl ancestor of whales [15] and may tell us, whether odontocete asymmetry could represent genetic heritage rather than being an adaptation to hearing in the water space.
We also provide qualitative images of micro-anatomical dissections with accompanying descriptions of the ear regions, discuss structure and function of the middle ears of sheep and cat, and highlight the likely contribution of the tensor tympani muscle of these two species in the acoustic reflex and in transmission of high frequency sound through the ossicular chain to the perilymph of the vestibule. The morphology of the medial surface of the tympanic membrane is described and progression of knowledge of tympanic membrane function over the past 50 years is briefly reviewed.
MATERIALS AND METHODS
Collection of material
Quantitative study: Twelve healthy stray cats were collected in Jerusalem, Israel by various municipal officers in the veterinary services, and were euthanized in animal shelters (for reasons unrelated to this study) according to State and municipal legislation concerning the humane euthanasia of stray animals. Likewise, twelve heads of young Awasi male sheep (up to 1 year old), were collected from butchers in Bethlehem, Palestine.
Qualitative study: The specimens for micro-anatomical studies performed in Australia, were ethically acquired from the anatomy department of the School of Animal and Veterinary Sciences, Charles Sturt University, Wagga Wagga, Australia. For the cat, two frozen thawed heads were used. An adult ram was donated to the school by a commercial sheep breeder because the ram was no longer able to function as a sire. After humane euthanasia, the body was frozen, and later, the head was available for dissection.
Preparation of heads for removal of ossicles
Sheep: Fresh heads were split in the median plane with a band saw, frozen at -20°C and thawed at room temperature just before removal of the ossicles from the middle ear cavity of both ears with standard microsurgery tools.
Preparation of material for micro-dissection of middle ear structures
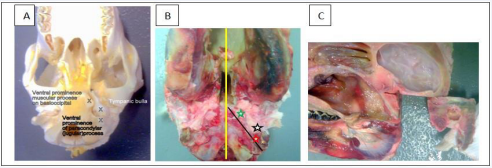
Sheep: Frozen /thawed heads were skinned and soft tissue removed from the caudo-ventral region of the skull (Figure 1).
Figure 1: Obtaining the temporal bone segment from the sheep skull with a band saw.
Landmarks to locate the tympanic bulla (Figures 1A,1B) were palpated, and then the head was sectioned in the mid-sagittal plane (Figure 1B, yellow line) with a band saw. Working on left and right sides in turn, the muscular process of the basioccipital bone (Figure 1B, green star) and paracondylar (jugular) process of the occipital bone (Figure 1B, red star) were identified again by palpation. Maintaining ventrodorsal positioning and a firm grip, the sagittally sectioned head was angled in the horizontal plane and the blade aligned on the medial side of these two processes (Figure 1B, black line). Maintaining this alignment, the head was tilted a few degrees medially to angle the blade away from the stapes and incus, then a second cut was made. The temporal bone segment containing the region of interest was isolated by two more cuts at right angles to each other Figure 1C).
Cats: The heads from frozen/thawed cats were skinned and the pinnae excised close to the skull. Eyes, lower jaw and masticatory muscles were removed. Care was taken to clear as much soft tissue as possible from the base of the skull, and laterally from the region of the temporal bone. The cat head is small enough to dissect using a dissecting microscope, and to photograph, without first having to remove blocks of auditory tissue, as was necessary in the sheep.
Frozen/thawed specimens were dissected immediately, or fixed in 10% Formalin for 7 days, rinsed with 20% ethyl alcohol then immersed in 20% ethyl alcohol for transport or storage for later dissection.
Microdissection and Photography
Micro-dissections were performed in the histopathology laboratory, School of Animal and Veterinary Sciences, Charles Sturt University, Wagga Wagga, NSW, Australia, using standard microsurgery tools and burrs. Photomicrographs of the sheep and cat middle ear dissections were obtained. Such an approach for recording micro-anatomical detail might reasonably be considered “historical”, being superseded now by micro-CT and 3-D computer modelling; but, as Mason [34] commented, “With the advent of modern multifocal imaging technology, standard micro-dissection is experiencing a new lease of life”. We had access to, and used, Leica Microsystems stacked image technology in the National Life Sciences hub at Charles Sturt University Wagga Wagga NSW. The clear 3D micro-anatomical detail obtained and recorded was a delight to behold and we believe could prove useful for fine tuning micro-CT and computer modelling algorithms.
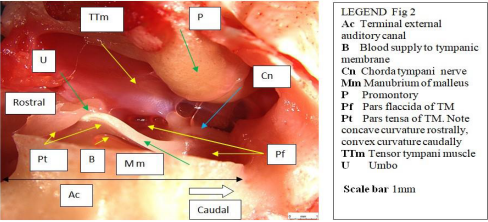
The terminal auditory canal within the tympanic bulla of the sheep is angled about 12° from a dorsal - caudolateral, to a ventralcraniomedial position (Figure 2).
Figure 2: Sheep: Block of fresh tissue ready to dissect (Ventral view right middle ear).
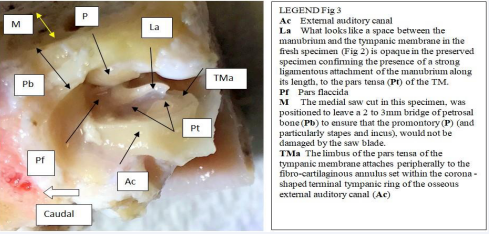
(Figure 3).
Figure 3: Sheep: Block of preserved tissue ready to dissect (Ventral view left ear).
The umbo is positioned approximately 2/3 the way across the longest diameter of the tympanic membrane.
RESULTS
Quantitative study
Measurement of mass and length of the ossicles and footplate area of the stapes.
For cat and sheep, we recorded the 10 most complete and whole set of ossicles, and measured the mass of each ossicle, length of the malleus and incus, and the stapes footplate area according to the procedure described by Tsur et al., [35]. The value of each parameter for each individual ossicle is the mean of three measurements. We took for each pair of ears (in the same head) the difference of the paramenter values for the left and right side. Paired – samples t-tests were performed on the means of these differences across animals (Figure 4).
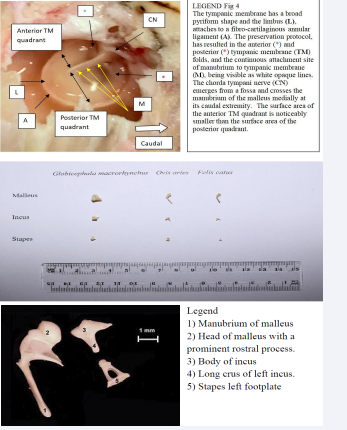
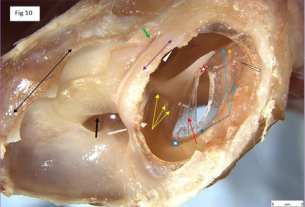
Figure 4: Figure ( i ) Ossicles of the three species we have dissected. Figure (ii) Ossicles from Felis catus processed for measuring and weighing. Microdissection middle ear sheep X30 magnification
(Figure 5).
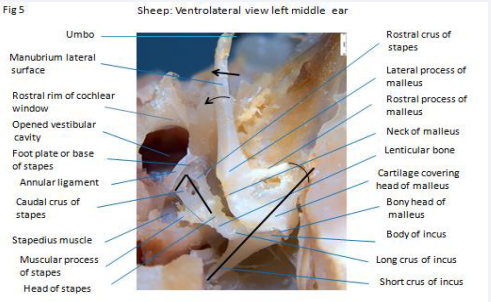
Figure 5: Auditory ossicles of the sheep
Auditory ossicles of the sheep (Figure 5) have a cartilage cover over the incudostapedial articulation, head of the stapes, long crus of the incus, neck , head, and anterior process of the malleus. A small right angled segment of cartilage with a concave articular surface was removed where it overlapped the edge of the large and plate-like head of the malleus to expose the shallow domeshaped, smooth, body of the incus at its articulation with the malleus. The features are those of a plate-like ball and socket joint (Merriam-Webster Medical dictionary). The rostral process of the malleus is shorter than that of the cat, but is well developed and is firmly attached to the skull by fibrous connective tissue. During dissection, the rostral process of the malleus was unintentionally damaged, such that when gentle medial pressure was applied to the manubrium (small black arrow), the attachment site avulsed from the skull. This release allowed the head of the malleus to rotate (small black curve) approximately 11° in an anticlockwise direction. Portion of the ventral floor of the promontory was removed to expose the vestibular space. Access was gained via the caudal rim of the cochlear window and a remnant of the rostral smooth edge of the cochlea window remains. The footplate of the stapes is flush with the vestibular window and is attached to a surrounding fibro-cartilaginous annular ligament that is set obliquely (angled lines) to the longitudinal axis of the stapes whose rostral crus is noticeably longer than the caudal crus. The apex of the long process of the incus is broad and flat and makes a sharp, almost right-angled turn before ending as a cartilage covered firm articulation with the head of the stapes. There is a shallow waist at the incudostapedial joint suggesting the presence of a lenticular cartilage or bone. The prominent muscular process of the stapes faces caudally and the associated stapedius muscle exerts a caudomedially directed force.
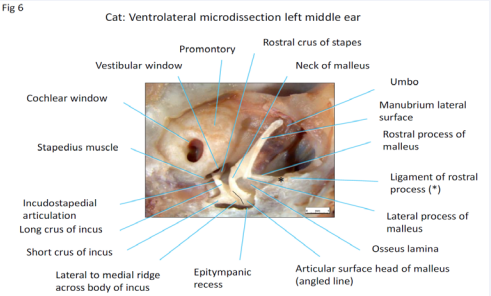
Cat: A cartilage cover is not present over the malleus and long process of the incus in the cat (Figure 6)
Figure 6: Microdissection middle ear cat X30 magnification.
so the exposed ossicles are sleek, clean, and streamlined. The manubrium of the malleus is long, and has a flat, curving, lateral facing surface that was attached to the medial convex surface of the pars tensa (removed in this specimen). The lateral process of the malleus is the caudal limit of attachment of the pars tensa to the manubrium and is the demarcation and anchor point for the fan-shaped, caudally directed pars flaccida that covers the tympanic incisure (pars flaccida removed in this specimen). At the apex of the manubrium is the region of the umbo. The anterior component of the head of the malleus is smooth, rounded and bulky (incompletely exposed in this specimen), giving the impression of a counter weight. The caudally positioned articular surface, (seen from the lateral side), has two parts to it, dorsolateral and ventrolateral, set at an angle of about 35° to each other. Therefore, a ridge must be present between these two articular surfaces oriented in a caudolateral to rostromedial direction (approximately in line with the manubrium). Movement therefore will be possible in the rostrocaudal and lateromedial directions. These features are those of a saddle joint (Merriam Webster Medical Dictionary). An extension of the tympanic cavity, the epitympanic recess, houses the head of the malleus, also body and beginning of the short crus of the incus. The malleoincudal articulation is not restrained and is positioned over the anatomical axis of rotation - the line joining the fossa incudis that houses the short crus of the incus and the ligament of the rostral process of the malleus. The rostral process is a substantial structure lying between the neck and head of the malleus. It consists of a thin but dense plate of bone, the osseous lamina that presents a flat ventral surface that is embedded in the tympanic membrane at the junction of the pars tensa and pars flaccida (both previously removed in this specimen). The rostral process is attached to the squamous temporal bone of the skull by a strong flexible ligament (*above and also Figure 8). The cochlear window has a deeply recessed and obliquely orientated cochlear membrane. The stapes too is deeply recessed, with the base plate also set at an oblique angle to the vestibular window. An impressively long stapedius muscle has a fine, flat, tendon of insertion attached to a muscular process located proximal to the caudal crus, on the neck, at its junction with the head of the stapes. The tendon bends around the smooth edge of the vestibular window (Figure 7)
Figure 7: Cat: Middle ear structure Ventral view left middle ear.
so a friction reducing device such as a tendon sheath or synovial bursa should be present at this site.
Novel observation
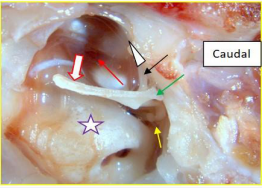
Visible structures include lateral process of the malleus (green arrow), rostral process (black arrow), and a clear but distant view of the incudo-stapedial joint (yellow arrow) line of tension of tensor tympani muscle (red arrow), promontory (purple star). After removing the pars tensa and the pars flaccida, and before opening the epitympanic recess, an unexpected complementary movement was seen. When the manubrium was displaced medially (red outlined white broad arrow), the rostral process arced upwards (white arrow head) in a curved trajectory towards the viewer in concert with the degree of medial displacement of the manubrium, and then, in a controlled manner, promptly returned to the resting position as the pressure was relaxed. The manoeuvre and response was repeatable. Could a physiological event with such a flamboyant type of movement occur in the live situation? Before proposing an answer, look first at the caudoventral view of the ossicles.
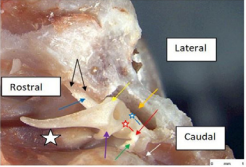
The rostral process (blue arrow) is attached to the skull by a strong ligament (black arrows). Part of the lateral process (yellow arrow) was unintentionally removed when burring near the epitympanic recess (orange arrow).The tensor tympani muscle (white star) is long (contraction therefore generates much movement), strong (relates to large cross-sectional area), and has a large hook-shaped muscular process (violet arrow). Force vectors on the process will therefore be rostromedial and dorsally inclined. The blue star is adjacent to the articular surface of the short crus of the incus and the red star is adjacent to the articular surface of the long crus of the incus (red arrow). Also visible are the head of the stapes (green arrow), and the stapedius muscle attached to the caudal crus of the stapes, (white arrow). For the rostral process to arc up as smoothly as it did when a dorsally inclined, caudomedially directed force was applied to the manubrium (Refer to red-rimmed white arrow Figure 7)the neck of the malleus would have to have rocked toward the vestibular window (Figure 6) and be slightly medially inclined (Figure 8).
Figure 8: Cat: Dissected malleo-incudal articulation. Caudoventral view left middle ear.
The pivot point for this motion would have been the slightly raised area on the articular surface of the malleus (Figure 6) adjacent to the neck. The extent of forward rocking of the neck of the malleus could be limited by at least two structures; the first, elastic resistance offered by the ligament of the rostral process (Figure 8), and the second (in life), the number of TTM fibres recruited , meaning that the response could be graded or maximal. Rocking due to a maximal TTM contraction would disengage all the articular surface of the malleus, and at the same time, medially subluxate the ossicle. Such motion might be pre-emptive, or be an accompanying protective acoustic reflex, independent of stapedius muscle contraction but dependent on the timing of the reflex motor input to each muscle. An unlikely third limitation to forward rocking could be the manubrium impacting an overshoot buffer such as the medial wall of the promontory (in the region of the manubrial shadow Figure 6). A graded TTM response might infer another possible function for this muscle, fine tuning of tension in the malleo-incudal articulation to receive and transmit very high frequency sound waves to the stapes. This thought will be expanded later.
While using rongeurs in this specimen to remove part of the squamous temporal bone in the vicinity of the petrous temporal bone, the shock wave generated as the instrument snapped closed, dislocated the incudo-stapedial articulation (blue star head of the stapes, red star long crus incus). A shiny, smooth, ball-shaped nipple of bone (black arrow) was exposed on the apex of the tapering long crus of the incus – indicating that this joint was a synovial joint. The narrow contact point and tapering long crus, gives the incudo-stapedial joint of the cat a distinctly hour-glass appearance (Figures 6-8). A similar bony nipple was seen by Malkemper et al., [36] at the apex of the long crus of the incus in the incudo-stapedial joint of a red fox and was referred to as a “ lenticular apophysis that articulates with the stapes”. This structure in humans also had the earlier name of Sylvian apophysis [2].
The graceful and masterful tapering of the incus suggests an amplification factor and contrasts with the broad, flat, incudostapedial joint in the sheep (that can still transmit frequencies in excess of 40 kHz). The tapering amplification therefore, might contribute to the upper frequency hearing edge of at least 30 kHz that the cat has over the sheep (Table 1).
Table 1: Means and standard deviations of ossicular parameters in the domestic cat (Felis catus).
|
|
Mean |
N |
Std. Deviation |
|
|
Pair 1 |
L_M_wt |
.009784 |
10 |
.0014564 |
|
R_M_wt |
.009613 |
10 |
.0014623 |
|
|
Pair 2 |
L_I_wt |
.003927 |
10 |
.0005696 |
|
R_I_wt |
.003889 |
10 |
.0005686 |
|
|
Pair 3 |
L_S_wt |
.000426 |
10 |
.0000891 |
|
R_S_wt |
.000426 |
10 |
.0000771 |
|
|
Pair 4 |
L_M_lngth |
7.712667 |
10 |
.1699295 |
|
R_M_lngth |
7.719000 |
10 |
.1862432 |
|
|
Pair 5 |
L_I_lnght |
2.678333 |
10 |
.0959327 |
|
R_I_lnght |
2.693000 |
10 |
.0959096 |
|
|
Pair 6 |
L_SFPA |
1.547998 |
10 |
.0806873 |
|
R_SFPA |
1.538150 |
10 |
.2091105 |
|
Abbreviations: L: Left, R: Right, M: Malleus, I: Incus, S: Stapes, Wt: Weight, lngth: Length, SFPA: Stapes Footplate Area
(Table 2).
Table 2: Paired samples t-test for left-right differences between ossicular parameters of the domestic cat.
|
|
t |
df |
Sig. (2-tailed) |
|
|
Pair 1 |
L_M_wt - R_M_wt |
.826 |
9 |
.430 |
|
Pair 2 |
L_I_wt - R_I_wt |
.886 |
9 |
.399 |
|
Pair 3 |
L_S_wt - R_S_wt |
-.027 |
9 |
.979 |
|
Pair 4 |
L_M_lngth – R_M_lngth |
-.487 |
9 |
.638 |
|
Pair 5 |
L_I_lngth – R_I_lngth |
-1.637 |
9 |
.136 |
|
Pair 6 |
L_SFPA – R_SFPA |
.222 |
9 |
.829 |
(Table 3).
Table 3: Means and standard deviations of ossicular parameters in the domestic sheep (Ovis aries).
|
|
Mean |
N |
Std. Deviation |
|
|
Pair 1 |
L_M_wt |
.011231 |
10 |
.0014579 |
|
R_M_wt |
.011131 |
10 |
.0014721 |
|
|
Pair 2 |
L_I_wt |
.008277 |
10 |
.0016210 |
|
R_I_wt |
.008432 |
10 |
.0013973 |
|
|
Pair 3 |
L_S_wt |
.001703 |
10 |
.0005419 |
|
R_S_wt |
.001698 |
10 |
.0005419 |
|
|
Pair 4 |
L_M_lngth |
8.330000 |
10 |
.3819249 |
|
R_M_lngth |
8.315667 |
10 |
.4105072 |
|
|
Pair 5 |
L_I_lngth |
3.872000 |
10 |
.1551359 |
|
L_R_lngth |
3.932333 |
10 |
.1912664 |
|
|
Pair 6 |
L_SFPA |
2.291622 |
10 |
.4047704 |
|
R_SFPA |
2.258583 |
10 |
.4312708 |
|
(Table 4).
Table 4: Paired samples t-test for left-right differences between ossicular parameters of the domestic sheep (Ovis aries).
|
|
t |
df |
Sig. (2-tailed) |
|
|
Pair 1 |
L_M_wt - R_M_wt |
1.335 |
9 |
.215 |
|
Pair 2 |
L_I_wt - R_I_wt |
-.545 |
9 |
.599 |
|
Pair 3 |
L_S_wt - R_stp_wt |
.105 |
9 |
.919 |
|
Pair 4 |
L_M_lngth – R_M_lngth |
.653 |
9 |
.530 |
|
Pair 5 |
L_I_lngth – R_I_lngth |
-1.699 |
9 |
.124 |
|
Pair 6 |
L_SFPA – R_SFPA |
1.392 |
9 |
.197 |
In the cat, the tympanic membrane, also known as the ear drum or myringa, is not as easy to visualize in the sequence - external auditory meatus, tympanic membrane, tympanic cavity, as it is in the sheep (Figure 2). In the sheep, the opening of the auditory meatus into the tympanic cavity stands out as a bony, flared, corona with an inset tympanic membrane (Figure 3). The medial view of the terminal auditory meatus in the cat (Figure 9,10),
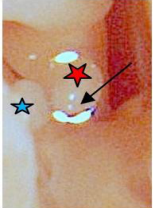
Figure 9: Cat: Shock wave dislocation of the incudo-stapedial joint.
(Figure 10),
Figure 10: Cat: tympanic membrane Ventro-caudal view.
looks like a truncated sphere, capped by an overriding dome-shaped tympanic membrane, reminiscent of the ‘whispering gallery’ in St Pauls cathedral London, causing one to ponder the associated acoustics [37] . The pars tensa (long red arrow) is thin and semitransparent. The gentle curve in the embedded blood vessel in the adventitia of TM (head of red arrow) indicates that at this location the TM presents a concave surface to incoming sound pressure waves. A deliberately placed radial incision in the pars tensa resulted in gentle outward bowing of the rostral edge of the incision. When a small anteriorly directed ‘back-cut’ was made close to the manubrium on the rostral side of the radial incision (red star), the rostral edge of the back-cut folded circumferentially outwards in proportion to its proximity to the back-cut. This indicates that both radial and circumferential forces are acting on the TM; where radial tension decreased, medial convex bowing increased. Conversely, at the point of maximum tension (umbo), medial concave bowing of the TM was seen (orange arrow). The limbus of the pars tensa of the tympanic membrane attaches peripherally to a fibro-cartilaginous annulus (blue arrows) set within the terminal tympanic ring of the osseous external auditory canal (blue star). At one location (narrow white arrow head), the limbus, at near maximum tension (in the region of the umbo), has pulled away from the annulus. The cause of this event was not immediately apparent but might have accompanied an unintentionally strong, medially directed force being applied when performing the back-cut in the TM close to the manubrium. Never-the-less, it does indicate that such an event can happen, and if so, less extreme movement at this location might even be physiological. The pars flaccida, also known as Shrapnell’s membrane [38], (yellow arrows), is pink, more vascular therefore, and thicker than the almost transparent pars tensa, and occupies the dorsomedial component of the TM. The tympanic cavity proper (dorsolateral bony compartment) has two communications with the larger ventromedial cavity. One, (white arrow), is a window adjacent to the cochlear window (black arrow), the other (green arrow) is a thin slit, midway along the fusion line of the tympanic cavity proper with the promontory. The cut medial edges of the tympanic septum (violet double arrow) and cut medial edge of the wall of the ventromedial compartment (black double arrow) are indicated. At the rostral extremity of the tympanic cavity proper (out of view) is the location of the ostium of the auditory (formerly Eustachian) tube (white arrow head) [39]. The membranous auditory tube opens into the nasopharynx and is supported medially by a plate of hyaline cartilage and laterally by the tensor veli palatini muscle. This muscle is innervated by the mandibular branch of the trigeminal nerve, as is the tensor tympani muscle. When these two muscles contract, the auditory tube opens and pressure is equalized on either side of the tympanic membrane.
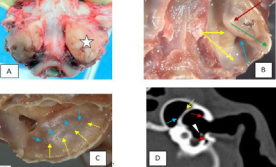
Tympanic bullae in the cat are prominent features of the caudoventral region of the skull. Figure 11A
Figure 11: Cat: Tympanic bullae, 11 A, B, C, caudoventral view, 11D, CT transverse plane..
therefore shows the left tympanic bulla (white star) to the right of the image. Figure 11B illustrates the left bulla after the shell of the ventromedial compartment has been removed. The striking feature now is a dorsolaterally positioned second compartment (dark red arrow), within the first, that almost completely surrounds the auditory ossicles and tympanic membrane. This second contained space is the tympanic cavity proper. The cochlea window (blue arrow) is visible, but much of the promontory (yellow arrows) is still covered by the seemingly complete bony septal wall of the second compartment (ignore the small iatrogenic opening). The ventral surface of the septum has an apex (head of dark red arrow) the caudal wall of which tapers in a caudolateral direction (green arrow).The umbo of the manubrium is located a short distance beneath and rostral to the apex. The cranially directed wall of this septum is quite steep. In Figure 11C, the bulla has been outwardly rotated slightly to show the medial, ventral and lateral surfaces of the septal wall. Closure of this bony cavity would have occurred by centripetal growth from the bony perimeter - the lateral component of which would have originated from the tympanic region of the temporal bone (yellow arrows) and the medial component from the petrosal region of the temporal bone (blue arrows).These connections are well illustrated in Figure 11D, a CT image of the normal outer, middle and inner ear of a cat, sourced from Fitzpatrick Referrals [40], and modified with permission. Although the image is a copy and is not sharp, it can be seen that bony spurs from the tympanic bone (yellow arrow) have linked up with bony spurs from the petrosal bone (blue arrow) to form a reverberation chamber that attaches near the vestibule of the promontory. The arrow head indicates where the manubrium of the malleus is embedded in the TM. Red arrows indicate the site of attachment of the limbus of the tympanic membrane (TM) to the auditory canal. The diameter of the TM in the dorsoventral direction is large due to flaring of the terminal part of the bony external auditory meatus. A histological transverse section of the middle ear of the cat may be viewed in Sula et al. [41] where the image mirrors the CT shown in Figure 11D, confirming the position of the flared attachment site of the tympanic membrane to the bony auditory canal of the cat.
Tympanic bullae function
The narrowness and depth of the sheep bulla and the breadth of the cat bulla probably are serving a similar purpose to increase cavity volume. However, because the cat bulla has an almost enclosed septum forming a separate dorsal compartment, one could argue that the cat tympanic bulla proper, is really quite small, but is accompanied by surrounding secondary enclosures such as the ventral bulla and the dorsal epitympanic recess . So what is the purpose of these cavities? This will be discussed under a number of sub-headings:
Regulation of high-pass filtration of tympanic function by bulla size (low frequency gate control)
In terms of the physics of compressible motion such as sound waves in air, the smaller the tympanic cavity, the more attenuated will be the lower frequency spectrum. This is because impedance to flow of air that is moving away from the tympanum as it is forced in, is equal to the absolute unidirectional resistance to flow (which is infinite because the cavity is essentially completely enclosed), plus the reactance (which is accounted for by the decreasing resistance flow with increasing frequency). The frequency-related variation, accounts for the ability of a tympanum to vibrate in sympathy with incoming sound. Thus, in the very simplest case, increasing the volume of the bulla will decrease the lower threshold frequency of the audible spectrum that can be made available to the stapes and thus vestibular window. As a cat is a very efficient nocturnal hunter, it should have very acute hearing, particularly, in the high frequency range of squeaky voiced prey. Cats are reported to be able to hear up to 85 kHz [42], considerably higher than the sheep at 42 kHz [12], and both higher than human at about 22 kHz [30], Whether this is because of bulla reverberation amplification or because the cat has a better cochlear structure has been an open question for some time. The recent work of Malkemper et al. [36] spoke into this question and virtually ruled out better cochlear function as the reason for superior high frequency hearing in the cat. They showed that the lengths of the basement membranes of the cochlea in dog, cat and fox were virtually the same; the total hair cell numbers were highest in the fox, then dog, followed by cat; and hair cell densities were of the same order - fox, dog and cat with little variation along the cochlear duct. These authors also found that although foxes, dogs and cats had similar behavioural audiograms, cats had better high frequency hearing, and fox audiograms were sharply tuned to 4 kHz. Why 4 kHz? It is known that the natural frequency of the sheep middle ear is near 4.8 kHz [43] suggesting perhaps that this is the natural frequency of the call of a young lamb for its mother [44] - to which a fox would be glad to overhear.
On bulla resonance frequencies and their intrinsic band-pass filtration and high-Q amplification effects (amplification of specific frequencies of interest
In practice, however, shapes and sizes can be important. The concept of intrinsic resonance frequencies that might be favoured in the tympanic cavity is not invalid, and there is no doubt that at intrinsic resonance frequencies, we would reasonably expect punctilia (relating to a point of time) increases (with logarithmic falloff on each side) in compressibility of air, and thus tympanic and ossicular chain frequency response. This would have the effect of selective amplification of the tympanic membrane function at those frequencies, potentially with considerable strength (“high-Q”). Of course, this is very neat: we expect that as well as seeing an enclosed chamber reverberating in sympathy with incoming sound, and given that it is an enclosed cavity with one functional opening, the tympanum, that it will also produce harmonic reverberations at the following frequencies: (fundamental being 0.5 times the wavelength amplified), 1.5, 2.5, 3.5, 4.5, etc, (rather similar to the wavelength harmonics of a reed instrument or brass instrument). These frequencies may, of course be chosen to coincide with intrinsic mechanical frequencies of the ossicular chain and indeed of the perilymph itself, to produce several, or one, or perhaps to cancel out the effects of reverberation so that the bulla may indeed serve ONLY to increase lower range of hearing.
On bulla multiple frequencies caused by shape variation
As mentioned above, shapes may also play a part: If we imagine a cavity to be a rectangular prism with three different dimensional sizes (for example, 1 * 1.5 * 3 mm), we can also imagine that there will be potential for propagation of compression waves in three dimensions, and that in each direction, the amplification Q frequency will differ due to the individual path length (the width and height relative to any given dimension being comparatively irrelevant). In this way we may produce several fundamental frequencies for which any given cavity may reverberate with punctilia frequency amplification effects that if close enough, may merge, producing a selective improvement in hearing across a range of frequencies within the possible transmission and sensing capability of the apparatus. In a biological cavity which is likely to be rounded, the low Q (that is, wide selection of frequencies for amplification, as compared with “high Q”: a tight frequency point selected by a single strong reverberating frequency) amplification is more likely to be indistinct and provide a selected range in which amplification occurs, along with the basal increase in low frequency range. Coupling this with other small chambers (such as the association of the bulla with the main chamber and with the epitympanic recess) will serve to increase the possible ranges, potentially with multiple points of select hearing.
DISCUSSION
Asymmetry in Globicephala (pilot whale), Tadarida (bat), and Tyto (barn owl)
Odontocetes have asymmetrical skulls that deviate to the left. In addition, ossicular asymmetry was shown by Tsur [28] to be present in Globicephala, with the left side ossicles, on the average, being heavier, and the angle between the incus and stapes also greater in the left ear. As a result, the effective pressure was, on average, greater on the left side than the right. Based on the middle ear- model of Hemila et al. [12], they showed that such differences could result in a significant elevation – dependent different signal, between the two ears. Could this asymmetry be associated with the medium in which they hear? Water transmits sound about five times faster than doe’s air, but sound velocity in water also varies with depth due to temperature, salinity and pressure gradients; so the question is not easy to answer. The bat Tadarida is an aerial hunter and interestingly, its ossicles show directional asymmetry too, being heavier, like the pilot whale, on the left side. Skull asymmetry has not been reported in bats. In many owl species the placement of the external auditory meatus on the skull is asymmetrical and that is genetically programmed. Remarkable spatial resolution in the vertical plane, enabled by this asymmetry, has been carefully studied in the barn owl Tyto [45,46].
Sensory spaces of pilot whale, cat and sheep
Pilot whale: Dolphins have good visual ability [47] as well as excellent hearing and echolocation, and are able to catch fish in the air and recognize their trainers. On the other hand, they routinely forage at depths where there is too little light for vision to be useful [48].
Odontocetes have lost their sense of smell while mysticetes still retain it [49]. There are two hypotheses why this is so [50]. The filter feeder hypothesis, suggests that mysticetes retain their olfaction to be able to smell the krill in the ocean. The echolocation – priority hypothesis suggests that echolocators – odontocetes, lost their olfaction because it was not needed. Most cetaceans have almost totally lost their sense of taste and this absence was present before mysticetes and odontocetes split [51].
Cat: The sense of smell in carnivores is said to be generally good [49], however, olfaction is better in dogs than in cats. When vision, olfaction and hearing were compared, the domestic cat had vision and hearing clearly above average, and olfaction also, trended higher than the average among mammals [33].
Sheep: Sheep have a narrow field of binocular vision in front of their head and wide peripheral fields of monocular vision – with its head down grazing, the sheep can see in all directions but depth perception is poor. Colour perception is poor, particularly reds. Sheep can see greens and yellows. Their hearing is excellent, and for locating sound they direct their ears at the source of the sound. Sense of smell is excellent to different predators, ewes can locate their lambs, and rams find ewes in heat by smell. Sheep also use smell to locate water. Taste has lower importance. Touch is an important interaction between sheep, and lambs keep bodily contact with their mother even when sleeping. Ewes can recognize their own lambs call [44]. Lambs recognize siblings through vocal, visual and olfactory cues [52]. Ewes and lambs can recognize their own young or mother, and another sheep, based solely on the individual call.
Muscles involved in the Acoustic reflex
Acoustic reflex in medical parlance usually means contraction of the stapedius muscle in response to a loud incoming noise [53]. Much has been written in medical literature about the acoustic reflex with the emphasis on its use as a hearing test [54]. In the debate about whether or not the tensor tympani muscle (TTM) had a role in the acoustic reflex, Mukerji et.al, [55], noted that the TTM muscle did contract in response to self-generated noise such as swallowing and chewing, and Jones et al., [56], reported that a tensor tympani reflex does occur in some people in response to a loud noise, but its functional significance in sound transmission was questioned. This prompted Hain [54], a clinical hearing neurologist (Chicago Dizziness and Hearing), to further comment that the neuroanatomy of the participation of the TTM in the acoustic reflex “has been generally ignored in most discussions”.
Sheep : Stapedius muscle
The stapedius muscle of sheep (Figure 5) has a relatively large cross-sectional area and therefore would be able to contract with considerable force at its tendinous attachment to the caudal crus of the incus [57]. The most common reason proffered for stapedius muscle contraction is to tense the ossicular chain [55,58] and thereby protect the cochlea from damage should a loud environmental noise impact the tympanic membrane. The robust dimensions and ‘in-line’appearance of the ovine incudo-stapedial joint (Figure 5), suggests that strong pressures are exerted on the footplate of the stapes. Sound at low to medium frequencies is transmitted by movement about the anatomical, rostro-caudal, axis of rotation (black line Figure 5). The compressive inline movement of the stapes footplate and the vector for inward pull of the stapedius muscle are set at about 45° to each other. Therefore, in the absence of stapedial muscle contraction, relatively unimpeded inward movement of the footplate should be present at moderate to high sound frequencies. But the dorsocaudal tension generated by the stapedius muscle in the event of wide amplitude (loud), incoming sound waves, will result in increased oscicular chain resistance and it is this that is widely recognized as the protective acoustic reflex.
Sheep: Tensor tympani muscle
The tensor tympani muscle (TTM) of sheep (Figure 2) has a broad delta shape and is attached to a robust, muscular process. It is capable therefore of contracting strongly; but for what purpose? The answer might again be as part of a protective acoustic reflex. Stapedius muscle contraction in this role (as mentioned above), doesn’t seem to be enough for such an important job. But if the TTM also contracted about the same time, this would greatly enhance the total protective response. The malleoincudal (MI) articulation of the sheep, described in Figure 5, has a concave dish-shaped outer cartilage cover on the malleal head that overlaps a shiny, smooth, convex articular surface of the body of the incus. These are features of a synovial ball and socket joint. The straight black arrow in Figure 5 indicates caudo-medial movement of the manubrium that occurs in the presence of an incoming sound pressure wave, and since rotation is a possible movement for a ball and socket joint, and since the rostral process of the malleus in the sheep has a fibro-cartilaginous attachment to the skull, a strong, (dorsally inclined), caudo-medially directed anticlockwise force will be exerted on the head of the malleus. According to Zimny 1988 [59], “If a joint has an intra-articular structure, mechanoreceptors undoubtedly are present within it. The concentration of mechanoreceptors appears greater in areas related to the extremes of movement and probably represents the first line of defence in sensing these extremes.” Therefore, if an incoming loud (high amplitude) sound pressure wave was to exceed a certain threshold, pressure sensors in the joint capsule might detect the threat and trigger a TTM response that would be dorsally inclined and rostro-medially directed; that is, a strong clockwise counter force, which conceivably could neutralize the threat.
Cat: Stapedius muscle
Because of the presence of a mobile incudo-stapedial joint, contraction of the stapedius muscle in the cat will draw the head of the stapes probably even more caudally than did this action in the sheep, but the outcome would be the same; “increasing middle ear impedance and attenuating the intensity of sound energy reaching the inner ear” [55]. In other words, stapedius contraction in cats will pull on and stiffen the fibrous annular ligament and so reduce sound transmission particularly in the low frequency range [60].
Cat: Tensor tympani muscle
We described above, a novel observation involving arcing up of the rostral process of the cat in response to medial displacement of the manubrium (Figure 7), and found that this observation led to an explanation of how the TTM might be involved in the protective acoustic reflex in the cat (Figure 8). It was argued that for “arcing up” to occur, the neck of the malleus would have to rock caudomedially in the frontal plane. The fulcrum for this action would most likely be a raised rostrally angled transverse ridge that was observed between the neck and articular surface of the malleus (Figure 6). Sufficient forward rocking would disengage the MI articular surfaces and stop transmission of that sound wave.
Role of middle ear muscles in high frequency hearing Sheep:
Stapedius muscle: It is proposed that another purpose for stapedial muscle contraction might be to fine-tune stapes footplate tension in order to preferentially transmit high frequency sound pressure waves to the perilymph of the cochlea. As mentioned previously, the approximate upper limit of hearing is 22 kHz for humans, 42 kHz for sheep, and 78 kHz for cats. Therefore, humans do not hear ultrasound (>30 kHz) but this phenomenon is common amongst mammals [31], including sheep and particularly cats. Caudally directed sideways tension generated by the stapedius muscle at the proximal end of the caudal crus of the stapes would tend to compress the footplate over the caudal crus and relax the footplate at its rostral extremity; that is, generate low amplitude, high frequency rocking of the footplate. If stapedial muscle contractions were not always maximal, (as they most likely would be in an acoustic reflex), variable contraction strength might facilitate transmission of high frequency, in-coming, sound pressure waves. Thus, short travel rocking of the footplate could be the means by which high frequency sound pressure waves arriving at the stapes are transmitted to the perilymph in the vestibule.
Sheep: Tensor tympani muscle: We have previously noted that sheep can hear frequencies as high as 42 kHz in spite of their bulky auditory ossicles. We have also argued that rotational movements at the MI articulation are possible in sheep and stretching of the circumferential joint capsule might activate pressure sensors. Therefore, incoming sound pressure waves might invoke a graded TTM reflex contraction that would preselect optimum tension in the joint capsule for the MI joint to oscillate in harmony with the frequency of sound waves selectively sourced from the vibrating tympanic membrane. Each impulse, when it reached the head of the malleus, might induce “normal” rocking along the anatomical axis of rotation, impact the incudo-stapedial articulation and transmit sound to the cochlea. However, if the critical upper frequency limit for this mode of transmission was exceeded, another mode of transmission for high frequency sound waves to the stapes and cochlea would be required.
Puria and Steele [14] have addressed this question. In a study of high frequency hearing, they reported the use of micro CT imaging and 3-D reconstruction to calculate centres of mass and moments of inertia in the auditory ossicles of a number of species. They showed that at low frequencies (less than a few kHz), hinging motion about the anatomical axis was dominant in humans, cats, guinea pigs and chinchilla, but at high frequencies, the axis of rotation changed to a twisting, inferior-superior axis of the malleus in humans and cats. The predicted motion for humans and cats was multi-resonance vibration at the tympanic membrane that became a “biological bevel-gear-like response” at the MI articulation. This change of axis of vibration was not seen in guinea pigs and chinchilla whose MI articulations were fused. The MI joints of humans and cats contained a fluid of high viscosity and were therefore deemed to be mobile. To address the question on how twisting motion of the malleus might be transferred to the incus, an hypothesis was advanced that the saddle shaped MI joints of humans and cats (first described by Helmholtz in 1868) [61], behaved like a helical or bevel gear.
This hypothesis was tested by Willi et al., [62] who confirmed the mobility, but the predicted vibratory pattern was inconclusive. To drive this type of movement, a further hypothesis of an asymmetrical tympanic membrane was advanced by Puria and Steele [14]. Reconstructions from their data were obtained to calculate surface area between the manubrium and tympanic annulus on the anterior and posterior sides. They found that posterior surface area was larger than anterior surface area in humans and cats. It was therefore argued that asymmetry in the tympanic membrane produced a force differential on the two edges of the manubrium, generating a clockwise twisting motion at the MI joint, capable of transmitting the high frequency sound waves to the stapes and therefore to the perilymph of the vestibule.
Our microdissection study clearly showed that tympanic membrane asymmetry is present in the sheep middle ear (Figure 4), and we have argued that the malleoincudal articulation of the sheep is capable of oscillatory movement. Therefore, we support the concept proposed by Puria and Steele that at high frequencies, the manubrial twisting they proposed for cats and humans might, in conjunction with malleoincudal oscillation, also be the means for transmission of high frequency vibratory energy from the tympanic membrane to the stapes in sheep.
Cat: Stapedius muscle: The stapedius muscle in the cat has a high innervation ratio of 1:1.6 [55], so should be able to exert precise motor control to optimize stapedius muscle tension to receive high frequency sound pressure waves, in accord with the manubrial twisting hypothesis of Puria and Steele.
Cat: Tensor tympani muscle: Since the maleoincudal joint of the cat is a saddle joint, movement of the malleus is possible in the transverse plane as well as the frontal plane (outlined above). Given the mass of the malleus, one could argue that to minimize ossicular inertia, medial movement of the malleus has to be a normal physiological event to align the centre of mass of the malleus over the long crus of the incus. The tasks of forward rocking and medial subluxation of the malleus could be performed by the tensor tympani muscle (TTM) at the same time. A graded reflex contraction of the TTM could apply sufficient tension on the hook-shaped muscular process to tilt the neck of the malleus forward, with counter tension provided by the rostral ligament until equilibrium was achieved. At the same time, it could subluxate the malleus in a rostro-medial direction to achieve optimum reduction in moment of inertia. Now, the contact surface of the malleus might only be the narrow ridge (knife edge), resting on the caudal extremity of the articular surface of the rostral (long) crus of the incus (Fig 6) in equilibrium with tension generated in the rostral ligament as it stretched . Such reflex adjustment of malleo-incudal surface contact, mediated by the TTM, could filter out competing middle and low frequency sound. If this malleoincudal configuration could be activated by the twisting motion of the manubrium such as proposed by Puria and Steele, very high frequency sound transmission could possibly be achieved.
Neural pathways for the middle ear muscle (MEM) reflexes
Ascending and descending limbs of the stapedius (cranial nerve VII) and tensor tympani muscle (cranial nerve V) reflex pathways are well characterized, but the identity of the reflex inter-neurons is not known [55]. Inputs from the auditory nerve travel to inter-neurons in the cochlear nucleus located in the brainstem and project directly or indirectly to MEM reflex motor neurons found near the motor nuclei of the facial nerve or trigeminal nerve. Within the haze of what is “unknown”, perhaps the afferent arm could begin with inputs not originating from the auditory nerve, and pass to as yet unknown interneurons, and project to MEM reflex motor nuclei of the facial nerve and trigeminal nerve to complete the reflex.
The genesis of the following thought came from illustrations in Evans and de Lahunta [63], showing that the skin lining the bony compartment of the external auditory meatus of the dog was innervated by the external acoustic meatus nerve, a branch of the auriculo-tremporal nerve, arising from the mandibular branch of the trigeminal nerve. This nerve is sensory to the skin in the region, with a ramus going to the tympanic membrane. Also of interest in the same illustration, is that the skin lining the concave surface of the external ear of the dog is innervated by the facial nerve through its internal auricular branch which carries general somatic afferent nerve fibres. Therefore, if mechano-receptors were present in the skin of these two regions, the trigger for an auditory reflex might possibly occur even before sound waves reach the tympanic membrane and activate the acoustic nerve!
Innervation of the human cavum conchae has been precisely described by Berniejo et al., [64]. These authors believe that such innervations would be of maximal interest for the design of transcutaneous auricular nerve stimulation devices.
muscular contraction.De Vreese et al., [65] described the innervations of the external ear canal in a number of odontocetes using histological, immunohistochemical and electron microscopy methods. They found large numbers of lamellar corpuscles and associated myelinated nerve fibres. Lamellar corpuscles function as mechano-receptors and respond to changes in pressure, setting into motion physiological responses for the whale to safely negotiate a foraging dive. Similar lamellar corpuscles are found in the skin of land mammals but only in association with hair. The immune-reactivity of the central axon in odontocete lamellar corpuscles was found to be similar to Paccinian corpuscles found in the ear canal of humans that were associated with a reflex mechanism that could expel foreign objects by means of glandular secretion and muscular contraction.
The ear canal of the domestic dog (and probably cat) is well innervated with connections via the trigeminal and facial nerves to various ganglia and beyond [63]. Just as De Vreese and colleagues discovered an ancillary role of the ear canal as a depth gauge and physiological trigger for odontocetes deep dives, perhaps there are grounds to further investigate numbers and densities of Paccinian corpuscles in the skin lining ear canals, external ears, face, also tactile hairs and even the tongue, to see if electronic stimulation of these sites can trigger an auditory reflex in the cat, sheep and dog for example. Because of the close relationship of the chorda tympani nerve to the tympanic membrane near the attachment of the flexible rostral ligament of the rostral process of the malleus [39], loud-noise vibrations might mechanically stimulate the chorda tympani nerve with the possibility of afferents in this nerve being able to trigger the acoustic reflex – at least in the stapes.
Tympanic membrane structure and function
In order to put into perspective possible functional implications of dissected and photographed structural features noted in our study in sheep and the cat, it was deemed appropriate to examine past reviews of human tympanic membrane (TM) structure [38,66], and recent work on TM vibratory function [67], Specifically:
Funnell and Laszlo [66] reported that there was a lack of knowledge about the tympanic membrane (TM) thickness, fibre distribution, three dimensional curvatures, post natal development, mechanical properties and attached structures. About vibratory patterns, it was generally agreed that at low frequencies, displacements of the manubrium were smaller than displacements of the surrounding TM. Lim [38] reviewed research advances made over the past three decades in understanding the tympanic membrane of laboratory animals and humans. Considerable variation in size and thickness of the pars tensa and pars flaccid were seen, with each of these parts consisting of an outer epidermal layer, middle fibrous lamina propria, and an inner mucosal epithelial layer. Fibrils in the fibrous layer were mostly type II and type III collagen with only a small amount of type I collagen. Large numbers of mast cells were found in Shrapnell’s membrane (pars flaccida) and it is suggested that these cells are responsible for middle ear effusion. The cellular basis for epidermal migration (tympanic membrane cleaning), the role of epidermal and fibroblast growth factors in epidermal cell proliferation and in wound healing, were defined. Szymanski et al., gave a contemporary review of the human tympanic membrane. A brief resume follows: The thin epidermis lining the lateral surface of the membrane is stratified squamous keratinized epithelium, continuous with the epidermis of the external acoustic meatus. Simple cuboidal epithelium lines the medial surface of the membrane and is continuous with the mucous membrane of the tympanic cavity. The lamina propria of the TM is composed of fibroelastic connective tissue, and within this layer is found the blood vessels and nerves of the tympanic membrane. The fibrous layer is thickened to form a tough ligamentous ring where the TM is lodged into the tympanic sulcus of the temporal bone. The manubrium of the malleus is attached to the medial surface of the TM and pulls its anterior and inferior portions medially giving it a conical shape. The central point of maximum depression is called the umbo and marks the end of the manubrium. When observing the lateral surface of the human TM (with an otoscope), superiorly and anteriorly from the umbo, a line, the malleolar stria, that corresponds to the manubrium of the malleus, can be seen. Directly above the stria, the lateral process of the malleus bulges and forms the malleolar prominence. That part of the tympanic membrane above the malleolar prominence is not taut as it bridges the tympanic sulcus and is therefore the pars flaccida (or Shrapnell’s membrane). The rest of the tympanic membrane below the prominence is taut - the pars tensa. On the medial surface of the tympanic membrane, the border between the pars flaccid and tensa is marked by the anterior and posterior malleolar folds, whose purpose is to hold the lateral process in place. These ligamentous bands (or more likely the fibroelastic lamina propria), also contain the chorda tympani nerve as it passes behind the tympanic membrane. (In our dissection of the sheep (Figs 2, 3, and 4), the chorda tympani nerve passed close to, but distinctly separated from the anterior and posterior malleolar folds as it crosses the medial surface of the malleus.) There are microstructural differences in the lamina propria of the pars tensa and flaccida. The fibres of the pars tensa are organized radial and circular fibres whereas those of the pars flaccida consist of loosely arranged fibroelastic tissue. The pars flaccida is well vascularised but the pars tensa lacks blood vessels in its semi-transparent major portion, and the pars flaccida is thicker than the pars tensa, is more elastic, and contains numerous mast cells. The embryological origin of the tympanic membrane is invagination of the first pharyngeal groove and the meeting of this ectodermal layer with the first pharyngeal pouch endoderm; that is, the tympanic membrane consists of two germ layers. As the fibrous middle layer is derived from neural crest mesenchyme, it too is ectodermal in origin. The tympanic membrane has a rather simple function, that of sound transmission and amplification. Similar to the membrane of a drum, the tympanic membrane vibrates as it encounters sound. It then transmits these vibrations to the ossicles of the middle ear to be further passed on to the cochlea of the inner ear for transduction.
Although true, exactly how it does this not so easy to explain, but recent advances in technology may help. For example, work at the Technion-Israel institute of Technology, Haifa 3200003, Israel, and at Rambam Healthcare Campus, Haifa 3109601, Israel, by Hamra [67], focused on rapid imaging of tympanic membrane vibrations in humans. Functional imaging of the human ear is an extremely challenging task because of minute anatomical structures and nanometer-scale motion in response to sound. This group developed and demonstrated non-invasive in vivo functional imaging of the human tympanic membrane under various acoustic excitations, using phase-sensitive spectraldomain interferometry. With this equipment, they were able to obtain high resolution functional imaging of the two dimensional tympanic membrane using a handheld imaging probe. Full vibration maps of the tympanic membrane were recorded noninvasively within a fraction of a second. They identified unique vibration patterns that varied between different healthy human subjects. As such, they see this piece of equipment as a powerful new tool for studying middle and inner ear physiology.
CONCLUSIONS
Quantitative measurement data has shown that sheep and cats do not have asymmetrical auditory ossicles as does the pilot whale.
Our approach to the qualitative study was to perform microanatomical dissections at X30 magnification using readily available microsurgery tools and photograph the specimens as the dissection progressed. Such an approach might reasonably be considered “historical”, being superseded now by micro-CT and 3-D computer modelling; but, as Mason [34,68,69] commented, “With the advent of modern multifocal imaging technology” (such as the Leica Microsystems equipment that we used), “standard micro-dissection is experiencing a new lease of life”. This is a sentiment with which we whole heartedly concur. The clear micro-anatomical detail obtained in this way could prove useful for fine tuning micro-CT and computer modelling algorithms.
Different topographical features of the tensor tympani muscle in both sheep (strength of contraction), and cat (strength of contraction and graded shortening), would appear to have a major role in effecting the acoustic reflex.
Further work on afferent neural pathways of the acoustic reflex, other than via the acoustic nerve, is likely to be a productive venture for hearing research in the cat, sheep and dog: Particularly, to investigate the numbers and densities of Paccinian corpuscles in the skin lining ear canals, external ears, face, tongue and those in association with tactile hairs, to see if electronic stimulation at these sites will trigger an acoustic reflex?
The topography of the tensor tympani muscle in the cat appears to be able to position the malleus on a knife-edge configuration with the incus, If such a malleoincudal configuration could be activated by a twisting motion of the manubrium, such as that proposed by Puria and Steele, competing frequencies could be filtered out and very high frequency sound transmission achieved in this species.
ACKNOWLEDGEMENTS
I am indebted to Dr. Sirpa Nummela, PhD, of Faculty of Biological and Environmental Sciences, Molecular and Integrative Biosciences Research Program, university of Helsinki, Helsinki, Finland, for her meticulous dissections of the sheep ossicles. She has spent her whole summer’s holiday in my surgery (dungeon), devoting all her time to produce the superbly clean and exact specimens for our measurement, and upon which our work is based.
We thank Ashleigh Van Oestrum, Former National Life Science and Health Technician, Charles Sturt University, Wagga Wagga NSW. Her expert help and technical advice on the use of Leica Microsystems stacked image technology to obtain photomicrographs of sheep and cat middle ear dissections, was much appreciated.
We also thank Charles Sturt University Wagga Wagga NSW, School of Animal and Veterinary Science, for providing access to specimens, and granting use of laboratory facilities and equipment to perform the micro-dissections of sheep and cat middle ears.
REFERENCES
- Hyrtl J. Vergleichend-anatomische Untersuchungen überdas innere Gehörorgan des Menschen und der S?ugethiere. 1845; 91-130.
- Doran A. Morphology of the Mammalian Ossicula auditus. The Transaction of the Linnean Society of London. 2nd Series, Zoology. 1879; 1: 371-497.
- Henson OW Jr. Some morphological and functional aspects of certain structures of the middle ear in bats and insectivores. Univ Kansas Science Bull. 1961; 42: 151-255.
- Fleischer G. Studien am Skelett des Gehörorgans der S?ugetiere, einschliesslich des Menchen. S?ugetierk. Mitt. 1973; 21: 131-239.
- Fleischer G. Evolutionary principles of the mammalian middle ear. Adv Anat Embryol Cell Biol. 1978; 55: 3-70.
- Rosowski JJ. Hearing in transitional mammals: Predictions from the middle ear anatomy and hearing capabilities of extant mammals. In: DB Webster, AN Popper, RR Fay (Eds.). The Evolutionary Biology of Hearing, Springer-Verlag, New York. 1992; 615–631.
- Rosowski, JJ. Outer and middle ear. In: AN. Popper, RR. Fay (Eds.). Comparative Hearing: Mammals, Springer-Verlag, New York. 1994; 172–247.
- Rosowski JJ. The middle and external ears of terrestrial vertebrates as mechanical and acoustic transducers. In FG Barth, JAC. Humphrey TW Secomb (Eds.), Sensors and Sensing in Biology and Engineering New York: Springer-Verlag. 2003; 59–69.
- Rosowski JJ. External and middle ear function. In PA. Fuchs (Ed.), The Oxford Handbook of Auditory Science: The Ear. 2010; 49–91.
- Rosowski JJ, Graybeal A. What did Morganucodon hear? Zool J Linn Soc. 1991; 101: 131-168.
- Nummela S. Scaling and modelling of the mammalian middle ear. Hear Res. 1995; 85: 18-30.
- Hemila S, Nummela S, Reuter T. What middle ear parameters tell about impedance matching and high-frequency hearing. Hear Res. 1995 ; 85; 31-44.
- Huang GT, Rosowski JJ, Flandemeyer DT, Lynch TJ, Peake WT. The middle ear of a lion: Comparison of structure and function to domestic cat. J Acoust SocAm. 1997; 101: 1532-49.
- Puria S, Steele C, Tympanic-membrane and malleus-incus-complex co- adaptations for high-frequency hearing in mammals. Hear Res. 2010; 263: 183-190.
- Thewissen JGM, Cooper LN, Clementz, MT, Bajpai S, Tiwari BN. Whales originated from aquatic artiodactyls in the Eocene epoch of India. Nature. 2007; 450: 1190-1195.
- Reysenbach de Haan FW. Hearing in whales. Acta Otolaryngol Suppl. 1957; 134: 1-114.
- Norris KS. Some problems of echolocation in cetaceans. In: WN Tavolga (Ed.), Marine Bio-Acoustics. Pergamon Press, New York. 1964; 317-336.
- Norris KS. The evolution of acoustic mechanisms in odontocete cetaceans. In: ET Drake (Ed.). Evolution and Environment. Yale Univ. Press, New Haven, CT. 1968; 297-324.
- Ketten DR. The marine mammal ear: specializations for aquatic audition and echolocation. In: DB Webster RR, Fay AN . Popper, editors. The evolutionary biology of hearing. New York , Springer. 1992; 717– 750.
- Ketten DR. Cetacean Ears, In Hearing by Whales and Dolphins, Au. WWL, Popper AN and Fay RR editors. Springer New York. 2000; 43- 109.
- Cranford TW, Amundin M, Norris KS. Functional morphology and homology in the odontocete nasal complex: Implication for sound generation. J of Morphology. 1996; 228: 223-285.
- Nummela S, Wäger T, Hemilä S, Reuter T. Scaling of the cetacean middle ear. Hear Res. 1999; 133:71-81.
- Hemilä S, Nummela S, Reuter T. A model of the odontocete middle ear. Hear Res. 1999; 133: 82-97.
- Tubelli AA, Zosuls A, Ketten DA, Mountain DC. Elastic modulus of cetacean auditory ossicles. Anat Rec. 2014; 297: 892-900.
- Yamato M, Pyenson, ND. Early development and orientation of the acoustic funnel provides insight into the evolution of sound reception pathways in cetaceans. PLoS One. 2015; 10: e0118582.
- Fahlke JM, Gingerich PD, Welsh RC, Wood AR. Cranial asymmetry in Eocene archaeocetes whales and the evolution of directional hearing in water. 201; 108 : 145-148 .
- Coombs EJ, J Clavel, T Park, M Churchill, A Goswami. Wonky whales:the evolution of cranial asymmetry in cetaceans. BMC biology. 2020.
- Tsur I. Directional Hearing under water: Morphology and Function of the Middle Ear of Globicephala machrorhynchus (Short-Finned Pilot Whale). DOCTORAL DISSERTATION. Faculty of Biological and Environmental Sciences, Molecular and Integrative Biosciences Research Program, University of Helsinki. 2020.
- Lifschytz T, Tchernov E, Werner YL. Directional asymmetry of middle- ear ossicles in the free-tailed Mexican bat Tadarida brasiliensis. J Zool. 2000; 46:166-167 .
- Fay RR. Hearing in Vertebrates: A Psychophysics Databook. Hill-Fay Associates. Winnetka IL.1988.
- Heffner HE, Heffner RS, The evolution of mammalian sound localization. Acoust. 2016; 12: 20-27.
- Jack W, Bradbury .Vehrencamp.Principles of Animal Communication, 2nd ed. Sinauer public. 2011.
- Nummela S, Pihlström H, Puolamäki K, Forelius M, Hemilä S, Reuter T. Exploring the mammalian sensory space: co-operations and trade-offs among senses. J Comp.Physiol. 2013; 199: 1077-1092.
- Mason M J.Of mice, moles and guinea-pigs: functional morphology of the middle ear in living mammals.Hear Res. 2013; 301: 4-18.
- Tsur I, Christie B. Middle ear morphology of the domestic cat (Felis catus) and the domestic sheep (Ovis aries): A comparative study with two echolocating mammals (Globicephala machrorhynchus and Tadarida brasiliensis Mexicana). 2020; 23-24.
- Malkemper EP, Mason MJ, Burda H. Functional anatomy of the middle and inner ears of the red fox, in comparison to domestic dogs and cats. J Anat. 2020; 236: 980-995.
- Cox T. Trevor Cox Sonic Wonderland. 2009.
- Lim DJ. Structure and function of the tympanic membrane: a review. Acta Otorhinolaryngol Belg. 1995; 49: 101-15.
- Howard Evans E , de Lahunta A. Miller’s Anatomy of the Dog 4th Ed.Elsevier: Sculptured medial view of the right middle ear of the dog. Shows proximity of cauda tympani nerve to the rostral ligament of the malleus. 2013; 20-9: 737.
- Fitzpatrick. ReferralsVestibular Disease. 2020.
- Sula MM, Njaa,BL, Payton ME. Histologic Characterization of the Cat Middle Ear: In Vet Pathol. 2014; 51: 951-67.
- Heffner RS, Heffner HE. Hearing range of the domestic cat. Hear Res. 1985; 19: 85-88.
- Péus D, Dobrev I, Pfiffner F, Hoon JS. Comparison of sheep and human middle-ear ossicles: anatomy and inertial properties. J Comp PhysiolA. 2020; 206: 683–700.
- Shillito-Walser E, Hague P, Walters E. Vocal recognition of recorded lambs voices by ewes of three breeds of sheep. Behaviour. 1981; 78: 260-272.
- Knudsen EI, Konishi M. Mechanisms of sound localization in the barn owl (Tyto alba). J Comp Physiol. 1979; 133, 13-21.
- Konishi M. Listening with two ears. Sci Am. 1993; 268: 66-73.
- Mass AM, Supin AY. Visual abilities of marine mammals. Encyclopedia of Marine Mammals (Third Edition). 2018; 1033-1044.
- Soto NA, Johnson MP, Madsen PT. Diaz F, Dominguez I, Brito A, Tyack
-
P. Cheetahs of the Deep Sea: Deep Foraging Sprints in Short-Finned Pilot Whales off Tenerife (Canary Islands). J Anim Ecol. 2008; 77: 936- 947
- Pihlström H, Fortelius M, Hemilä S, Forsman, R Reuter T. Scaling of mammalian ethmoid bones can predict olfactory organ size and performance. Proc Boil Sci. 2005; 272: 957–962.
- Kishida T, Thewissen, JGM. Evolutionary changes of the importance of olfaction in cetaceans based on the olfactory marker protein gene. Gene. 2012; 492: 349-53.
- Kishida T, Thewissen JGM, Hayakawa T, Imai H, Agata K. Aquatic adaptation and the evolution of smell and taste in whales. Zool Lett. 2015; 1:9.
- Halpin ZT. Kin recognition cues of vertebrates. In PG Hepper. (Ed.) Kin Recognition, Cambridge Univ. Press, Cambridge. 1991; 220-258.
- Margolis RH, SC Levine. “Acoustic reflex measures in audiologic evaluation.” Otolaryngology Clin North Am. 1991; 24: 329-347.
- Hain TC. Acoustic reflexes Chicago Dizziness and Hearing. 2021.
- Mukerji S, Windsor AM, Lee DJ. Auditory Brainstem Circuits That Mediate the Middle Ear Muscle Reflex. Trends Amplif. 2010; 14: 170– 191.
- Jones SE, Mason MJ, Sunkaraneni VS, Baguley DM. The effect of auditory stimulation on the tensor tympani in patients following stapedectomy. Acta Otolaryngol. 2008; 128: 250-4.
- Josephson RK. Extensive and intensive factors determining the performance of striated muscle. J Exp Zool. 1975; 194: 135- 154.
- Evans HE, de Lahunta A. Miller’s Anatomy of the Dog 4th Ed, Elsevier: Muscles of the ossicles of the dog. 2013; 739.
- Zimny ML. Mechanoreceptors in articular tissues. Am J Anat. 1988; 182: 16-32.
- Pang XD, Peake WT. How do contractions of the stapedius muscle alter the acoustic properties of the ear? In JB. Allen A. Hubbard SI, Neely A. Tubis (Eds). Peripheral auditory mechanisms.1986; 36-43. New York, NY. Springer-Verlag.
- Helmholtz H. Die Mechanik der Gehorknochelchen und des. Bone conduction - The influence of the middle ear. Acta Otol Suppl. 1868; 155: 1–99.
- Willi UB, Fernazzini MA, Huber AM. The incudo-malleolar joint and sound transmission loss. Hear Res. 2002; 174: 32-44.
- Evans HE, de Lahunta A. Miller’s Anatomy of the Dog 4th Ed Elsevier: Cutaneous areas of the rostral concave surface of the external ear of the dog. 2013; 719 -724.
- Bermejo P, M López, I Larraya, J Chamorro, JL Cobo, S Ordóñez, et al. Innervation of the Human Cavum Conchae and Auditory Canal. Anatomical Basis for Transcutaneous Auricular Nerve Stimulation. Biomed Res Int. 2017; 1–10.
- De Vreese S, AndréM, Cozzi B, Centelleghe C, van der Schaar M, Mazzariol S. Morphological Evidence for the Sensitivity of the Ear Canal of Odontocetes as shown by Immunohistochemistry and Transmission Electron Microscopy. 2020.
- Funnell WRJ, Laszlo CA. A Critical Review of Experimental Observations on Ear-Drum Structure and Function. ORL J Otorhinolaryngology Relat Spec 1982; 44: 181-205.
- Hamra M, Shinnawi S, Cohen-Vaizer M, Yelin D. Rapid imaging of tympanic membrane vibrations in humans. Biomed Opt Express. 2020; 11: 6470-6479.
- Evans HE, de Lahunta A. Miller’s Anatomy of the Dog 4th Ed Elsevier: Ligaments of the auditory ossicles of the dog. 2013; 739.
- Merriam-Webster. Saddle joint. 2021.