Comparative Characteristics of the Ultrastructure of the Pyramidal Neurons of the Parietal Cortex in Cerebral Ischemia of Varying Severity
- 1. Grodno State Medical University, Grodno, Republic of Belarus
Abstract
Introduction: The ultra-structural characteristics of neuron organelles are important indicators of the degree of brain damage during ischemic exposure, which necessitates the study of changes in the ultrastructure of brain neurons.
Methods: Models of subtotal, stepwise subtotal, partial and total cerebral ischemia were carried out on 24 outbred white male rats. Electron microscopy methods were used.
Results: The data obtained indicate that with total cerebral ischemia the disturbances are more pronounced than with partial cerebral ischemia and stepwise subtotal cerebral ischemia.
Conclusion: The data obtained, due to their novelty and relevance; represent a fundamental basis for further studies of neurons in various parts of the brain during cerebral ischemia, with subsequent implementation of the results into clinical practice.
Keywords
• Subtotal Ischemia
• Neuron Ultrastructure’s
• Parietal Cortex
CITATION
Bon E, Maksimovich N, Zimatkin S, Ostrovskaya O, Kokhan N (2023) Comparative Characteristics of the Ultrastructure of the Pyramidal Neurons of the Parietal Cortex in Cerebral Ischemia of Varying Severity. Ann Clin Cytol Pathol 9(1): 1147.
INTRODUCTION
With Cerebral Ischemia (CI), a chain of pathogen etic disorders develops in its structures, among which one of the leading ones is energy deficiency, which leads to the development of cellular pathology due to disturbances in homeostasis, enzyme activity, membrane integrity and energy pumps [1]. The ultra-structural characteristics of neuronal organelles are important indicators of the degree of brain damage during ischemic exposure, reflecting the severity of compensation, which necessitates the study of changes in the ultrastructure of brain neurons.
In the world literature, there are data according to which, with insufficient blood supply to the brain, a number of ultra structural disorders of the brain occur, namely: swelling of mitochondria and destruction of their cristae are observed in the cytoplasm of neurons, expansion of the cisterns of the endoplasmic reticulum and the Golgi complex, an increase in the number of free ribosomes that form extensive clusters in the cytoplasm. The total number of lysosomes increases, their sizes increase [1,2].
However, there are no data on the severity of these disorders depending on the type of ischemic injury and its severity. These studies, in our opinion, are relevant, as they will allow not only to study the nature of brain neuron disorders at the ultra-structural level, depending on the severity of ischemia, but also to assess the participation of compensatory mechanisms in preventing their occurrence [1,4,5]. This article describes the original stepwise cerebral ischemia model developed by the authors of the article. This model makes it possible to assess not only the severity of hypoxic neuronal damage, but also the degree of implementation of compensatory mechanisms, since, in our opinion; it is the compensatory mechanisms that should be given the most attention when detailing the pathogenesis of cerebral ischemia [2-5]. In this article, the aim was to study the organelles of pyramidal neurons in the field CA1 of the hippocampus of outbred white rats with stepwise subtotal cerebral ischemia.
Materials and Methods of Research
The experiments were carried out on 24 outbred male rats weighing 260 ± 20g. Permission to conduct the study was obtained from the ethical commission of the Grodno State Medical University (protocol No. 1 of 01/05/2022). Sodium thiopental was used for anesthesia; the solution was administered intravenously at a dose of 40 mg/kg. Total cerebral ischemia or TCI was modeled by decapitation of animals. The brain was collected 1 hour and 24 hours after decapitation. Subtotal cerebral ischemia or SCI was modeled by simultaneous ligation of both CCAs. The material was collected 1 hour and 24 hours after surgery. Partial cerebral ischemia or PCI was modeled by ligating one CCA on the right. The material was taken 1 hour after surgery. Stepwise subtotal cerebral ischemia or SSCI was performed by sequential ligation of both common carotid arteries with an interval of 7 days [6].
Six rats underwent only a sham operation; they constituted a sham group. The field CA1 of the hippocampus of beardless albino rats was selected for ultrastructural analysis. The rats were decapitated; the brain was removed from the skull. Immediately after the removal of the rat brain from the skull, the brain was cut along the median sagittal plane with a sharp blade, and the right half was used to take samples for electron microscopic examination. Fragments of the motor cortex about 1.0x1.0x2.0 mm in size, prismatic in shape, were taken from the anterior parietal region (at the level of slices 3-4), so that the long axis of the prism was perpendicular to the surface of the cortex and included all layers of gray matter, as well as adjacent to the latter a thin layer of white matter. When sampling the hippocampus, a slice about 1.5 mm thick was obtained from the right half of the brain in the frontal plane.
Samples were immersed in 1% osmium tetroxide solution (Osmiumtetroxid ≥ 99.95%, Carl Roth GmbH, Germany) in 0.1 M Milling’s buffer, pH 7.4, at + 40°C for 2 hours. After dehydration in ascending alcohols and acetone, the samples were embedded in Araldite resin (Araldite (Sigma-Aldrich Chemise GmbH, Germany)). During pouring, the cortical samples were oriented so as to obtain sections in a plane perpendicular to the surface of the cortex, the hippocampal samples were oriented so as to obtain sections of the CA1 nucleus in the sagittal plane. Semi thin (0.35 μm thick) and ultrathin (40 nm) sections were made on a Leica EM UC7 ultra microtome (Leica, Germany) using glass knives. The first sections were stained with methylene blue. Semi-thin sections of the cortex were used to select the area containing the cells of the inner pyramidal layer. At the same time, to obtain ultrathin sections, we chose the area containing the largest (in comparison with the rest of the neurons in the section) pyramidal neurons along with the thickest dendritic profiles running perpendicular to the cortical surface. To obtain ultrathin sections, only lateral sections containing the described neurons and characterized by satisfactory fixation were selected. At the same time, no more than 3 pyramidal neurons corresponding to the requirements could be found in this area at the same time. Therefore, to visualize a sufficient number of neurons, 5-6 series of ultrathin sections with an interval of 15 μm were obtained from a site selected in the described way, sharpened by a «pyramid». Each series (6-8 adjacent sections) was mounted on a separate copper grid.
From each sample of hippocampus embedded in resin, 3 series of ultrathin sections were obtained with an interval of 10 μm. Ultrathin sections were made with the microtome feed set to 40 nm (rarely 35 nm) and were gray or silver in color, indicating slice thicknesses of about 60 and 100 nm, respectively. All sections were counterstained with saturated urinal acetate and lead citrate according to Reynolds E.S. and studied under a JEM-1011 electron microscope (JEOL, Japan). To obtain images, a complex from an Olympus Mega View III digital camera (Japan) and the item program (Version 5.0 (Build 1224); Serial Number A3766900-7E852FAB) was used. On sections of the cortex, for the description and morphometric of mitochondria, neurons were selected that had a pyramidal shape with a clearly defined apical dendrite and a maximum transverse width of the perikaryon (usually the width at the base of the pyramidal perikaryon) of at least 15 μm, as well as a nucleus shape close to round or oval. Thus, neurons captured on one grid could not be counted again on adjacent grids [7,8]. Neuronal organelles and their parameters were studied using the Image Warp image processing program (Bit Flow, USA).
The data obtained by morphometric were processed using the Statistical 10.0 program for Windows (Stat Soft, Inc., USA) and presented as Me (LQ; UQ), where Me is the median, LQ is the value of the lower quartile; UQ is the value of the top quartile (since the experiment used small samples that had a non-normal distribution, the analysis was carried out using nonparametric statistics methods). Differences between groups were considered significant at p < 0.05 (Kruskal-Wallis test with Bonferoni correction) [9].
RESEARCH RESULTS
When studying the ultrastructure of neurons in the brain of rats with stepped cerebral ischemia (SSCI), the following data were obtained (Table 1). Giant mitochondria were found in the cytoplasm of rat hippocampal neurons in the 1st subgroup of SSCI with an interval between ligation of both common carotid arteries of 7 days. The number of free ribosomes in the cytoplasm of parietal cortex neurons increased by 67 (62.72)%, p < 0.05, compared to the values in the control group.
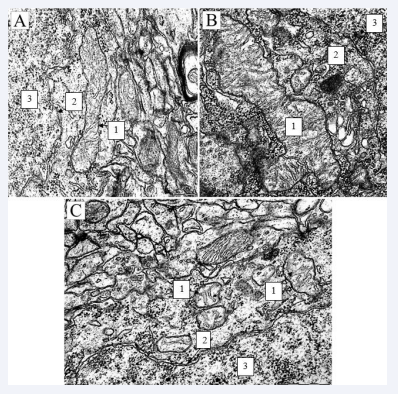
Disorganization and expansion of the cisterns of the Golgi complex were noted, the sizes of lysosomes did not differ from the sizes in the control group, however, their density in the cytoplasm of neurons of the parietal cortex of SSCI rats, compared with the indicators in the control group, was 50% more, p < 0.05. Compared to the parameters in the control group, in SSCI rats 1 day with an interval between CCA ligations of 1 day, in the parietal cortex, the mitochondrial elongation factor decreased by 55 (47.59)%, p < 0.05, while the forms -factor increased by 28 (23.34)%, p < 0.05. The density of mitochondrial cristae and their length were less by 42 (35.46)%, p < 0.05 and by 50 (46.55)%, p < 0.05, respectively. Compared with the indicators in SSCI with an interval between CCA ligations of 7 days, in SSCI rats 1 day with an interval between CCA ligations of 1 day, in the parietal cortex, the mitochondrial elongation factor decreased by 45 (35.47)%, p < 0.05, and form factor – increased by 26 (21.32)%, ? < 0.05. The density of mitochondrial cristae and their length decreased by 41 (35.47)%, p < 0.05 and by 67 (63.75)%, p < 0.05, respectively [Figure 1A,B].
Figure 1 Mitochondria of neurons in the parietal cortex of the control (1 - mitochondria; 2 - nuclear envelope; 3 -core) scale bar 0.5, rats×50000. Electron diffraction pattern; Mitochondria of neurons in the parietal cortex of SSCI (1 - mitochondria; 2 - nuclear envelope; 3 - core) scale bar 0.5, rats×50000 rats×50000. Electron diffraction pattern; Mitochondria of neurons in the parietal cortex of rats with 1-hour total cerebral ischemia (1 - mitochondria; 2 - nuclear envelope; 3 - core) scale bar 0.5, rats×50000. Electron diffraction pattern.
The number of free ribosomes in the cytoplasm of neurons in the parietal cortex of SSCI 1 day rate increased by 72 (67.79)%, p < 0.05, compared with the values in the control group. The number of free ribosomes in the cytoplasm of parietal cortex neurons in SSCI and SSCI 1 day rats did not differ (p > 0.05). The cisterns of the endoplasmic reticulum and the Golgi complex were vacuolated, the number of lysosomes was 75 (67.79)% more than in the control group - in the parietal cortex (p < 0.05) and 64 (53.69) %, area - by 91 (87.98)%, ? < 0.05. Compared with SSCI, the density of lysosomes in SSCI 1 day increased by 50 (47.59)% in the parietal cortex (p < 0.05), and their area increased by 91 (85.96)%, p < 0, 05.
The parameters of ultramicroscopic morphometric of organelles of parietal cortex neurons in SSCI rats with an interval between CCA ligations of 7 days were similar to those in PCI, except for a higher density of cristae in the mitochondria of the parietal cortex of SSCI rats (by 17 (11.23)%, p < 0.05), the number of free ribosomes in the parietal cortex (by 20 (15.28)%, p < 0.05) and the density of lysosomes in the parietal cortex (by 50 (46.54)%, p < 0.05). The number of free ribosomes in the cytoplasm of neurons in the parietal cortex of SSCI 1 day rats increased by 33 (27.38)%, p < 0.05, compared with the indices in the PCI group. In SSCI 1 day, the density of lysosomes increased by 75 (68.79)% in the parietal cortex (p < 0.05), and the area increased by 91 (87.98)%, p < 0.05. At 1-hour TCI, in comparison with the indicators in the control group, in the parietal cortex, the area of mitochondria decreased by 58 (52.63)%, p < 0.05, the elongation factor (elongation of the organelle) - by 63 (59.69 )%, p < 0.05, and the form factor (roundness of the organelle) increased by 28 (22.33)%, p < 0.05, which indicates rounding of mitochondria in the studied structures (Figure 1C).
The density of mitochondrial cristae and their length decreased in the parietal cortex by 40 (36.45)%, p < 0.05 and by 58 (52.61)%, p < 0.05, respectively. Compared with the indicators in the control group, the number of free ribosomes at 1-hour TCI in the parietal cortex increased by 71 (66,75)%, p < 0.05. The ratio of the number of bound and free ribosomes decreased from 3.43 (2.83; 4.12) in the control group to 0.11 (0.04; 0.15) in the parietal cortex, p < 0.05. There was a significant decrease in the density of lysosomes. At 1-hour TCI, only single lysosomes were observed in the cytoplasm of parietal cortex neurons (p < 0.05). Compared with the indicators in the control group, with 1-hour SCI, in the parietal cortex, the area of mitochondria did not change (p > 0.05), the elongation factor decreased by 53 (49.59)%, p < 0.05, and form factor - increased by 29 (22.32)%, p < 0.05, reflecting an increase in their sphericity. The density of mitochondrial cristae and their length in the parietal cortex decreased by 29 (27.33)%, p < 0.05 and by 50 (46.58)%, p < 0.05, respectively.
Compared with the indicators in the control group, with 1-day SCI in the parietal cortex, there was a decrease in the area of mitochondria by 58 (53.62)%, p < 0.05, the elongation factor - by 63 (58.67)%, p < 0.05 and an increase in the form factor by 31 (26.35)%, p < 0.05. The density of mitochondrial cristae and their length decreased by 40 (37.44)%, p < 0.05 and by 58 (52.63)%, p < 0.05, respectively. In addition, vacuolization of mitochondrial cristae occurred during 1-day SCI, as in TCI. Compared with TCI, with SCI lasting 1 hour, in the parietal cortex, the average area of mitochondria was greater by 39 (32.43)%, p < 0.05, the elongation factor was by 22 (18.26)%, p < 0.05. Changes in other indicators were of a similar nature (p > 0.05). Compared with the indicators in the control group, the number of free ribosomes at 1-hour SCI in the parietal cortex increased by 73 (67.77)%, p < 0.05. Unlike the TCI group, rats with SCI showed an increase in the density of lysosomes in the cytoplasm of neurons and their sizes.
Thus, compared with the indicators in the “control” group, in the “SCI 1 hour” group, the density of lysosomes in the cytoplasm of parietal cortex neurons increased by 76 (68.79)%, p < 0.05, and their area increased by 94 (89.97)%, p < 0.05. In PCI, compared with the values in the “TCI 1 hour” group, in the parietal cortex, the area of mitochondria was 59 (55.62)% larger, p < 0.05, the elongation factor was 62 (57.67)%, p < 0.05, and the form factor is 21 (16.28)% less, p < 0.05. At the same time, the density of mitochondrial cristae and their length per unit area were higher by 27(21.33)%, p < 0.05 and by 50 (47.58)%, p < 0.05, respectively. Compared with the values in the “SCI 1 hour” group, with PCI, in the parietal cortex, the elongation factor was higher by 51 (48.57)%, p < 0.05, while the form factor was lower by 23 (20.28)%, p < 0.05. The density of mitochondrial cristae and their length were higher by 29 (21.33)%, p < 0.05 and by 50 (47.58)%, p < 0.05, respectively.
However, in the cytoplasm of neurons in the parietal cortex of rats with PCI, an increase in the number of free ribosomes was noted - by 58 (52.65)%, p < 0.05, which indicates the switching of protein synthesis to the cells’ own needs and underlies hyperchromia of neurons in PCI. The ratio of bound and free ribosomes decreased from 3.4 in the control group to 0.8 in the parietal cortex, p < 0.05. The number of free ribosomes during PCI in the cytoplasm of neurons of the parietal cortex was 29(21.33)% less, p < 0.05, compared to the values in the “TCI 1 hour” group, p < 0.05, and compared to the “SCI 1 hour” group - by 33 (27.39)%, p < 0.05. The ratio of bound and free ribosomes was greater by 87 (82.91)%, (p < 0.05) compared to the values in the “TCI 1 hour” group, and by 76 (72.81)%, p < 0.05. The density of lysosomes, compared with the group “SCI 1 hour” in the parietal cortex was 76 (73.79)% less, p < 0.05, and the area of lysosomes was 93 (87.99)% less, p < 0.05.
DISCUSSION
Cerebral ischemia, according to the literature, causes disturbances in the energy exchange of neurons. At the ultrastructural level, this reflects mitochondrial edema and degradation of their cristae. We observed these changes during ligation of the common carotid arteries with a 7-day interval, the parameters of mitochondria did not differ from the control group. In addition, at the 7-day interval between dressings, giant mitochondria were observed as a sign of hypertrophic changes in neuronal organelles [10-17]. At the same time, with a 7-day interval between dressings, these ultra-structural changes were minimal, even hypertrophy of the cistern of the endoplasmic reticulum was noted. Thus, based on the above, a 7-day interval between ligation of the common carotid arteries was sufficient for the implementation of compensatory mechanisms, which were reflected at the ultra-structural level with the preservation of the normal structure of mitochondria and hypertrophy of synthetic transport organelles [2,18 -26].
Thus, with a interval between ligation of the common carotid arteries of 7 days, the number of mitochondria and their cristae did not differ from those in the group “control”, hyperplasia of the endoplasmic reticulum occurred, as a reflection of the activation of compensation mechanisms during hypoxia. There was not enough time to implement compensatory mechanisms.
Thus, changes in the ultrastructure of neurons in “SSCI”, “SSCI 1 day” were multidirectional: in “SSCI” with a maximum interval between CCA ligation of 7 days, the number of mitochondria and their cristae did not differ from those in the “control” group, hyperplasia of the endoplasmic networks, as a reflection of the activation of compensation mechanisms during hypoxia. As the time interval between CCA dressings decreased, the structure of organelles approached that observed with simultaneous SIGM lasting 1 day, indicating the insufficiency of switching on the compensation mechanisms in these methods of IGM modeling. However, there was an increase in the number of free ribosomes, disorganization and expansion of the cisterns of the Golgi complex. The sizes of lysosomes did not differ from the sizes in the control group, however, their average number in the cytoplasm of neurons increased.
As the time interval between CCA dressings for SSCI 1 day decreased, the structure of organelles was similar to that for SCI 1 hour, indicating insufficient inclusion of compensation mechanisms in these types of SSCI modeling. However, with 1-day SIGM, changes in the ultrastructure of parietal cortex neurons were similar to changes in parameters in the TCI group (p > 0.05). The number of free ribosomes in the “SCI” groups of 1 hour and in TCI rats did not differ. Also, with SCI, the number of lysosomes and their size increased, compared with the indicators in the control and TCI groups.
The mitochondria of rats with PCI did not differ in size and shape from the mitochondria of rats in the control group, except for a lower density of mitochondrial cristae in parietal cortex neurons. However, in the cytoplasm of neurons in the parietal cortex of rats with PCI, an increase in the number of free ribosomes was noted, but to a lesser extent than in SCI and TCI. The average number and size of lysosomes did not differ from those in the control group. When modeling PCI, blood circulation is compensated in the circle of Willis, which explains the smaller changes in the ultrastructure of neurons compared to “TCI” and “SCI”.
CONCLUSION
Thus, it has been established that the study of the structure of neurons in various parts of the cerebral cortex is important and promising, and makes it possible to make a comprehensive assessment of the state of the brain. As a result of qualitative and quantitative morphometric of neurons in the parietal cortex and hippocampus, it was revealed that the most profound changes in cerebral ischemia are observed with total ischemia (swelling of mitochondria, destruction of the brain, disorganization). Similar changes occur with subtotal cerebral ischemia, and the least pronounced disorders are observed with partial cerebral ischemia and subtotal stepped cerebral ischemia with an interval between ligations of the common carotid arteries of 7 days. The data obtained, in terms of their novelty and relevance; represent a fundamental basis for further studies of neurons in various parts of the brain during cerebral ischemia, with subsequent implementation of the results into clinical practice.
ACKNOWLEDGMENT
All authors meet the authorship criteria; funding: state research programs 4 «Translational medicine», subprogram 4.1 «Experimental medicine», research work 4.1.1 «To study the processes of damage and adaptation of the brain during its ischemia and the use of correction».
Conflict of Interest
The authors declare no conflict of interest. The requirements of the Directive of the European Parliament and of the Council No. 2010/63/EC of September 22, 2010 on the protection of animals used for scientific purposes (protocol attached) have been complied with. All authors gave their informed consent prior to their inclusion in the study.
REFERENCES
4. Maksimovich NE, Bon EI, Zimatkin SM. The rat brain and its response to ischemia: monograph. Grodno: GrSMU. 2020.
10. Rebrova O Yu. Statistical analysis of medical data. Using the Statistica application package. Moscow: Media Sphere. 2003; 312.
11. Semchenko VV. Postanoxic encephalopathy. Omsk. 1999; 446.
13. Belenichev IF. Neuroprotection and neuroplasticity: monograph. Kyiv: Polygraph Plus LLC, 2014. 512.