Accumulation and Migration of Heavy Metals in Hydrobionts of Lake Markakol, Kazakhstan
- 1. Senior Lecturer, Al-Farabi Kazakh National University, Kazakhstan
- 2. Senior Lecturer, Al-Farabi Kazakh National University, Kazakhstan
- 3. Faculty of Geography, Philipps-Universität Marburg, Germany
- 4. Al-Farabi Kazakh National University, Almaty, Kazakhstan
- 5. Kazakh National Agrarian Research University, Kazakhstan
Abstract
The research is devoted to the exploration of heavy metal (HM) accumulation in hydrobionts of Lake Markakol, located in Eastern Kazakhstan. The objective of the research was to evaluate the levels of HMs accumulation in aquatic sections, food organisms and fishes, as well as to determine their impact on the ecosystem and potential risks to human health. The study analyzed concentrations of copper, zinc, lead, cadmium, nickel and cobalt in macrophytes, zooplankton, macrozoobenthos and fish tissues (uskuch, Brachymystax lenok). The results show significant exceedances of the maximum permissible concentrations (MPC) for all tested metals, especially in food organisms and fish. The highest concentrations were recorded in liver, gills and muscles, with nickel content reaching 55.6 mg kg-1, and cobalt – 111 mg kg-1, which exceeds MPC by tens and hundreds of times, which indicates a high pollution degree of the aquatic ecosystem. It was established that heavy metals actively migrate along trophic chains, accumulating in hydrobionts tissues, which results in a risk to human health when consuming fish. The obtained data emphasize the necessity for a regular monitoring of Lake Markakol’s pollution and for the development of measures to reduce the anthropogenic load on the ecosystem.
KEYWORDS
- Heavy metals
- Hydrobionts
- Bioaccumulation
- Aquatic macrophytes
- Trophic chains
- Toxicity
CITATION
Ismukhanova L, Madibekov A, Opp C, Sultanbekova B, Zhadi A (2025) Accumulation and Migration of Heavy Metals in Hydrobionts of Lake Markakol, Kazakhstan. Ann Aquac Res 7(1): 1050.
INTRODUCTION
Heavy metals (HM), including lead, cadmium, mercury, zinc, copper, nickel and cobalt, pose a serious threat to aquatic ecosystems due to their high toxicity, bioaccumulative capacity and biomagnification. Their sources can be of natural origin (rock weathering, volcanic activity) and anthropogenic influences (industrial emissions, agricultural runoff). As soon as heavy metals enter into the aquatic environment, they immediately become participants of complex migration and transformation processes, which occur at different levels and under the influence of multiple factors [1,2]. Bioaccumulation of heavy metals exacerbates the problem of their impact on the ecosystem, since these elements have high resistance to biodegradation.Their accumulation ability in living organisms, including aquatic plants and hydrobionts, poses a risk of toxic effects at various levels of the trophic chain. Entering biological systems, heavy metals can cause a wide range of physiological disorders, including toxic damage to the organs, violation of metabolic processes and cancer [3,4]. A common sequence in the aquatic environment is the HM accumulation in bottom sediments, their migration into water and biological organisms, causing toxic effects in hydrobionts.
The interaction of hydrobionts with HMs leads to various dysfunctions in their biological systems. Scientific studies show that under the influence of these pollutants, aquatic organisms suffer from damage to the nervous, digestive and respiratory systems, while in aquatic plants the processes of photosynthesis are disturbed under their influence [5].
Aquatic plants, being the beginning of the trophic chain, plays a key role in biofiltration processes by absorbing heavy metals from the aquatic environment and bottom sediments. The intensity of metal absorption and accumulation by plants varies depending on their concentration in water, as well as on surrounding factors such as pH, redox potential and temperature, which determine the dynamics of migration and bioavailability of these elements in the aquatic ecosystem. Macrophytes are important elements of the food chain in aquatic ecosystems. Thus, most hydrobionts feed on algae and aquatic plants, which in its turn become food for fish. Macrophytes are also fed by herbivorous fish, which serve as food for predatory fish [6]. Therefore, HMs entering a water body are able to participate in the cycle of substances actively and migrate through food chains to people by consuming fish products [7,8].
The next link in the trophic chain consists of fish. Even when concentrations are low, heavy metals and trace elements have a negative impact on phytoplankton and zooplankton, settling in bottom sediments and affecting benthic organisms. The accumulation of harmful substances in fish tissues is a potential threat to human health from fish consumption. That is why the study of the ability to accumulate trace elements by hydrobionts allows not only to determine the level of pollution of the aquatic environment, but also to trace the biological consequences of anthropogenic impacts.
Considering the ability to accumulate in biological components of ecosystems, including through the transfer across trophic networks, heavy metals pose a potential danger to human health risks, since the consequences of such accumulation are often observed in the fish consumption–one of the main components of the food chain [9,10]. As an example, high concentrations of copper in the aquatic environment lead to atrophy of fish tissues, disturbance of metabolic processes and development of endemic anemia. Excesses of this heavy metal prevent normal hematopoiesis and gas exchange, as well as weakens the ion-osmotic regulation and bone mineralization processes [9,10].
According to a review of multiple scientific studies, it was revealed that fish tissues differ in the degree of absorption and accumulation of toxic metals, whereas metabolically active organs, such as gills, liver and kidneys, are characterized by the most intensive retention of these elements. In these organs there are synthesized specific proteins–metallothioneins, which play a key role in binding and detoxification of metal ions. At the same time, lower concentrations of toxic metals are found in skin and muscle tissues. The muscles are a subject of special scientific interest because they are the main object of human consumption and have a variable bioaccumulation capacity depending on the species of fish [11,12].
Therefore, the study of the ability to accumulate trace elements by hydrobionts allows not only to determine the level of pollution of the aquatic environment, but also to trace the biological consequences of anthropogenic impacts.
The present research objectives are to determine the levels of heavy metal accumulation in hydrobionts of Lake Markakol, to reveal their influence on physiological and biochemical processes of aquatic organisms, as well as to assess possible ecological and toxicological risks for the aquatic ecosystem and human health.
MATERIAL AND METHODS
Study area. Lake Markakol, located in a tectonic basin in Eastern Kazakhstan, is the largest lake in the Altai Mountains (Figure 1).
Figure 1: Location of Lake Markakol.
It has the shape of an irregular ellipse, stretching from northeast to southwest, with a catchment area of 1180 km² and a surface area of 454.1 km². The maximum depth of the lake reaches 23.8m and the water volume is 6.5 km³. There are 27 streams and rivers flowing into the lake, the main ones being Topolevka, Matabai, Elovka, Tikhushka, Zhirelka and Urunhaika, while the only Kalzhyr River flows out. The lake is classified as a freshwater and flowing lake. The southern shore is bordered by steep mountain slopes, while the northern shore of the lake is characterized by a fan-like sediment accumulation lake bank [13]. The lake was formed as a result of tectonic uplift, which overlapped the valley of the Kalzhyr River. The relief of the Markakol lake basin is divided into three zones: highlands (alpine relief), midlands and the lake valley. Besides the tectonic basin formation, the origin of the lake is related to glacial phases of the Quaternary period. Its geological structure is represented by metamorphic rocks, granites, tuffs and volcanic breccias.
The flora of the basin has about 700 species of higher plants, including 15 rare species included in the Red Book of Kazakhstan. The lake is located on the territory of the Markakol National Nature Reserve, established in 1976 to preserve the natural complexes of the southern part of the Altai Mountains. The reserve is included in the list of 200 priority ecological regions of the world according to WWF classification, and it is part of the UNESCO World Network of Biosphere Reserves.
However, long-term agricultural activities in the surrounding areas have caused intensive anthropogenic load on aquatic ecosystems, first of all, manifested in increasing the degree of natural water pollution, including such toxicants as HMs [14]. The main sources of pollution include industrial and household effluents, agricultural outflows, the use of fertilizers and pesticides, as well as the activities of mining and metallurgical industries. HMs are accumulated in water, bottom sediments and hydrobionts, with little or no chemical or biological degradation, which aggravates their toxicity [13,15-17].
Research methods
Nowadays, modern methods of analyzing the content of heavy metals (HM) are based on highly sensitive and accurate instruments such as atomic absorption spectrometry (AAS) and inductively coupled plasma mass spectrometry (ICP-MS). These techniques allow the quantification of metals with a high degree of accuracy and reproducibility, making them indispensable in studies of the environmental status of aquatic ecosystems and assessment of pollution levels.
Biological methods, including biotesting and bioindication, are an important complement to chemical analyses, allowing the assessment of the impact of HM on biota. An integrated approach studying the pollution of aquatic ecosystems includes the determination of metal concentrations in water, bottom sediments and hydrobionts, the analysis of their sources, the study their migration and accumulation mechanisms, as well as the assessment of potential risks to human health and ecosystem sustainability.
The technique of metal determination in water samples and bottom sediments using a flame atomic absorption spectrometer includes several steps [13].
Sample preparation of hydrobionts: The selected samples were chopped (with a knife made of inert material to avoid contamination) and dried at 60-105°C to remove moisture according to GOST 26929-94, ST RK ISO 8288- 2005 [18,19].
The 5g wet weight of samples of various environmental objects (Figure 2),
Figure 2: Sampling of hydrobionts.
biological materials (hydrobionts) placed in quartz cups were burned until the formation of gray or white residue (ash) by heat treatment of the sample in a heat chamber in the range of 450-550°C on the device Temos-EXPRESS. The complete destruction of disturbing organic substances by thermal treatment is combined with HNO3 (chemical pure) in different temperature regimes 50- 650°?, during the determination of metal concentration of toxic elements (Cd, Pb, Zn, Cu, etc.) by atomic-absorption spectrometry.
After the complete destruction of the organics, the ash was dissolved in a minimum volume of concentrated HNO? (the solution becomes transparent), the volume of cooled and filtered solution, was brought to 100 ml with bidistilled water [20,21].
Quality control and calibration:
State Standard Samples (SSS), registered in the Register of the State Measurement System of the Republic of Kazakhstan in different concentrations, are used for the construction of calibration graphs (r=0,99) for the spectrometric determination of metals:
- aqueous solution of copper ions (3K-1) SSS 7998-93 in concentrations CCu = 0.0125; 0.025; 0.05; 0.1 mg/L;
- aqueous solution of zinc ions SSS 7837-2000 in concentrations CZn = 0.0125; 0.025; 0.05; 0.1 mg/l mg/L;
- aqueous solution of lead ions (SSS 7012-93 (2K-1) in concentrations CPb = 0.0125; 0.025; 0.05; 0.1 mg/L;
- aqueous solution of cadmium ions (SSS 6690-93 (1K- 1) in concentrations ??d = 0,0125; 0,025; 0,05; 0,1 mg/L;
- aqueous solution of cobalt ions (SSS 7880-2001 (NK-EC) in concentrations CCo = 0.1; 0.15; 0.2; 0.5 mg/L;
- aqueous solution of nickel ions (SSS 7873-2000 (NK-EC) in concentrations CNi = 0.1; 0.15; 0.2; 0.5 mg/L.
State standard samples and calibration graphics are necessary for reliability of heavy metals content in water and bottom sediment samples.
Atomic absorption analysis:
The determination of HMs was done using the flame atomic absorption spectrometric method (ST RK ISO 8288-2005) on atomic absorption spectrophotometer AA- 7000 (Shimadzu company, Japan) operating on acetylene- air mixture [18]. The method is based on the property of metal atoms to absorb in the basic state light of certain wavelengths (Cu – 324.7 nm; Zn – 213.9 nm; Cd –228.8 nm; Pb – 283.3 nm; Co–240.7 nm; Ni–232.0 nm), which they emit in their initial activity.
The obtained atomic absorption analysis data were used to estimate metal content in water and bottom sediment samples.
RESULTS AND DISCUSSION
Importance and function of macrophytes
Macrophytes play a key role in the bioindication of aquatic ecosystems, since they are a habitat-forming component [22], which emphasizes the necessity of a detailed study of their floristic composition and dynamics under anthropogenic impact. These aquatic plants form an important link in the system of water self-purification, but they are highly sensitive to environmental changes. Although macrophytes have a certain resistance to short- term fluctuations in environmental conditions, their long-term changes may indicate the ongoing processes of anthropogenic transformation of water bodies. Thus, the study of the macrophyte community of aquatic ecosystems has a special significance in ecological monitoring studies, allowing us to identify trends in changes in the aquatic environment and to assess the impact of anthropogenic factors on the ecosystem in the long-term perspective.
The aquatic plants of Lake Markakol are mostly represented by macrophytes of air-water and submerged ecological groups: (lat.) Elodea canadensis, Potamogéton amphibious, Persicaria amphibia, Elodea canadensis), Potamogéton amphibious, Persicaria amphibia, Ceratophyllum demersum, and Myriophyllum aquaticum.
Macrophytes play both positive and negative roles in shaping water quality [23]. They are powerful agents of water purification from salts. At the same time, their abundant development leads to siltation, excessive development of periphyton, zooplankton and phytoplankton, contributing to the eutrophication of the water basin. In addition, because of decomposing in the fall-winter period, macrophytes also serve as a source of additional pollution of the aquatic environment. Air-water and submerged plants can grow under high dynamic loads where the maximum benthic speed is unable to move the soil. Plants with floating leaves are able to form phytocenoses in areas where benthic maximum speeds do not exceed 0.2m/s, but in conditions of increased nutrient content (such as nitrogen and phosphorus), which is typical for eutrophic water bodies, their rapid growth can cause negative effects.
Macrophytes, are the most important component of ecosystems of continental water bodies, especially in water bodies experiencing significant anthropogenic load, as it plays a major role in maintaining the biotic balance, participating in cleaning the water body from pollution by absorption. Therefore, aquatic and near-water plants can provide a very informative indicator of the degree of pollution in the ecosystem of a water body [24].
Considering the selective ability of macrophytes to absorb various substances, aquatic plants can be used as indicators of the presence of chemical substances in the aquatic environment [25]. However, plants show high resistance to short-term pollution outbreaks and can accumulate pollutants in their tissues in large quantities without visible functional changes. This applies most of all to HMs, which, unlike organic pollutants, are not able to degrade to safe forms [26]. Therefore, the content of HMs in the plants ash is an important characteristic of the state of ecosystem pollution.
The results of toxicological analysis of aquatic plants are considered in total amount: Elodea canadensis, Potamogéton amphibious, Persicaria amphibia, Ceratophyllum demersum, Myriophyllum aquaticum.
It is known that aquatic plants is the initial link in the trophic chain and performs the function of “biofilter”, absorbing heavy metals from water and bottom sediments [27]. The uptake and accumulation of metals by plants can be varied depending on the concentration of metals in water and the environmental conditions (pH, reduction potential, temperature).
Metal concentrations in macrophytes of Lake Markakol tributaries and the outflowing Kalzhyr River
According to the results of the analyses, copper concentration in aquatic plants from 8.8 to 14.8mg kg-1. High values are registered in the estuaries of the rivers Zhirelka – 14.8 mg kg-1, Matabai – 14.0mg kg-1, Topolevka and Tikhushka – 11.4 mg kg -1. The estuary of the Kalzhyr River is less vulnerable to pollution - 8.8 mg kg-1, indicating a moderate accumulation capacity of plants, apparently due to the outflowing nature of the river (Table 1).
Table 1: Heavy metal concentrations in macrophytes of Lake Markakol tributaries and of the outflowing Kalzhyr River.
|
River |
Cu |
Zn |
Pb |
Cd |
Ni |
Co |
|
mg kg-1 |
||||||
|
Urunhaika |
10.5 |
44.6 |
16.7 |
1.7 |
54.6 |
85.3 |
|
Matabai |
14.0 |
51.7 |
12.5 |
2.1 |
64.0 |
87.0 |
|
Kalzhyr |
8.8 |
36.0 |
17.7 |
0.0 |
47.2 |
88.6 |
|
Yelovka |
9.6 |
34.0 |
17.2 |
0.0 |
47.2 |
91.8 |
|
Zhirelka |
14.8 |
46.5 |
15.1 |
0.0 |
37.8 |
102 |
|
Topolevka |
11.4 |
54.0 |
17.2 |
0.0 |
30.3 |
93.4 |
|
Tikhushka |
11.4 |
52.1 |
14.6 |
0.0 |
32.2 |
88.6 |
Although there is no direct threat, the high bioavailability of copper may lead to its accumulation in organisms feeding on plants. In the long term, this may affect physiological processes in plants such as photosynthesis and respiration [27-29].
The average concentration of zinc in macrophytes is 45.6 mg kg-1, reaching high values up to 54.0 mg kg-1 in the Topolevka and Matabai rivers, up to 44.6 mg kg-1 in the Urunhaika and Zhirelka rivers, which indicates significant zinc accumulation and pollution of these rivers. Actively taken up by plants, zinc plays a key role in the metabolism of hydrobionts, but its excess can provoke toxic effects.
Lead concentrations in aquatic plants range from 12.5 to 17.7 mg kg-1, with a mean value of 15.9 mg kg-1. Compared to copper and zinc, lead has no biological role and being a highly toxic metal, it causes oxidative stress and damage to membrane structures in plants [30].
The content of cadmium is recorded in low values up to 1.7 mg kg-1 on the Urunhaika River and up to 2.1 mg kg-1 on the Matabai River. Cadmium was not detected in the remaining samples taken from the rivers Kalzhyr, Yelovka, Zhirelka, Topolevka and Tikhushka. The average cadmium values were 1.90 mg kg-1, which is low. However, cadmium can be easily absorbed by plants and cause serious toxic effects even in low concentrations, impairing growth, development and photosynthesis, inhibiting enzymatic systems [25].
Nickel content in high values is recorded in the rivers Kalzhyr, Elovka – 47.2 mg kg-1, Urunhaika – 54.6 mg kg-1 and Matabai – 64.0 mg kg-1. Nickel uptake by macrophytes in high concentrations (up to 44.8 mg kg-1 on average) negatively affects photosynthesis processes in plants, which in turn will affect the organisms feeding on aquatic plants [31].
High concentrations in aquatic plants were also characterized by cobalt, which amounted to 90.9 mg kg-1 on average. Maximum high concentrations were recorded in the Zhirelka River – 102mg kg-1, Topolevka River – 93.4 mg kg-1, Elovka River – 91.8 mg kg-1. Nickel and cobalt in aquatic plants in high concentrations, can cause inhibition of photosynthesis, accumulation of free radicals and damage to cellular structures [26].
Thus, aquatic plants regardless of their belonging to different ecological groups in their life process can accumulate elements in rather high concentrations, for example, zinc – 54.0 mg kg-1, nickel – 64.0 mg kg-1 and cobalt – 102 mg kg-1. The study of aquatic plants is a necessary component of water bodies monitoring, since components of the natural environment demonstrate different responses to anthropogenic interference. The ability of aquatic plants to accumulate heavy metals is the primary source of pollution for food organisms, increasing the risks of biomagnification. But, at the same time, they can be used for bioremediation of polluted water bodies, as they are able to extract metals from water and concentrate them in their biomass.
Thefoodorganismsis represented byvariousorganisms: zooplankton (lat. Zooplankton), macrozoobenthos (lat. Macrozoobenthos) and other invertebrates such as daphnia (lat. Daphnia) (genus of the family Daphniidae), rotifers (lat. Rotifera) (animal type), paddlefish (lat. Copepoda) (subclass of crustaceans) and branchiopod crustaceans (lat. Cladocera) (subclass of crustaceans), which consume aquatic plants and are important carriers of pollutants along the trophic chain.
These species of organisms directly absorb metals from water and plants, becoming a source of their accumulation in fish.
When assessing the content and accumulation of HMs in food organisms, consider their maximum permissible concentrations (MPC) in food products, including fish and seafood. According to SanPiN RK 2.3.2.1078-01 [20], in Kazakhstan, the content of HM in feed organisms is regulated by sanitary norms and rules established by the Ministry of Health, which determine the toxicity of these elements (in mg kg-1wet weight):
- Copper (Cu): 10.0 mg kg-1;
- Zinc (Zn): 40.0 mg kg-1;
- Lead (Pb): 1.0 mg kg-1;
- Cadmium (Cd): 0.2 mg kg-1;
- Nickel (Ni): 0.5 mg kg-1;
- Cobalt (Co): 0.1 mg kg-1.
The accumulation of HMs in aquatic prey organisms is a significant environmental problem affecting the health of ecosystems and the safety of food chains. Aquatic food organisms, such as zooplankton, macrozoobenthos, paddlefish and branchiopods, are the main links in food chains, so their bioaccumulation ability plays a key role in the transfer of toxic elements to higher trophic levels. HMs become accumulated in the tissues of hydrobionts through absorption from water and food. Studies [32,33] show that such species as daphnia are able to accumulate metals in significant amounts, which leads to bioconcentration in their organisms. Depending on the chemical properties of metals and environmental conditions, their toxic effects on hydrobionts are manifested in the disruption of enzymatic activity, damage to cells and tissues, reduced reproductive function and increased mortality [34]. For example, cadmium and lead have direct toxic effects on metabolism, while copper and zinc can impair osmoregulation and respiratory processes [35].
The accumulation of metals in hydrobionts has a significant impact on ecosystems as a whole. Biomagnification of HMs by trophic levels leads to their accumulation in fish and other aquatic animals, which poses a risk to humans when consuming them for food. Studies conducted by Galatova EA et al. [36], describe the mechanism of metal intake through feed and their further distribution in the food chain. The research by Gadzhieva SR et al. [37], emphasizes the importance of monitoring the content of toxic elements in feed organisms to assess the state of ecosystems and identify sources of pollution. They emphasize too, that HM concentrations in organisms of hydrobionts depend on the level of environmental pollution and species affiliation of organisms. In addition, the study of Gadzhieva SR et al. [37], noted that the bioconcentration of metals in zooplankton is related to the chemical characteristics of elements and their solubility in water.
The direct toxic effect of metals on aquatic organisms is associated with the presence of these metals in ionic forms. The toxicity of elements for hydrobionts is sometimes higher by several orders than for terrestrial animals, and especially increases in low saline waters [38].
The results of the toxicological analysis of heavy metal concentrations in the food base show a significant exceedance of the maximum permissible concentrations (MPC) for all studied elements in both the eastern (up to 5m) and western (up to 24m) sections of the lake. Samples were taken considering the differences in lake depth and river inflows, which influence the distribution of pollutants. Comparing the two sections of the lake in terms of metal concentrations, certain differences were identified. In the eastern shallower section of the lake, were the Topolevka, Zhirelka, Tikhushka, and Urunhaika rivers flow into the lake, higher concentrations of copper, zinc, lead, and cobalt were recorded (Table 2).
Table 2: Heavy metal concentration in feed organisms of Lake Markakol (in mg kg-1).
|
Lake area |
Cu |
Zn |
Pb |
Cd |
Ni |
Co |
|
Western section |
20.9 |
45.2 |
15.1 |
1.0 |
49.0 |
96.7 |
|
Eastern section |
14.8 |
39.6 |
14.6 |
0.0 |
50.9 |
87.0 |
|
MPC |
10.0 |
40.0 |
1.0 |
0.2 |
0.5 |
0.1 |
The copper content in the western section of the lake is 20.9 mg kg-1, and in the eastern section – 14.8 mg kg-1, which exceeds the established MPC (10 mg kg-1) by 2.09 and 1.48 times, respectively. The zinc concentration in the western section reaches 45.2 mg kg-1, which exceeds the MPC (40 mg kg-1) by 1.13 times, while in the eastern section the content of this metal is slightly lower – 39.6 mg kg-1, almost corresponding to the standard (0.99 of the MPC). According to the authors [39], copper ions are the most toxic to daphnia, and at a concentration of 0.03 mg/L, they suppress all mentioned functions, so at levels of 17.9 mg kg-1, they become lethal and critical. A similar situation was observed with zinc.
Lead concentrations in the western and eastern sections of the lake are similar in value – 15.1 and 14.6 mg kg-1, respectively, which in both cases exceeds the standard by almost 15 times. Cadmium was detected only in the western section of the lake at a concentration of 1.0mg/ kg, which exceeds the MPC (0.2 mg kg-1) by 5 times, and in the eastern section it was not detected. The most critical excess of standards is observed for nickel and cobalt, the content of which is very high in both the western and eastern sections of the lake. The concentration of nickel in the western section is 49.0 mg kg-1, and in the eastern section it is even higher (50.9 mg kg-1), which exceeds the established MPC by 98.0 and 102 times, respectively. The content of cobalt reaches extremely high levels: in the western section its concentration is 96.7 mg kg-1, and in the eastern section – 87 mg kg-1, which exceeds the standard value (0.1 mg kg-1) by almost a thousand times.
The obtained data indicate a high degree of contamination of the food organisms by HMs, which is probably due to both natural and anthropogenic factors. Main sources of metal intake can be caused by processes of rock weathering and soil pollution in the catchment, while the contribution of agricultural and industrial discharges, based on the volumes, is less significant [40]. The most significant excess was recorded for cobalt, which may indicate the natural geochemical background in the rocks of the region, contributing to its high content in the environment. Since the main processes controlling the forms of metal migration in natural waters are adsorption on mineral and organic particles. The form of migration of these metals depends on the physical and geographical features of the catchment area. The suspended form of copper is most often transported by mountain river. It migrates in surface waters in a dissolved state up to 89.5%, while the proportion of suspended forms of zinc is on average 88.8% [41-44]. Increased concentrations of cobalt and nickel in the aquatic environment, especially their localization in deep bottom layers, may indicate various processes and factors affecting the structure of these metals in the lake, such as the geological structure and composition of surrounding rocks containing minerals rich in these metals, which can lead to their leaching into the water. In particular, rich deposits of cobalt-nickel ores in Eastern Kazakhstan, such as the Ni-Co ore deposits of Gornostaevskoye and Belogorskoye, with a Ni content (up to 1782g/t on average) and Co (up to 60g/t on average), have the opposite effect, contributing to natural and anthropogenic pollution of the ecosystems of water bodies in the eastern region [45-47].
However, considering the level of HM accumulation in aquatic organisms, it is necessary to explore in further studies the mechanisms of their migration and potential risks for trophic network of aquatic ecosystems.
The high level of HMs in the food organisms of aquatic ecosystems has a significant ecological impact. The accumulation of metals in the organisms of hydrobionts leads to bioconcentration and biomagnification along the trophic chain, which can affect their vital activity negatively, reducing reproductive capacity and increasing mortality. These processes pose a threat to the sustainability of ecosystems, contributing to the reduction of biodiversity and disturbance of ecological balance.
In addition, HMs accumulated in aquatic organisms pose a potential risk to human health. Their intake through consumption of fish and other aquatic bioresources can lead to long-term toxic effects, which emphasizes the need for comprehensive monitoring of both pollution of aquatic ecosystems and risk assessment for food security.
Fish, being at the upper level of the trophic chain, can accumulate the highest concentrations of HMs, which creates a potential risk for predators and humans consuming fish. The toxicological analysis of uskuch fish (lat. Brachymystax lenok), included muscle, liver and gills [48,49]. The samples included two adult specimens as well as four fry of this species. One adult specimen was sampled frozen, while the second specimen and fry species were at the stage of decomposition on the water surface (4 specimens).
The uskuch fish is a freshwater fish, belonging to salmonides, that is a benthophage, with its main food consisting of benthic invertebrates such as insect larvae (e.g., Ephemeroptera, Trichoptera and Diptera), as well as small crustaceans and other organisms living on the bottom of water bodies. Although this fish also can eat small fish and terrestrial insects, its basic diet is centered on benthic organisms [50].
The results of the analysis of HM concentrations in different organs of the uskuch fish, including fry, revealed significant differences in the levels of accumulation due to physiological features of tissues and their functions. The highest concentrations of metals were recorded in metabolically active organs such as liver and gills, which is associated with their role in detoxification and metabolism.
Concentrations of copper, zinc, lead, nickel and cobalt significantly exceed the maximum permissible concentrations (MPC), indicating pronounced pollution of the aquatic environment and increased accumulation of metals in hydrobionts (Table 3).
Table 3. Heavy metal concentrations in uskuch fish (Brachymystax lenok).
|
Fish |
Organs |
Cu |
Zn |
Pb |
Cd |
Ni |
Co |
|
mg kg-1 |
|||||||
|
Fry |
- |
13.1 |
42.1 |
17.7 |
0.0 |
28.5 |
98.3 |
|
Uskuch (found decomposed) |
Muscles |
15.7 |
120 |
17.7 |
7.5 |
37.8 |
102 |
|
Gills |
20.9 |
1280 |
17.2 |
2.9 |
32.2 |
87.0 |
|
|
Liver |
12.2 |
731 |
16.2 |
1.7 |
34.1 |
91.8 |
|
|
Uskuch (frozen) |
Muscles |
18.8 |
46.6 |
12.8 |
0.0 |
37.9 |
104 |
|
Gills |
19.2 |
227 |
14.4 |
0.0 |
55.6 |
100 |
|
|
Liver |
21.8 |
372 |
12.5 |
0.0 |
35.0 |
111 |
|
These results demonstrate the high level of anthropogenic impact on the ecosystem and the need for further monitoring, taking into account the potential risks to human health when consuming fish that has accumulated toxic elements.
In the fry tissues, metal levels are lower than in adult fish, which may be due to a shorter life span and a lower time period for metal accumulation. However, zinc and cobalt concentrations in fry already exceed MPC by dozens of times, indicating early exposure to pollution.
Muscles of decomposing fishes show elevated levels of metals, especially zinc at 120 mg/kg-1 and nickel at 37.8mg kg-1, which may be related to metabolic disturbances leading to altered distribution of toxic substances. The gills also show particularly high zinc concentration of 1280mg kg-1, which is attributed to their function of water filtration and direct contact with the polluting environment. In contrast, the liver shows high concentrations, especially of zinc–731mg kg-1, reflecting the accumulation ability of this organ. Literature reviews [51,52] showed that HMs are accumulated in different organs of fish selectively, and the most intensive accumulation of elements occurs in scales and gills, i.e., in organs in direct contact with water.
In thefrozen fish taken foranalysis, metal concentrations in organs are lower than in decomposing fish, which confirms the toxic effect of HMs as a factor contributing to mortality. The highest concentrations of zinc are recorded in the gills up to 227 mg kg-1, which is associated with their role in gas exchange and osmoregulation. In the liver there is also a significant accumulation of copper up to 21.8 mg kg-1, zinc up to 372 mg kg-1, reflecting the importance of this organ in detoxification [53].
The lead concentration in fish tissues, in values from 12.5 to 17.7 mg kg-1, exceeds the MPC by ten times. The highest levels are observed in muscles and gills of decomposing fish, which may be related to metabolic disturbance after the death of the organism and redistribution of the metal in tissues, indicating the significant effect of lead on the main vital functions of fish, including respiration and metabolism [54,55].
Cadmium was detected only in the tissues of decomposing fish at concentrations of up to 7.5 mg kg-1 in muscle and up to 1.7 mg kg-1 in liver, which exceeds the MPC by more than 30 times. Cadmium in muscle indicates high toxicity and the ability of cadmium to penetrate muscle tissues despite their low level of metabolic activity, which also emphasizes the potential risk of the most dangerous metals for ecosystems [53,56].
Nickel in fish tissues ranges from 28.5 mg kg-1 in fry to 55.6 mg kg-1 in the gills of adult fish, exceeding MPC more than 100 times. Maximum concentrations are recorded in gills, which is explained by their constant contact with water and filtration function. High concentrations of nickel in the liver up to 37.9 mg kg-1 reflect its role in the accumulation and detoxification of toxic substances [57].
Cobalt was founded in the highest concentrations of all metals, reaching 111 mg kg-1 in frozen fish liver, exceeding the MPC by more than one thousand times. High levels of cobalt in all tissues studied emphasize its
strong accumulation and significant environmental threat. Especially noticeable is its effect on gills up to 100 mg kg-1, which may be associated with respiratory organ disorders [58].
Exceeding MPC of metals, poses a threat to the ecosystem and human health through food chains. High concentrations of metals impair the function of fish organs, leading to toxic effects such as cell damage, reduced reproductive capacity and increased mortality [59-63].
To conduct a scientific analysis of the system “surface water layer–bottom water layer–bottom sediments”, it is necessary to consider the pathways of migration and accumulation of heavy metals in various components of the ecosystem, as well as their impact on living organisms.
The system “surface water layer – bottom water layer – bottom sediments” is a dynamic interconnected structure where heavy metals migrate between components, accumulate in different media and affect the ecosystem. The distribution of metals is determined by their chemical properties, solubility, ability to sorption, migration and bioaccumulation, also in the cycles of biogeochemical processes.
The gradient distribution of HM concentraions in the “system” shows the following trend:
surface water layer: Co > Ni > Pb > Cu > Zn > Cd;
benthic water layer: Co > Ni > Pb > Pb > Cu > Cu > Zn= Cd;
bottom sediments: Zn > Co > Pb > Cu > Cd > Ni;
macrophytes (hydrophytes): Co > Zn > Ni > Pb > Cu >Cu > Cd;
food organisms: Co > Ni > Zn > Cu > Cu > Pb > Cd; fish: Zn > Co > Ni > Cu > Pb > Cd.
In the surface water layer, the highest concentrations are observed for cobalt and nickel, which may be due to their high mobility in water and lower tendency to precipitate. They are followed by lead and copper, which may be partially precipitated or bound to organic matter. Low concentrations of zinc and cadmium are explained by their more active participation in the processes of migration to deeper layers or by their binding to particles.
The leading role of cobalt and nickel in the bottom water layer remains, which indicates their continued migration from the surface layer. Lead and copper occupy intermediate positions, possibly due to sorption processes
on sediment particles. Zinc and cadmium are present in equal concentrations, reflecting their partial deposition from the water mass.
In bottom sediments, the highest concentration is characteristic of zinc, which is actively sorbed on mineral particles and organic matter, as well as cobalt and lead, which have a high capacity for accumulation in sediments. Copper, cadmium and nickel are less represented, which may be related to their lower ability to bind in anaerobic conditions of bottom layers.
Cobalt continues to dominate in macrophytes, reflecting its biological importance and high availability to aquatic plants. Zinc and nickel, also accumulated in significant amounts, are involved in physiological processes of plants. The lower concentrations of copper, lead and cadmium may be due to their limited bioavailability or competition with other metals for uptake.
Cobalt continues to dominate in the hydrobiont food organisms, indicating its active inclusion in trophic chains. Nickel and zinc also have high concentrations due to their ability to accumulate in microorganisms and invertebrates. Copper, lead and cadmium are present in lower amounts, indicating lower trophic availability or lower levels of their inputs.
In fish tissues, the highest concentration is observed for zinc, which confirms its importance in biological processes and active accumulation at higher trophic levels. Zinc is followed by cobalt and nickel, showing a predisposition to bioaccumulation. Copper, lead and cadmium have a lower representation, which may be related to metabolic control or excretion mechanisms.
The interaction of the components of the “system” shows that cobalt, nickel and zinc play a key role in the migration and accumulation of heavy metals in the aquatic ecosystem. The high content of these metals in biota emphasizes their ecological significance and the need to control the sources of their inputs.
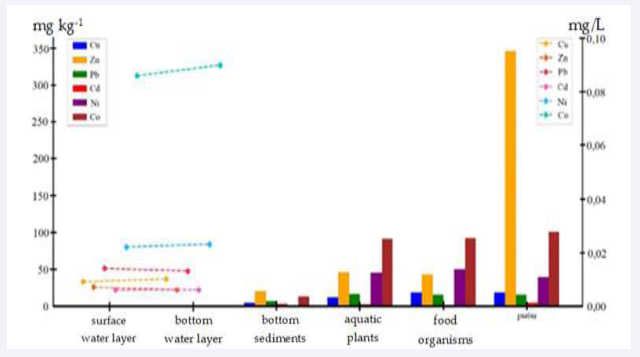
As can be seen from Figure 3,
Figure 3: Heavy metal distribution in different ecosystem components.
the surface water layer contains the lowest concentrations of metals for copper– 0.009mg/L, for zinc–0.007mg/L, which is associated with water dilution and active hydrodynamic processes. Bottom water layer has similar composition, but concentrations of some metals, such as cobalt –0.090mg/L, are slightly higher due to proximity to bottom sediments and low rate of vertical transport.
Bottom sediments serve as the main depot of HMs, where their concentrations are much higher, since due to sedimentation processes, they are transformed into insoluble forms, under the influence of anaerobic conditions or pH changes, and their secondary dissolution and migration back to the bottom water layer is possible.
Aquatic plants, being in contact with water and bottom sediments, can accumulate metals in concentrations exceeding their content in the aquatic environment, which makes plants an important link in the transfer of metals to the food organisms, including zooplankton and macrozoobenthos, where metal concentrations are also high. Fish, being at the top of the trophic chain, show a significant cumulative effect, especially for zinc–346mg kg-1 and cobalt –101mg kg-1, which is explained by long- term accumulation of metals through feeding.
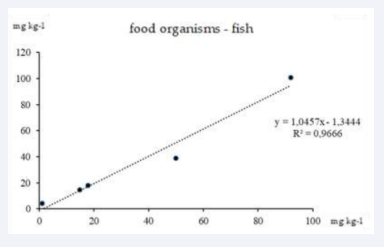
The analysis of correlation dependencies presented in the graphs (Figures 4-6) shows the relationship between ?M concentrations in abiotic and biotic components of the ecosystem.
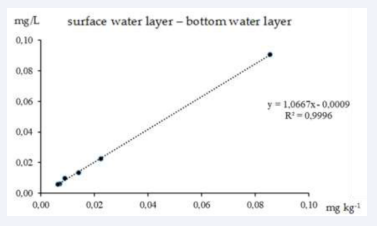
Figure 4: Correlation between overall heavy metals concentrations in surface and bottom water layers.
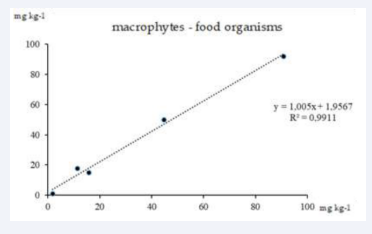
Figure 5: Correlation between overall heavy metals concentrations in macrophytes and food organisms.
Figure 6: Correlation between overall heavy metals concentrations in food organisms and fish.
These dependencies reflect the mechanisms of transfer and accumulation of pollutants, starting from the aquatic environment and ending with biological organisms at different levels of the food chain. The first graph (Figure 4) shows an almost perfect correlation between HM concentrations in surface and bottom water layers (R2=0.9996), indicating a close relationship between these layers. The high degree of correlation indicates that pollution entering the surface water layer is effectively transferred to the bottom layers, which may be due to the processes of vertical water circulation, adsorption of metals on suspended particles and their deposition on the bottom due to downward gravimetric movement and the charge-caused sorption on the negatively surplused humic bottom sediments.
The regression equation confirms that the concentration of metals in the bottom layer is in proportional dependence on their content in the surface layer, with a small deviation, which indicates the presence of sedimentation factors or the influence of bottom sediments.
The second graph (Figure 5) demonstrates the correlation between HM concentrations in macrophytes and fodder organisms (R2=0.9911), where the biological process of metal accumulation in primary products of the ecosystem (macrophytes) can be clearly traced. The linear regression equation indicates a directly proportional relationship, on the role of macrophytes in the transfer of pollutants to the next trophic level – forage organisms.
The third graph (Figure 6) shows the correlation relationship between HM concentrations in food organisms and fish (R2=0.9666). Despite the slightly lower value of the coefficient of determination compared to the other graphs, the correlation remains high, confirming a significant transfer of metals from one level of the trophic chain to another. The regression equation shows that the HM concentration in fish rises with increasing concentrations in food organisms, indicating a biomagnification effect in which metal concentrations increase at higher trophic levels.
In general, the data presented emphasize not only a relation between different lake layers, but also a high degree of interaction between abiotic and biotic environments, where HM concentrations in water directly determine their content in macrophytes, food organisms and ultimately fish. This mechanism reflects the characteristic processes of the biogeochemical cycle, including transport, deposition and bioaccumulation. High correlation values in all plots confirm the identified patterns, indicating strong interconnection between ecosystem components and active processes of pollutant transfer through food chains.
CONCLUSION
The results confirm a significant accumulation of heavy metals in the hydrobionts of Lake Markakol, which indicates a pronounced anthropogenic and natural load on the aquatic ecosystem. Analysis of food organisms, aquatic plants and fish tissues showed exceedance of maximum permissible concentrations (MPC) for a number of toxic elements, including copper, zinc, lead, nickel and cobalt. During the study, we found that macrophytes, which play an important role in the self-purification of water bodies, demonstrated the ability to accumulate heavy metals in significant amounts. In particular, the concentration of zinc, nickel and cobalt in aquatic plants reached 54.0 mg kg- 1, 64.0 mg kg-1 and 102 mg kg-1, respectively, indicating a high level of pollution in the aquatic environment.
The forage study revealed bioconcentration and biomagnification of metals, which is a threat to the ecosystem as a whole. Indicators of lead and cadmium concentrations and the accumulation in organisms at the lowest trophic levels (zooplankton, macrozoobenthos) exceed MPC 14.9 and 5 times, respectively. The highest concentrations were registered for nickel (50 mg kg- 1) and cobalt (91.9 mg kg-1), which indicates their active accumulation in aquatic organisms.
Toxicological analysis of the tissues of the uskuch fish (Brachymystax lenok) demonstrated the variability of metal accumulation depending on the functional features of organs. The highest concentrations were recorded in liver, gills and muscles, with nickel content reaching 55.6 mg kg-1, and cobalt – 111 mg kg-1, which exceeds MPC by tens and hundreds of times. Taking into account that metal concentrations in the tissues of fry were lower than in adults, the levels of zinc and cobalt already at the early stages of development exceeded the permissible norms by dozens of times.
Exceeding the MPC of heavy metals in hydrobionts indicates a high level of pollution in Lake Markakol, which may lead to unfavorable environmental consequences, including disruption of fish reproductive capacity, reduction of biodiversity and degradation of the aquatic ecosystem. Given the identified concentrations of toxic elements, there is a potential risk to human health through food chains when consuming contaminated aquatic organisms. The obtained results emphasize the need for regular monitoring of heavy metals in the hydrobionts of Lake Markakol and the environment for timely identification of pollution sources and development of measures to preserve the ecological sustainability of the water body.
ACKNOWLEDGMENTS
We acknowledge the support of the Science Committee of the Ministry of Education and Science of the Republic of Kazakhstan.
REFERENCES
- Geng N, Xia Y, Li D, F Bai, C Xu. Migration and Transformation of Heavy Metal and Its Fate in Intertidal Sediments: A Review. Processes. 2024; 12: 311.
- Briffa J, Sinagra E, Blundell R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon. 2020; 6: 9.
- Mitra S, Chakraborty AJ, Tareq AM, Emran TB, Nainu F, Ameer K et al. Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity. J King Saud University – Sci. 2022; 34, 3: 101865.
- Haidar Z, Fatema K, Shoily SS, Sajib AA. Disease-associated metabolic pathways affected by heavy metals and metalloid. Toxicology Reports. 2023; 10: 554–570.
- Malik DS, Sharma AK, Sharma AK, Thakur R, Sharma M. A review on impact of water pollution on freshwater fish species and their aquatic environment. Advances in Environmental Pollution Management: Wastewater Impacts and Treatment Technologies. 2020; 2.
- Skorbi?owicz M, Sidoruk M. Assessment of Heavy Metal Content and Identification of Their Sources in Bottom Sediments and Various Macrophyte Species of the Narew River (Poland). Minerals. 2025; 15: 8.
- Saravanan P, Saravanan V, Rajeshkannan R, Arnica G, Rajasimman M, Baskar G, et al. Comprehensive review on toxic heavy metals in the aquatic system: sources, identification, treatment strategies, and health risk assessment. Env Res. 2024; 258: 119440.
- Biedunkova O, Kuznietsov P. Dataset on heavy metal pollution assessment in freshwater ecosystems. Sci. 2024; 11: 1241.
- Anishchenko OV, Gladyshev MI, Kravchuk ES, Sushchik NN, Gribovskaya IV.Distribution and migration of metals in trophic chains of the Yenisei ecosystem near Krasnoyarsk City. Water Resour. 2009; 36: 594–603.
- Jaishankar M, Tseten T, Anbalagan N, Blessy B. Mathew, Krishnamurthy N. Beeregowda. Toxicity, mechanism and health effects of some heavy metals. Interdisciplinary Toxicology. 2014; 7: 60–72.
- Zuliani T, Vidmar J, Drin?i? A, Janez Š?an?ar, Milena Horvat, Marijan Ne?emer, Marina Piria, et al. Potentially toxic elements in muscle tissue of different fish species from the Sava River and risk assessment for consumers. Sci Total Environ. 2019; 650: 958–969.
- Rajeshkumar S, Li X. Bioaccumulation of heavy metals in fish species from the Meiliang Bay, Taihu Lake, China. Toxicology Reports. 2018; 5: 288–295.
- Madibekov A, Ismukhanova L, Amirgaliev, N, et al. Ecological sustainability of Lake Markakol under anthropogenic impact. Monograph. Almaty: Smart University Press. 2024: 278. ISBN 978-601-08-4760-6 (In Rus.)
- Alam M, Rohani MF, Hossain MS. Heavy metals accumulation in some important fish species cultured in commercial fish farm of Natore, Bangladesh and possible health risk evaluation. Emerging Contaminants. 2023; 9, 4: 100254.
- Madibekov A, Ismukhanova L, Zhadi A, Sultanbekova B, Zhumatayev S, Madibekova A. Assessment of the Level of Pollution of the Aquatic Ecosystem of Lake Markakol with Mobile Forms of Copper and Zinc. Evergreen. 2024; 11: 1568–1579.
- Ismukhanova L, Madibekov A, Opp C, Askhat Z, Botakoz S, Serik Z. Status and Migration Activity of Lead, Cobalt and Nickel in Water and in Bottom Sediments of Lake Markakol, Kazakhstan. Appl Sci. 2024; 14: 7487.
- Madibekov A, Ismukhanova L, Zhadi A, Sultanbekova B, Zhumatayev S, Karimov A, et al. Assessment of lake Markakol’s physical and chemical condition with consideration of eutrofication. Central Asian Journal of Water Research. 2024; 10: 58–78.
- GOST 26929-94 Raw materials and food products. Sample preparation. Mineralization for determination of toxic elements content.
- ST RC ISO 8288-2005. Water quality. Determination of cobalt, nickel, copper, zinc, cadmium and lead. Flame atomic absorption spectrometric methods (ISO 8288:1986). Committee on Technical Regulation and Metrology of the Ministry of Industry and Trade of the Republic of Kazakhstan. Astana. 2005: 23.
- Sanitary rules “Sanitary-epidemiologic requirements for public catering facilities. Order of the Minister of Health of the Republic of Kazakhstan from February 17, 2022 ? KR DSM-16. Registered with the Ministry of Justice of the Republic of Kazakhstan on February 21, 2022; 26866.
- Technical Regulations of the Eurasian Economic Union “On the Safety of Fish and Fish Products”. The decision of the Council of the Eurasian Economic Commission of October 18, 2016; No 162.
- Vinokurova NV, Solovykh GN, Donskova SA. Macrophyte structure of the formation of ecosystems of the Ural River. Bulletin of the Orenburg State University “Problems of ecology of the Southern Urals”. 2015; 10: 108–111.
- Loginova EVV, Lopukh PS. Hydroecology: a course of lectures. Minsk: BSU. 2011: 300. (In Rus.)
- Kochetkova AI. About some regularities of heavy metals accumulation by higher aquatic vegetation on the Volgograd reservoir. Bulletin of Volgograd State University. Ecology. Biology. 2012; 1: 305–309. (In Rus.)
- Lychagin NYu. Biochemistry of macrophytes in aquatic landscapes. GIS of the Astrakhansky Reserve. Geochemistry of landscapes of the Volga delta. Moscow: Geogr. Fact. Moscow State University. 1999; 3: 141–164. (In Rus.)
- Shashulovskaya EA. On the accumulation of heavy metals in the higher aquatic vegetation of the Volgograd reservoir. Volga Ecological Journal. 2009, 4: 357–360. (In Rus.)
- Shabbir Z, Sardar A, Shabbir A, Abbas G, Shamshad S, Khalid S, et al. Copper uptake, essentiality, toxicity, detoxification and risk assessment in soil-plant environment. Chemosphere. 2020; 259.
- Cao Q, Steinman AD, Wan X, Xie L. Combined toxicity of microcystin- LR and copper on lettuce (Lactuca sativa L.). Chemosphere. 2018; 206: 474–482.
- Ameh T, Sayes CM. The potential exposure and hazards of copper nanoparticles. A review. Environmental Toxicology and Pharmacology. 2019; 71.
- Gupta M, Dwivedi V, Kumar S, Patel A, Niazi P, Yadav VK. Lead toxicity in plants: mechanistic insights into toxicity, physiological responses of plants and mitigation strategies. Plant signaling & behavior. 2024; 19(1).
- Ahmad MS, Ashraf M. Essential roles and hazardous effects of nickel in plants. Reviews of environmental contamination and toxicology. 2011; 214: 125–167.
- Shilova NA, Rogacheva SM, Gubina TI. Influence of biogenic metals on the life activity of Daphnia magna. Izvestia Samara Scientific Center of the Russian Academy of Sciences. Environmental Monitoring. 2010; 1: 1951–1953. (In Rus.)
- Shashkova TL, Grigorieva YS. The effect of heavy metals on the trophic activity of daphnia depending on the feeding conditions and age of the crustaceans. Siberian Ecological Journal. 2013; 6: 885–890. (In Rus.)
- Silkina ENN, Silkin YuA, Silkin MYu, Stolbov AYu, Silkina AYu. Effects of heavy metals on functional and biochemical indicators of marine hydrobionts as bioindicators of the ecological state of the environment. Modern Problems of Science and Education. 2016; 6:120. (In Rus.)
- Polistovskaya PA. Physiological mechanisms of fish adaptation to heavy metals. FGBOU VO “St. Petersburg State University of Veterinary Medicine”. St. Petersburg. 2021: 115. (In Rus.)
- Galatova EA. Peculiarities of accumulation and distribution of heavy metals in the system of water, bottom sediments, hydrobionts: on the example of the river Uy. FGOU VPO “Ural State Academy of Veterinary Medicine”. Ekaterinburg. 2007: 191. (In Rus.)
- Gadzhieva SR, Rustamova UNN, Alieva TI, et al. Heavy metals in aquatic ecosystems as an indicator of anthropogenic impact. Young Scientist. 2019: 115–118. (In Rus.)
- Makarenko TV. Distribution of heavy metals in biotic and abiotic components of aquatic ecosystems of Gomel and adjacent territories. Minsk: (b. i.). 2010: 28. (In Rus.)
- Cabral-Lares M, Rentería-Villalobos M, Mendieta-Mendoza A, Ortíz-Caballero Z, Montero-Cabrera E, Vioque I. Partitioning and Availability of Metals from Water Suspended Sediments: Potential Pollution Risk Assessment. Water. 2022; 14: 980.
- Said M, Shah MT, Khan S. Health risk assessment of heavy metals and their source apportionment in drinking water of Kohistan region, northern Pakistan. Microchem. J. 2011; 98: 334–343.
- Hart BT, Davies SH. Trace metal speciation in three Victorian Lakes. Ibid. 1981, 2. pp. 175–189.
- Pic AJ, Eckert JM, Williams KL. Speciation of iron, copper and zinc in the Hawkesbry River. Austr. Journ. Mar. Freshwater Res. 1982; 33, 6: 971–977.
- Rocha JC, Desene JJ, Dossantos A, Toscano IAS, Zara LF. Aquatic humus from an unpolluted Brazilian dark brown stream – general characterization and size fractionation of bound heavy metals. J. Environ. Monit. 2000; 2, 1: 39–44.
- Gordeev VV, Lisitsyn AP. Microelements. Ocean Chemistry. M.: Nauka. 1979; 1: 337–375. (In Rus.)
- Kozhakhmetov SM, Kwiatkowski SA, Sadykov SB, et al. Smelting of cobalt-nickel oxidised ores of Gornostayevskoye deposit for Ferronickel. Metallurgy. Complex utilization of mineral raw materials. 2015; 1: 25–30. (In Rus.)
- Amralinova BB. Laws of formation and assessment of prospects of nickel-cobalt weathering crusts of East Kazakhstan. Dissertation for the degree of Doctor of Philosophy (PhD) on speciality 6D070600 – Geology and Exploration of Mineral Deposits. Ust-Kamenogorsk. 2017: 145. (In Rus.)
- Production of nickel-cobalt products in the Republic of Kazakhstan. Astana. 2015: 113. (In Rus.)Moshkin NV. Heavy metals in organs and tissues of Siberian grayling r. Yenisei. Youth and Science: Collection of materials of the VII All-Russian scientific and technical conference of students, postgraduates and young scientists dedicated to the 50th anniversary of the first manned space flight [Electronic resource]. Krasnoyarsk: Siberian Federal University, 2011. (In Rus.)
- Popov PA, Androsova NV. The content of heavy metals in the muscle tissue of fish from reservoirs of the Ob River basin. Bulletin of Tomsk State University. Biology. 2014; 4(28): 108-122. (In Rus.)
- Bogutskaya NG, Naseka AM. Cyclostomata and fishes of Khanka Lake drainage area (Amur River basin). An annotated check-list with comments on taxonomy and zoogeography of the region. Zool. Inst. Russ. Acad. Sci. 1996: 89.
- Rodríguez G, Castañeda-Chávez MDR, Lango-Reynoso F. Geoacumulation of Heavy Metals in Sediment of the Fluvial-Lagoon- Deltaic System of the Palizada River, Campeche, Mexico. International Journal of Environmental Research and Public Health. 2020; 17: 969. https://doi.org/10.3390/ijerph17030969
- Ju?kiewicz W, Gierszewski P. Toxic metal pollution of aquatic ecosystems of European Union nature protection areas in a region of intensive agriculture (Lake Gop?o, Poland). Aquatic Sciences. 2022; 84: 52.
- Besser JM, Mebane CA, Mountet DR, Ivey CD, Kunz JL, Greer IE, et al. Sensitivity of mottled sculpins (Cottus bairdi) and rainbow trout (Onchorhynchus mykiss) to acute and chronic toxicity of cadmium,copper, and zinc. Environmental Toxicology and Chemistry. 2007; 26: 1657–1665.
- Shah N, Khan A, Ali R, Marimuthu K, Uddin MN, Rizwan M, et al. Monitoring Bioaccumulation (in Gills and Muscle Tissues), Hematology, and Genotoxic Alteration in Ctenopharyngodon Idella Exposed to Selected Heavy Metals. BioMed research international. 2020: 17 April.
- Ghaffar A, Hussain R, Aslam M, Abbas G, Khan A. Arsenic and urea in combination alters the hematology, biochemistry and protoplasm in exposed rahu fish (Labeo rohita) (Hamilton, 1822). Turkish Journal of Fisheries and Aquatic Sciences. 2016; 16, 2: 289–296.
- Davidova S, Milushev V, Satchanska G. The Mechanisms of Cadmium Toxicity in Living Organisms. Toxics. 2024; 12: 875.
- Bozorgzadeh P, Shamsaie Mehrgan M, Pourang N, et al. Effects of nickel on liver and bone metabolic functions, biochemical and histopathological responses in common carp (Cyprinus carpio). Iranian Journal of Fisheries Sciences. 2023; 22: 526–546.
- Hantoush A, Al-Najare G, Amteghy A, Al-Saad H, Abd Ali K, et al. Seasonal variations of some trace elements concentrations in Silver Carp Hypophthalmichthys molitrix Consolidated from farms in central Iraq. Marsh Bulletin. 2012; 7: 126–136.
- Jamil Emon F, Rohani MF, Sumaiya N, Jannat F, Akter Y, Shahjahan MD, et al. Bioaccumulation and bioremediation of heavy metals in fishes. A review. Toxics. 2023; 11: 510.
- Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ. Heavy metal toxicity and the environment. Mol. Clin. Environ. Toxicol. 2012; 3: 133–164.
- Ali MM, Ali ML, Proshad R, Islam S, Rahman Z, Kormoker T. Assessment of trace elements in the demersal fishes of a coastal river in Bangladesh: a public health concern. Thalassas: Int J Mar Sci. 2020; 36: 641–655.
- Wahiduzzaman M., Islam MM, Sikder AHF, Parveen Z. Bioaccumulation and heavy metal contamination in fish species of the Dhaleswari River of Bangladesh and related human health implications. Biol Trace Elem Res. 2022; 200: 3854–3866.
- Santhosh K, Kamala K, Pasiyappazham R, Musthafa MS, Almujri SS, Basheeruddin Asdaq SM, et al. Unveiling the silent threat: Heavy metal toxicity devastating impact on aquatic organisms and DNA damage. Marine Pollution Bulletin. 2024: 200.