A Case Report: Blastic Plasmacytoid Dendritic Cell Neoplasm (BPDCN) Treated with Venetoclax, Hypomethylating agent, and Tagraxofusp
- 1. Hematology and Bone and Marrow Transplant, City of Hope/Cancer Treatment Centers of America Arizona, USA
- 2. Hematology/Oncology, Mayo Clinic Arizona, USA
- 3. Department of Laboratory Medicine and Pathology, Mayo Clinic Arizona, USA
Abstract
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is an extremely rare subtype of myeloid neoplasm, often presenting as cutaneous skin lesions and nodules in elderly males. The diagnosis of BPDCN is often challenging as it can resemble lymphoblasts or small immunoblasts, requiring the utilization of large panel antibodies to characterize the disease. As BPDCN remains a rare disease, there is no consensus on optimal therapy. We present a case report that describes the clinical presentation, pathologic diagnosis, treatment, and outcome of a patient with BPDCN.
Keywords
• Blastic Plasmacytoid Dendritic Cell Neoplasm
• Cutaneous Lesions
• Tagraxofusp
• Capillary Leak Syndrome
Citation
Granroth G, Francisco M, Kelemen K (2023) A Case Report: Blastic Plasmacytoid Dendritic Cell Neoplasm (BPDCN) Treated with Venetoclax, Hypomethylating agent, and Tagraxofusp. Ann Clin Pathol 10(1): 1162.
INTRODUCTION
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is an extremely rare subtype of myeloid neoplasm that is derived from precursors of plasmacytoid dendritic cells [1]. BPDCN most commonly affects adults over the age of 60 and exhibits a male predominance [2]. It is a clinically aggressive tumor that often initially presents as cutaneous skin lesions, nodules, and plaques that vary in size. The skin lesions can be associated with erythema, hyperpigmentation, purpura, or ulceration [3]. The histology of the cutaneous lesions is characterized by diffuse monomorphic infiltrates of medium-sized blast cells with fine chromatin and small irregular nuclei [3]. Skin biopsies of the lesions have shown diffuse infiltration of these cells in the dermis, sparing the epidermis, but expanding into the subcutaneous fat [3]. As the disease progresses, it rapidly involves the bone marrow, lymph nodes, exhibits leukemic dissemination [1], and can also affect the central nervous system [4]. Due to its aggressive nature, the overall median survival is 12 months [5]. The diagnosis is often challenging as neoplastic cells can resemble lymphoblasts or small immunoblasts; and require the use of a large panel of antibodies including those against CD4, CD56, CD123, CD303, TCL1, and TCF4 [1]. BPDCN is characterized by the expression of both CD4 and CD56, in addition to one or more antigens specific to pDCs including CD123, BDCA-2, TCL1, and CD2AP, along with the absence of lineage- specific markers for B-cells, T-cells, and myeloid and monocytic cells [2]. CD68, CD7, and CD33 are also commonly positive with BPDCN [2]. Given the rarity of the disease, BPDCN is often characterized by an inherent resistance to standard chemotherapies [1]. Although there is no consensus on optimal therapy for BPDCN due to its overall low incidence and rarity [3], pre- clinical investigations have shown some promise utilizing venetoclax (an oral BCL-2 inhibitor) in combination with a hypomethylating agent [6].
CASE PRESENTATION
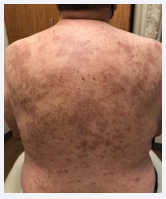
A 65-year-old male with a past medical history significant for hypertension, hyperlipidemia, and depression, presented to the Emergency Department with two weeks of drenching night sweats, fever, and worsening rash. Initially the rash developed as a single white raised lesion, 3-4 cm in diameter, on his right buttocks. The rash further developed into a maculopapular rash with raised lesions and components of plaques at the center, which were also erythematous, blanchable and convalescing extending to both upper and lower extremities, chest, back, and neck [Figure 1].
Figure 1: Cutaneous Skin Lesions. Pruritic maculopapular rash with raised lesions and components of plaques at the center, which were erythematous, blanchable and convalescing
Over time the rash became pruritic in nature. He was found to have punctate lesions on the palms of his hands, but no lesions on the soles of his feet. He also started to develop migratory joint pain. A CBC at time of presentation showed leukopenia with absolute neutropenia, and macrocytic anemia.
Several infectious disease tests were obtained and found to be negative for HSV, VZV, Cocci, Measles, Rubella, parvovirus, HIV, and syphilis [7-10]. A CT of the chest was obtained and found to have prominent bilateral axillary and left supraclavicular lymph nodes. A CT of the abdomen was obtained and showed enlarged portal and retroperitoneal lymph nodes concerning for malignancy. A PETCT showed hypermetabolic lymphadenopathy above and below the hemidiaphragms, involving the bilateral cervical, axillary, inguinal, periportal, and retroperitoneal stations, as well as subcutaneous nodule in the right upper back. Findings were suggestive of a lymphoma/lymphoproliferative disorder.
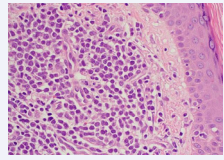
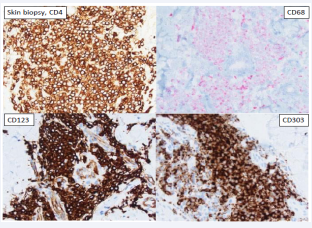
He underwent a skin biopsy which revealed Blastic Plasmacytoid Dendritic Cell Neoplasm [Figure 2, Figure 3].
Figure 2: Skin biopsy, H&E, 500x. Skin biopsy, hematoxylin & eosin (H&E), 500x magnification. The skin biopsy shows intradermal nodular infiltrates of uniform intermediatesize mononuclear cells with visible nucleoli, resembling blasts.
Figure 3: Skin biopsy, CD4, CD68, CD123, and CD303. Immunohistochemical stains show the skin infiltrate is positive with CD4, CD68, and strongly positive with plasmacytoid dendritic cell markers CD123 and CD303. The skin infiltrate was negative with CD34, CD117, TCL1 and TdT. The diagnosis of Blastic Plasmacytoid Dendritic Cell neoplasm was rendered.
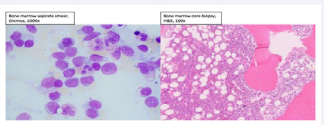
A bone marrow biopsy was performed and found to be hypercellular with 50% involvement by AML with phenotype expression of dim CD4, CD13, dim CD15, CD34, dim CD117, CD123, HLA-DR, and dim TdT but not myeloperoxidase, CD10, cytoplasmic CD3, or cytoplasmic CD22 felt to be possibly related to BPDCN due to expression of CD123, but absence of CD56 atypical for BPDCN [Figure 4].
Figure 4: Bone marrow aspirate smear (Giemsa, 1000x magnification) and bone marrow core biopsy (H&E, 100x magnification). Bone marrow aspirates smear (Giemsa, 1000x magnification) and bone marrow core biopsy (H&E, 100x magnification). The bone marrow aspirate and core biopsy show a hypercellular bone marrow with increased numbers of blasts representing more than 50% of the bone marrow cells. Trilineage hematopoiesis is present.
The cytogenetic and molecular findings of the bone marrow are as follows: Routine karyotype analysis showed an abnormal male karyotype: 47, XY, +13[9]/46, XY [11]. Molecular studies were negative for FLT3 mutation and NPM1 mutation. Next generation sequencing performed on the bone marrow showed three mutations: RUNX1 (Variant Allele Frequency 15%), SFRB1 (VAF 38%), SRSF2 (VAF 34%).
The bone marrow biopsy was interpreted as an acute myeloid leukemia. A right axillary lymph node biopsy was performed and found to be positive for BPDCN and myeloid sarcoma.
An in-depth discussion was carried out with the patient regarding the differences between the diagnosis and treatment approaches for Acute Myeloid Leukemia and Blastic Plasmacytoid Dendritic Cell Neoplasm. Treatment options and approaches included interleukin3 chemotherapy (Tagraxofusp), AML directed chemotherapy, and ALL directed chemotherapy options. Risk and benefits of each approach was discussed, and questions were answered to the patient’s satisfaction. Since the AML expressed CD123 it was initially decided he would undergo treatment with Tagraxofusp, as it showed a 90% response rate as upfront therapy [7].
Unfortunately, the patient experienced significant transaminitis and Tagraxofusp was not administered with his first cycle of treatment. He was therefore treated with 75 mg/m2 of Vidaza and 100 mg of Venetoclax. He underwent two cycles of Vidaza and Venetoclax. After his second cycle of therapy a bone marrow biopsy was performed and showed no evidence of disease. He had a repeat skin biopsy which continued to show BPDCN. At this point in time his liver enzymes had returned to normal, and the patient and his primary hematologist decided he would undergo treatment with Tagraxofusp for his third cycle of therapy.
The patient received Tagraxofusp-EXZS 12 mcg/kg daily for a total of 3 doses. He tolerated the infusions well. The day after treatment completed, he started to show classic signs of capillary leak syndrome with weight gain, elevated liver enzymes, and ascites. He received standard treatment for capillary leak syndrome with albumin and Lasix 2-3 times a day. Unfortunately, he continued to have worsening capillary leak syndrome, and started to have other organ involvement. He developed atrial fibrillation with RVR and had a heart rate in the 180s. He received IV metoprolol, which was ineffective. Cardiology was consulted and placed the patient on an amiodarone drip, as well as a heparin drip, in case he needed to be cardioverted. He started to experience pain and myalgias in his shoulders and was started on 1 mg/kg of IV methylprednisolone. His clinical course continued to worsen, as he experienced lactic acidosis with a lactate of 6.2, mottled skin, and was transferred to the ICU for cardiogenic shock, where he was started on milrinone for a SVR of 2,000. He received further albumin given the findings of volume depletion in the setting of capillary leak syndrome, and beta blockers and amiodarone were discontinued due to chronotropic incompetence. As the patient became more hemodynamically stable the milrinone was able to be weaned off. Unfortunately, his BUN and creatinine continued to worsen, therefore an HD line was place, and he was initiated on CRRT. He continued to have worsening shoulder pain, and was found to have severe rhabdomyolysis with an up-trending CK. His liver enzymes also worsened, and he became coagulopathic, requiring treatment with Vitamin K. His clinical course continued to worsen, and he experienced sudden onset of acute hypoxic respiratory failure and hypotension, requiring intubation and increased pressor support. At this point in time, he was hemodynamically unstable, and was on 4 pressors with MAPS between 50-60s. Repeat labs showed a lactate of 23, profound liver failure with ALT and AST of 7,000, and persistent rhabdomyolysis and mitochondrial dysfunction with a CK of 21,000. Due to his rapid clinical decline and overall poor prognosis given his complications post Tagraxofusp, his family opted for comfort measures. The patient passed away 10 days after receiving treatment with Tagraxofusp.
DISCUSSION
Blastic Plasmacytoid Dendritic Cell Neoplasm is a clinically aggressive myeloid malignancy that has typically demonstrated overall poor outcomes [1]. A variety of treatments have been used in induction therapy over the years including HCVAD-based (including venetoclax), AML-based induction, hypomethylationbased (including venetoclax), CHOP, bortezomib-based, and clinical trial/targeted therapy (including Tagraxofusp). Based on ongoing clinic analysis, treatment with clinical trial targeting the CD123+, has become the most commonly employed therapeutic strategy for patients with BPDCN [8].
Unfortunately, there are common risks and complications with any treatment options.
In the study that approved Tagraxofusp for the treatment of BPDCN, 55% of the patients experienced capillary leak syndrome with grade > 3 events occurring in 9% of those patients, including 2% fatal events [9]. The severity of this adverse reaction warranted a black boxed warning for CLS and inclusions of monitoring and CLS sign/symptoms-based management guidelines in the prescribing information [9]. Edema accumulation can occur with capillary leak syndrome leading to rhabdomyolysis and compartment syndrome [10]. Another significant side effect included hepatotoxicity, with elevated transaminases occurring in 88% of patients treated with Tagraxofusp [9]. Less common side effects of Tagraxofusp include pain, tachycardia, hypotension, and weight gain.
As capillary leak syndrome is a common and potentially life-threatening side effect of Tagraxofusp, it should be treated immediately upon a patient showing signs and symptoms. Fluid management, with crystalloid fluids, is the most important component of therapy in patients with capillary leak syndrome [11]. Vasopressors can be administered to patients remaining hypotensive despite adequate fluid resuscitation [10]. Diuretics should be instituted once a patient is hemodynamically stable to help reduce the risk for pulmonary edema and respiratory distress syndrome. Steroids are effective to help reduce cytokine production and may help reverse the capillary leak [10]. Patients with severe signs and symptoms should be treated immediately in an intensive care setting, as they may need pressor support, CRRT, and mechanical intubation and ventilation [11].
REFERENCES
- Maria Rosaria Sapienza, Alessandro Pileri, Enrico Derenzini, Federica Melle, Giovanna Motta, Stefano Fiori, et al. Blastic Plasmacytoid Dendritic Neoplasm: State of the Art and Prospects. Cancers (Basel). 2019; 11: 595.
- Robert S Ohgami, Phyu P Aung, Alejandro A Gru, Mohammad Hussaini, Kunwar Singh, Christiane Querfeld, et al. An Analysis of the Pathologic Features of Blastic Plasmacytoid Dendritic Cell Neoplasm Based on a Comprehensive Literature Database of Cases. Arch Pathol Lab Med. 2022; 1-10.
- Yang Shi, Endi Wang. Blastic Plasmacytoid Dendritic Cell Neoplasm, a Clinicopathologic Review. Arch Pathol Lab Med. 2014; 138: 564-9.
- Amy M Trottier, Sonia Cerquozzi, Carolyn J Owen. Blastic plasmacytoid dendritic cell neoplasm: challenges and future prospects. Blood Lymphat Cancer. 2017; 7: 85-93.
- F Julia, T Petrella, M Beylot-Barry, M Bagot, D Lipsker, L Machet, et al. Blastic Plasmacytoid Dendritic Cell Neoplasm: Clinical Features in 90 patients. Br J Dermatol. 2013; 169: 579-586
- Naseema Gangat, Marina Konopleva, Mrinal M Patnaik, Elias Jabbour, Courtney DiNardo, Aref Al-Kali, et. al. Venetoclax and hypomethylating agents in older/unfit patients with blastic plasmacytoid dendritic cell neoplasms. Am J Hematol. 2021; 97: E62-E67.
- Naveen Pemmaraju, Andrew A Lane, Kendra L Sweet, Anthony S Stein, Sumithira Vasu, William Blum, et al. Tagraxofusp in Blastic Plasmacytoid Dendritic-Cell Neoplasm. N Engl J Med. 2019; 380: 1628-1637.
- Pemmaraju Naveen, Kantarijan Hagop, Khoury Joseph D, Sanam Loghavi, Susan O’Brien, Jorge E. Cortes, et al. Blastic Plasmacytoid Dendritic Cell Neoplasm (BPDCN) Commonly Presents in the Setting of Prior Concomitant Hematologic Malignancies (PCHM): Patient Characteristics and Outcomes in the Rapidly Evolving Modern Targeted Therapy Era. Blood. 2019; 134.
- Emily Y Jen, Xin Gao, Liang Li, Luning Zhuang, Natalie E Simpson, Baikuntha Aryal, et al. FDA Approval Summary: Tagraxofusp-erza For Treatment of Blastic Plasmacytoid Dendritic Cell Neoplasm. Clin Cancer Res. 2019; 26: 1-5.
- Eric Siddall, Minesh Khatri, Jai Radhakrishnan. Capillary Leak Syndrome: etiologies, pathophysiology, and management. Kidney Int. 2017; 92: 37-46.
- Kirk M Druey, Philip R Greipp. Narrative Review: The Systemic Capillary Leak Syndrome. Annals of Internal Medicine. 2010; 153: 90- 98.