Olfactory Dysfunction in SARS-CoV-2 Infection: Potential Benefits of Including Smell Tests in COVID-19 Patients
- 1. Department of Biomedical Sciences, East Tennessee State University, USA
ABSTRACT
Olfactory dysfunction has been documented in the viral infection of the respiratory system, including SARS-CoV-2 that causes COVID-19. Sudden loss of the sense of smell is widely considered a core symptom of COVID-19 infection. Animal and human models of post-viral olfactory dysfunction provide insight behind the mechanism of SARS-CoV-2 entry and action. Including smell tests in COVID-19 patients and studying the mechanism underlying olfactory dysfunction could provide potential strategy to prevent COVID-19 infection and spread as well as reveal potential therapeutic intervention to restore the sense of smell. In addition, we do note that while dexamethasone is commonly used to treat severe cases of COVID-19, it may delay olfactory function recovery.
KEYWORDS
• Olfactory dysfunction
• Clinical olfactory function test
• COVID-19
• Dexamethasone
CITATION
Caviness D, Rodriguez-Gil D, Jia C (2022) Olfactory Dysfunction in SARS-CoV-2 Infection: Potential Benefits of Including Smell Tests in COVID-19 Patients. Ann Otolaryngol Rhinol 9(1): 1283.
INTRODUCTION
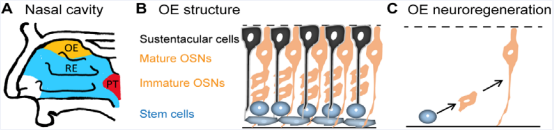
Loss of olfactory function can readily be overlooked in a clinical setting. In fact, how many patients partake in olfactory assessments as a mandatory component of their routine exam? In most cases, symptomatic patients recover the sense of smell without any treatment due to the intrinsic neuroregenerative capacity in adult olfactory epithelium [1]. While respiratory epithelium comprises the majority of the nasal cavity, the olfactory epithelium is located at the roof of the nasal cavity (Figure 1A)
Figure 1: The olfactory epithelium: anatomy and adult neuroregeneration. A) Sagittal view of the human nasal cavity. OE, olfactory epithelium, is highlighted in yellow. RE, respiratory epithelium, is highlighted in blue. PT: Pharyngeal Tonsils. B) The structure of olfactory epithelium. Sustentacular cells (black), supporting cells, are located in the apical layer that faces the nasal cavity. Olfactory sensory neurons (OSNs, orange), including immature and mature, are in the middle layer. Olfactory stem cells (blue) reside in the basal layer. C) Adult neuroregeneration in the olfactory epithelium. Olfactory stem cells proliferate to produce new olfactory sensory neurons throughout life.
and contains olfactory sensory neurons responsible for odorant detection (Figure 1B). The supporting cells, i.e. sustentacular cells, cover the apical layer of the olfactory epithelium that faces the nasal cavity, while olfactory stem cells reside in the basal layer (Figure 1B). The olfactory stem cells produce new olfactory sensory neurons to replace dying neurons throughout life (Figure 1C), which is required for normal smell function. Damaging of olfactory sensory neurons leads to reduced olfactory function and loss. However, the olfactory function can be automatically recovered via upregulated neuroregeneration. The substantial prevalence of olfactory dysfunction following viral infection of the respiratory system leads many clinicians to consider this physical dysfunction as a subjective symptom rather than a diagnostic and pathologic sign amenable to analysis. Better understanding of the mechanisms behind olfactory dysfunction is expected to increase its clinical importance. Through analyzation of human and mouse specimens, one is able to gain insight into viral-induced olfactory dysfunction on a pathophysiologic level. These murine and human models provide a better understanding of mechanisms and reveal potential therapeutic targets. This is becoming increasingly useful considering the high prevalence of sudden olfactory loss among COVID-19 patients. Here, we discuss the process of olfactory loss following viral infection, the possible mechanism of sudden olfactory dysfunction in COVID-19 infection, the possible benefits of clinical smell tests in COVID-19 patients, and a potentially overlooked consequence on olfactory function using dexamethasone in treating COVID-19 patients.
Olfactory dysfunction, a common symptom following respiratory system viral infection
The loss or deficit of olfactory function has been documented in the viral infection of the respiratory system. For example, in chronic rhinosinusitis, olfactory dysfunction occurs ranging from 30-80% of cases depending on the clinical olfactory function tests [2]. The post-viral olfactory dysfunction could be due to an inflammatory effect and/or a direct effect of virus infection on olfactory sensory neurons. An inflammatory theory suggests that the loss of smell is attributed to the effect of inflammatory infiltrate following viral insult. A genetic mouse model of chronic rhinosinusitis, by temporarily expressing a pro-inflammatory cytokine tumor necrosis factor-alpha (TNF-α) in sustentacular cells, results in loss of olfactory function [3]. TNFα expression induces a progressive infiltration of inflammatory cells into the olfactory epithelium, which leads to apoptosis of olfactory sensory neurons and reduction of olfactory epithelium neuroregeneration. The structure of the olfactory epithelium and olfactory function recovers when TNFα expression is extinguished. Three distinct phases of olfactory dysfunction and regeneration are demonstrated in this study. First, marked decreased smell sensation without gross histological changes to the epithelial layer was noted. Next, drastic rearrangement of the olfactory epithelium following the invasion of inflammatory cells was observed. Lastly, a dramatic recovery in the structure was accompanied by the return of smell sensation after inflammatory cytokine expression had stopped. These results conceptually support the idea that disruption of the olfactory epithelium by inflammatory mediators can induce olfactory insufficiency. However, asynchronous sensation loss and invasion of inflammatory cells could suggest that inflammatory mediators only play a secondary role in olfactory dysfunction. A competing theory poses that loss of olfaction following viral infection credits direct accumulation of viral proteins in the olfactory sensory neurons. This theory supports the idea that olfactory dysfunction is one of the earliest symptoms following viral infection, including SARS-CoV-2. This suggests that the olfactory epithelium, olfactory bulb, and cranial nerve 1 are a potential route for viral infection of the central nervous system which can manifest as other neurological symptoms, such as headache and seizure, reported in COVID-19 patients. In a mouse model of postviral olfactory dysfunction with a Sendai virus, that is parallel to the Human Parainfluenza Virus family, the virus persists in the olfactory epithelium and bulb and impairs olfactory function via increasing apoptosis and decreasing olfactory stem cell proliferation [4]. Importantly, olfactory sensory neurons have a diminished response to odorants when Sendai virus F proteins are seen in the cytoplasm of olfactory sensory neurons [4]. This supports the idea that the virus directly targets olfactory sensory neurons, which potentially play a major role in sudden olfactory dysfunction following viral infection. SARS-CoV-2 proteins have also been found in olfactory sensory neurons [5], supporting a possible hypothesis of neuronal insult for olfactory dysfunction in COVID-19 patients.
Sudden olfactory dysfunction in COVID-19 patients
Among the many signs and symptoms of SARS-CoV-2 infection, sudden anosmia has been documented in a significant number of cases, including in infected but otherwise asymptomatic individuals. The loss of smell is currently listed as one of the COVID-19 symptoms by the World Health Organization (WHO). A European Study of 417 patients with mild-to-moderate COVID-19 symptoms reports that 85% of patients had some variable level of olfactory dysfunction which appeared before the other symptoms, such as cough, fever, and difficulty to breath, in 12% of cases [6]. A cross-sectional study of 150 RT-PCR confirmed COVID-19 patients survey reported a loss of smell in 41% of cases with 74% of these reporting extremely severe [7]. Most patients (88%) recovered their sense of smell by two months. But nearly one out of ten have not recovered in two months. These reports of “loss of smell” were based on a self-reported symptom, not a quantified fact. To accurately document olfactory dysfunction in COVID research, clinical smell tests are needed, not necessarily for diagnostic purposes but for clinical follow-up to determine whether therapeutic intervention is needed to enhance olfactory function recovery. Indeed, a recent study assessed COVID-19-induced olfactory dysfunction using the University of Pennsylvania Smell Identification Test (UPSIT). This is a wellvalidated self-administered test with 40-odorant “scratch and sniff” scented strip and associated multiple-choice questions that was used in 60 COVID-19 patients and 60 age- and sex-matched controls. The result indicates that 98% of the patients exhibited different degrees of smell dysfunction, which is substantially higher than the 18% of the controls [8]. These data support that quantitative smell testing is useful to identify decreased smell function in COVID-19 patients. Since olfactory deficits in COVID-19 patients are evident for all 40 UPSIT odorants, the three-item Q Sticks Test, a simple and fast screening test that is sensitive to anosmia and differentiates between normosmic and hyposmia/ anosmia and easy for everyday medical practice [9], may also be used to fast screen olfactory function in different clinical settings. However, loss of the sense of smell can be caused by a variety of factors, including head trauma. A careful differential diagnosis of anosmia is required to rule out overdiagnosis of COVID-19, especially in patients at high risk for COVID-19. A complete history and physical, lab work, and even brain imaging would be appropriate to rule out COVID-related anosmia [10].
It is widely accepted that the mechanism of cellular injury by SARS-CoV-2 is through binding of viral Spike (S) protein to ACE2,a metalloproteinase ectoenzyme. In addition, priming protease TMPRSS2 is vital for uptake and replication. Substantial evidence presents that the olfactory epithelium is a potential entry target for SARS-CoV-2 that causes COVID-19 [11-13]. Sungnak et al., used single cell RNA sequencing from healthy donors to analyze expression of ACE2 and TMPRSS2 in various tissues of the human body. Their study revealed an overall low expression of ACE2 in the nasal epithelium with further reduced expression in the bronchi, lower airway club cells, and lung parenchyma, supporting that the upper, as opposed to the lower, airway is the initial site of SARS-CoV-2 infection. TMPRSS2 was highly expressed with a broader distribution, suggesting that ACE2 is the limiting factor for initial infection. Also, Chen et al., examined 23 (4 controls) nasal and 7 tracheal specimens from human patients suffering from chronic rhinosinusitis and undergoing endonasal surgical approaches and patients undergoing bronchoscopy for tracheal stenosis, respectively. Immunohistological analysis revealed expression of ACE2 along the apical surface of sustentacular cells in the olfactory epithelium. Thus, the olfactory epithelium may be a determinant of susceptibility of infection and symptoms and analysis of olfactory epithelium structures could provide not only mechanisms underlying olfactory dysfunction but also insight into the host’s immunological competence in the fight against virus entry and spread [14]. In addition, increases of ACE2 and TMPRSS2 expression in mouse olfactory epithelium from young adult to old age mice were identified [11], suggesting the olfactory epithelium in aged mice is more susceptible to SARSCoV-2 infection. If this is true for the human olfactory epithelium, then the older humans will be more vulnerable to SARS-CoV-2 infection. The pandemic of COVID-19 is continuing with SARSCoV-2 variants. Recent study indicates that the highly mutated Omicron variant found in November 2021 differs in the replication pathway compared to the Delta variant that has dominated the world since June 2021. Omicron variant infection is not enhanced by TMPRSS2 but is largely mediated via the endocytic pathway [15]. Thus, the difference in entry pathway between Omicron and Delta variants may explain why, anecdotally, the sense of smell in patients infected with the Omicron variant seems less hindered compared to Delta variant infection.
COVID-19-related olfactory dysfunction seems to be attributed to damage to the olfactory epithelium rather than blockade of the nasal cavity [6]. These findings have also been further supported by studies in rodents [11,16]. In SARS-CoV-2 infected hamsters, immunostaining reveals a heavy viral load in the cytoplasm of mature and immature olfactory sensory neurons along with sustentacular cells. In addition, electron microscopy study shows a deciliation of olfactory sensory neurons as early as 2 days postinoculation attributes to viral impact. At 14 days post-inoculation, all hamsters in the experimental group regain ciliation and the sense of smell [16]. The detection of viral particles in olfactory sensory neurons and supporting cells was similarly demonstrated in seven COVID positive humans in the same study. In four COVID-19 positive patients who reported long-lasting/relapsing loss of smell after diagnosis. All nasopharyngeal samples showed no detectable SARS-CoV-2 RNA through RT-qPCR. However, all cytological samples of olfactory mucosa had detectable SARSCoV-2 RNA but lack evidence of active viral replication. This indicated that the olfactory epithelium of these patients remained infected with viral (but inactive) proteins, which may attribute to long-lasting/relapsing olfactory dysfunction. This also calls into question whether addition therapeutic intervention is needed to help restore the olfactory function. Loss of the sense of smell compromises human health and life quality and is a major safety issue, such as detecting gas leaking and spoiled food.
Treating COVID-19 patients with dexamethasone may delay recovery of olfactory function
As COVID-19 cases continue to rise and fall, researchers and clinicians still debate on appropriate treatment strategies for patients suffering from this respiratory illness. Proposed, although not always supported by evidence-based research, treatments range from antivirals to antiparasitics. Dexamethasone has shown to relieve symptoms in some patients with severe progression of COVID-19, and is considered by some as the “first drug shown to save lives” [17]. It is largely accepted as appropriate treatment for many cases of COVID-19. The WHO recommended the use of dexamethasone in patients who are seriously ill with COVID-19. Dexamethasone is a corticosteroid known for suppressing inflammatory response. While this medication is highly effective, it should be noted that dexamethasone treatment might delay the neuroregeneration of the olfactory epithelium, which may affect the olfactory recovery. In a mouse model of inflammation-mediated damage of olfactory epithelium following methimazole- or methyl bromide-induced olfactory lesions, dexamethasone treatment decreases olfactory stem cell proliferation [18]. Thus, dexamethasone treatment could diminish the regenerative abilities of the olfactory epithelium. The impact of long-term dexamethasone treatment in COVID-19 may be substantial but may impede the regeneration of the olfactory epithelium and stun the return of olfactory function. While we do not discourage dexamethasone treatment in severe cases of COVID-19, as such treatment has proven beneficial; we are simply trying to draw caution to a potentially overlooked consequence of dexamethasone.
CONCLUSIONS
Sudden olfactory dysfunction is strongly connected to SARS-CoV-2 infection. Current research supports that olfactory dysfunction commonly follows viral infection including SARSCoV-2. Better understanding mechanisms underlying olfactory dysfunction in early and late stages of COVID-19 infection would not only provide potential strategy to prevent COVID-19 infection and spread but also reveal potential therapeutic intervention to restore the sense of smell.
FUNDING
Medical Student Summer Research Program, Quillen College of Medicine, East Tennessee State University.
REFERENCES
- Berger T, Lee H, Thuret S. Neurogenesis right under your nose. NatNeurosci. 2020; 23: 297-298.
- Kohli P, Naik AN, Harruff EE, Nguyen SA, Schlosser RJ, Soler ZM. The prevalence of olfactory dysfunction in chronic rhinosinusitis. Laryngoscope. 2017; 127: 309-320.
- Lane AP, Turner J, May L, Reed R. A genetic model of chronicrhinosinusitis-associated olfactory inflammation reveals reversiblefunctional impairment and dramatic neuroepithelial reorganization.J Neurosci. 2010; 30: 2324-2329.
- Tian J, Pinto JM, Cui X, Zhang H, Li L, Liu Y, et al. Sendai Virus Induces Persistent Olfactory Dysfunction in a Murine Model of PVOD via Effects on Apoptosis, Cell Proliferation, and Response to Odorants. PLoS One. 2016; 11: e0159033.
- Meinhardt J, Radke J, Dittmayer C, Franz J, Thomas C, Mothes R, et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci. 2021; 24: 168-175.
- Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020; 277: 2251-2261.
- Printza A, Katotomichelakis M, Valsamidis K, Metallidis S, Panagopoulos P, Panopoulou M, et al. Smell and Taste Loss Recovery Time in COVID-19 Patients and Disease Severity. J Clin Med. 2021; 10: 966.
- Moein ST, Hashemian SM, Mansourafshar B, Khorram-Tousi A, Tabarsi P, Doty RL. Smell dysfunction: a biomarker for COVID-19. Int Forum Allergy Rhinol. 2020; 10: 944-950.
- Sorokowska A, Oleszkiewicz A, Minovi A, Konnerth CG, Hummel T. Fast Screening of Olfactory Function Using the Q-Sticks Test. ORL J Otorhinolaryngol Relat Spec. 2019; 81: 245-251.
- Nagamine T. Beware of traumatic anosmia in COVID-19 pandemic.CJEM. 2021; 23: 567-568.
- Bilinska K, Jakubowska P, Von Bartheld CS, Butowt R. Expression of the SARS-CoV-2 Entry Proteins, ACE2 and TMPRSS2, in Cells of the Olfactory Epithelium: Identification of Cell Types and Trends with Age. ACS Chem Neurosci. 2020.
- Chen M, Shen W, Rowan NR, Kulaga H, Hillel A, Ramanathan M Jr, et al. Elevated ACE-2 expression in the olfactory neuroepithelium: implications for anosmia and upper respiratory SARS-CoV-2 entry and replication. Eur Respir J. 2020; 56: 2001948.
- Sungnak W, Huang N, Becavin C, Berg M, Queen R, Litvinukova M, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020; 26: 681-687.
- Gori A, Leone F, Loffredo L, Cinicola BL, Brindisi G, De Castro G, et al. COVID-19-Related Anosmia: The Olfactory Pathway Hypothesis and Early Intervention. Front Neurol. 2020; 11: 956.
- Zhao H, Lu L, Peng Z, Chen LL, Meng X, Zhang C, et al. SARS-CoV-2 Omicron variant shows less efficient replication and fusion activity when compared with Delta variant in TMPRSS2-expressed cells. Emerg Microbes Infect. 2022; 11: 277-283.
- de Melo GD, Lazarini F, Levallois S, Hautefort C, Michel V, Larrous F, et al. COVID-19-related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Sci Transl Med. 2021; 13.
- Ledford H. Coronavirus breakthrough: dexamethasone is first drug shown to save lives. Nature. 2020; 582: 469.
- Chen M, Reed RR, Lane AP. Acute inflammation regulates neuroregeneration through the NF-kappaB pathway in olfactory epithelium. Proc Natl Acad Sci U S A. 2017; 114: 8089-8094.