Benign is a Relative Term: the Whipple Resection in Non- Oncologic Cases
- 1. Department of Pathology, University of Vermont/Fletcher Allen Health Care, USA
- 2. Department of Pathology, University of Chicago, USA
Abstract
The pancreaticoduodenectomy, commonly referred to as the Whipple resection, is the en bloc surgical resection of the pancreas head and uncinate process, duodenum, gallbladder, common bile duct, and common hepatic duct just proximal to the cystic duct takeoff with (conventional) or without (pylorus-sparing) the gastric antrum. Although generally an oncologic procedure, the Whipple resection can be warranted in benign or pre-neoplastic periampullary/ampullary lesions as the intimate structural relationship of the distal common bile duct, pancreatic head and ampulla of Vater creates an opportunity for otherwise benign processes to cause severe obstructive changes.
Keywords
• Whipple
• Pancreaticoduodenectomy
• Autoimmune pancreatitis
• Amyloidosis
• Paraduodenal pancreatitis
Citation
Crothers JW, Zhao L, Wilcox R (2014) Benign is a Relative Term: the Whipple Resection in Non-Oncologic Cases. Ann Clin Pathol 2(2): 1019
Historical Perspective
The history of the Whipple resection begins with an Italian surgeon by the name of Alessandro Codivilla who first removed the pancreas, duodenum, distal stomach and distal bile duct for a carcinoma of the pancreas. According to Codivilla’s Dal Monte1898 report (as cited in Schnelldorfer 2009) [1], continuity of the bowel was restored using a Roux-en-Y technique, but no anastomosis or closure of the pancreatic stump was performed and the patient died 18 days post-operative with severe cachexia and steatorrhea [1]. This case illustrates two prevailing opinions of the day, that the duodenum was essential for human survival and that the pancreatic duct could be permanently ligated. A year later in 1899 William Halsted, one of the great contributors to modern surgery, presented the first successful resection of ampullary cancer in which he excised a portion of the duodenum and pancreas. In explaining the procedure, Halsted described his technique in giving “the growth a wide margin, a large piece of duodenum was excised, about three-quarters of an inch of the common duct and a shorter piece of the pancreatic duct were excised” [2]. Unfortunately, this patient presented months later with reoccurrence and was too ill to be operated on again. At autopsy the tumor was found “to have recurred in the head of the pancreas, closing the common duct” [2].
The next decade saw additional surgical experimentation with removal of the pancreas and adjacent organs. In 1909, Walter Carol Kausch performed a two-step en bloc resection of the majority of the duodenum and a significant portion of the pancreas, a procedure that he documented in his 1912 publication Das Carcinom der Papilla duodeni und seine radikale Entfernung (as cited by Chandrakanth 2011) [3]. A few years later, described in his article Die Resektion des Duodenums mit der Papille wegen Karzinoms, Georg Hirschel reimplanted the pancreatic duct into the duodenum and bridged the common bile duct to the duodenum with the aid of a rubber tube in the first one-stage partial pancreaticoduodecomy (as cited by Whipple 1949 and Schnelldorfer 2008) [4,5]. The patient’s jaundice improved and he lived for another year. A major milestone occurred in 1918 when Lester Dragstedt demonstrated in dogs that complete excision of the duodenum was compatible with life [6]. Still, surgeons were resistant to complete duodenectomy and in 1922 Tenani, an Italian surgeon, described in his Contributo alla chirurgia della papilla del Veter an impressive two-stage resection for ampullary carcinoma in which he performed a partial gastrectomy, partial pancreaotecomy and removal of the common bile duct with re-anastomosis of the remaining stomach, gallbladder and pancreas to the last portion of duodenum. Following the surgery, Dr. Tenani’s patient lived an additional 3 years (as cited by Chandrakanth 2011) [3].
Overlapping with Dr. Tenani was the work of Dr. Allen Oldfather Whipple. After only 10 years of practice, Dr. Whipple obtained the position of Professor and Surgeon–in-Chief at Columbian-Presbyterian Medical Center in New York, where he would eventually lead the surgical department as the Valentine Mott Chair for 25 years. His first official report of a two-stage pancreaticoduodenectomy was presented at the American Surgical Association meeting in 1935. Among the 3 cases he presented, 1 patient underwent total duodenectomy (as cited by Chandrakanth 2011) [3]. This was the first successful total duodenectomy reported in humans and served as an important milestone in medical history, establishing the fact that total duodenectomy is compatible with life. Dr. Whipple’s next major milestone occurred in 1940 when he reported the first one-stage complete excision of the pancreatic head and entire duodenum. Six years later he published his 10-year experience with the procedure that bears his name in which he proposed various modifications, including complete resection of the duodenum and head of the pancreas, anastomosis of the remaining pancreatic duct with the jejunum, and choledochoenterostomy to avoid ascending biliary tract infections and stenosis [4]. Dr. Whipple performed a total of 37 pancreaticoduodenectomies over his career; 30 for periampullary carcinoma and 7 for chronic pancreatitis.
Current Application
Today the Whipple resection is a mainstay of treatment for pancreatic head and peri-ampullary malignancies. In direct relation to surgical experience, the mortality associated with the procedure has plummeted over the last half century. At almost 25% in Dr. Whipple’s era, mortality has been reduced to less than 5% at large surgical centers where the Whipple resection is routinely performed [7,8]. The indications for undergoing a Whipple resection have also shifted with time. In a retrospective analysis of the 2,050 Whipple resections performed at Massachusetts General Hospital since Dr. Whipple’s seminal 1935 paper, pancreatic ductal adenocarcinoma (PDAC) was the most frequent indication (36%), followed by cystic neoplasms and ampullary lesions (17% and 16%, respectively) [8]. Over time, PDAC steadily decreased to 32% in the 1980s as the predominate indication for pancreaticoduodenecomy until plateauing over the past 2 decades at around 35%. Inversely, chronic pancreatitis gained in proportion, reaching its peak in the 1970’s and 1980’s (20%) (8). Over the last 2 decades, the proportion of cystic neoplasms has emerged as the second most frequent indication for a Whipple resection (18%) [8].
Presumed Malignancy
Occasionally, a Whipple resection is performed for a presumed malignancy and final pathology reveals a benign underlying process. In a review of 494 pancreaticoduodenectomies performed for presumed malignancy at Duke University, 37 cases (7%) revealed a benign underlying pathologic process with no evidence of malignancy [10]. The majority of these cases had undergone pre-operative evaluation by endoscopic ultrasound (EUS) and fine needle aspiration (FNA). The most common finding by EUS in the patients with postoperative benign disease was a pancreatic head mass. The FNA diagnosis of “suspicious for cancer” or “indeterminate for cancer” was made in 63% of these patients [10]. Obstructive jaundice was the most common presenting symptom in patients with benign disease and 35% of this subset of patients had multiple additional symptoms including abdominal pain and weight loss [10], reflecting the observation that benign diseases affecting the pancreatic head or peri-ampullary region can present with mass effect on imaging and mimic an obstructive malignancy symptomatically. Herein, we discuss three case studies (from our institution) in which patients underwent Whipple resection for presumed malignancy and were ultimately found to have a benign process affecting the pancreatic head or peri-ampullary region.
Case 1
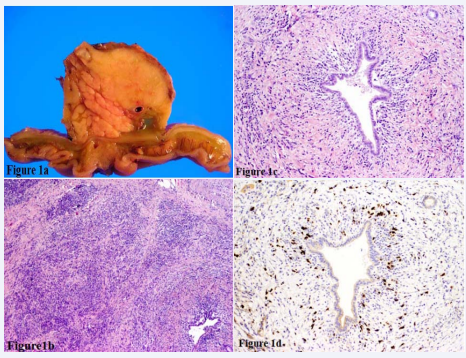
A 76-year-old man with a history of successfully treated autoimmune hemolytic anemia three years prior presented with painless jaundice accompanied by a 5-lb weight loss. CT scan revealed a 5 cm ill-defined region of fullness in the pancreatic head with dilatation of the pancreatic duct. The computed tomography (CT)-guided FNA was non-diagnostic, showing spindled stromal cells and chronic inflammation. The patient was referred to surgery and underwent a Whipple resection with curative intent. Consistent with imaging, gross examination revealed an ill-defined, firm, yellow mass centered around the pancreatic duct (Figure 1a).
Figure 1: A) Cut surface of the pancreaticoduodenectomy specimen highlights an ill-defined yellow mass in a background of normal pancreatic parenchyma. B) H&E 100X Low power view of the pancreatic parenchyma reveals loss of normal architecture due to a dense lymphoplasmacytic infiltrate admixed with storiform fibrosis. C) H&E 400X High power view of an individual duct highlights a concentric lymphoplasmacytic infiltrate and secondary scarring. D) H&E 400X Immunohistochemical staining confirms a large number of the inflammatory cell infiltrate to be composed of IgG4 positive plasma cells.
The remainder of the pancreas was normal without gross evidence of chronic pancreatitis. The entire mass was submitted for histologic review. Although there was no evidence of malignancy, the histologic sections were significant for storiform fibrosis admixed with a marked lymphoplasmacytic infiltrate (Figure1b). A concentric inflammatory infiltrate, predominantly composed of plasma cells, surrounded most interlobular ducts as well as veins and nerves (Figure 1c). These histologic features in conjunction with the large number (> 80 per single high power field) of IgG4 positive plasma cells, as proven by immunohistochemical staining (Figure 1d) led to a final diagnosis of autoimmune pancreatitis, Type 1.
Autoimmune pancreatitis (AIP), historically known as lymphoplasmacytic sclerosing pancreatitis, inflammatory pseudotumor and primary inflammatory sclerosis of the pancreas [11], is part of the greater constellation of IgG4-related disease. Classically characterized by fibroinflammatory mass lesions, storiform fibrosis and a dense lymphoplasmacytic infiltrate rich in IgG4 plasma cells, IgG4-related disease was first identified within the pancreas but is now recognized to effect many anatomic sites. Autoimmune IgG4-related pancreatitis is further classified as Type 1 and Type 2. Type 1 disease is much more common worldwide, exhibits the aforementioned classic histology, is commonly associated with extrapancreatic manifestations and is often associated with increased serum IgG4 levels. Type 2 disease exhibits a predominately neutrophilic infiltrate and occasional epitheiloid granulomas. Patients with type 2 disease often have normal IgG4 levels and no extrapancreatic involvement. While the precise pathogenesis of IgG4-realted disease is still under investigation, mounting evidence supports an autoimmune etiology; the disease can be associated with other autoimmune or immune deregulatory disease such as Sjogren’s syndrome [12,13], rheumatoid arthritis [14], inflammatory bowel disease [15] and primary sclerosing cholangitis [13,16]. Unlike most autoimmune pathologies however, autoimmune IgG4-related pancreatitis has a male predilection (77% in Type 1 and 55% in Type 2) [17].
Clinically, AIP can present as a focal mass lesion, diffuse pancreatic enlargement or strictures of the pancreatic duct leading to recurrent abdominal pain and/or obstructive jaundice.
Significant weight loss and severe chronic abdominal pain are rare [17,18]. Various diagnostic criteria have been suggested, including the HISORt criteria developed at the Mayo Clinic and the most recent 2012 International Consensus Diagnostic Criteria (ICDC) [17, 19]. While histology is considered the current “gold standard”, definitive diagnoses must also take into account imaging, extrapancreatic involvement, response to glucocorticoid therapy and IgG4 serology. A serum IgG4 level >140 mg/dL has a sensitively of 76% and a specificity of 93% in diagnosing AIP, based on a recent Mayo Clinic cohort study [17,20]. If measured in a population with a low prevalence of AIP or IgG4-related disease however, the positive predictive value of an elevated serum IgG4 level can be as low as 10% [21], thus exemplifying the importance of ordering this test in the appropriate clinical context, notably obstructive jaundice and/or workup for a pancreatic mass.
The importance of diagnosing AIP cannot be understated. As it is a benign disease process that responds well to treatment with corticosteroids, the diagnosis of AIP may allow patients to avoid major surgery and its inherent potential complications. In a review of pancreaticoduodenectomy performed for presumed malignancy, approximately one third of those found to have benign underlying pathology were diagnosed with AIP [22].
Case 2
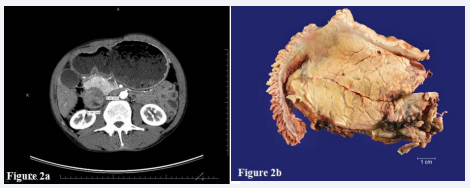
A 58-year old male with a history of alcohol dependence and a 20-pack-per-year smoking history was admitted with sharp epigastric pain radiating to the back, nonbilious emesis and a 15 lb weight loss. Contrast-enhanced CT identified a 6-cm complex mass in the head of the pancreas with associated intra and extra hepatic biliary dilatation. Although primarily solid, CT scan identified an associated cystic component to the lesion centered at the ampulla (Figure 2a).
Figure 2: A) Contrast-enhanced computed tomography (CT) identified a complex pancreatic head mass showing both solid and cystic components with extrinsic compression of the duodenum. B) Cut section of the pancreaticoduodenectomy specimen reveals a poorly defined white scar-like mass originating from the duodenal wall with extension into the underlying pancreas.
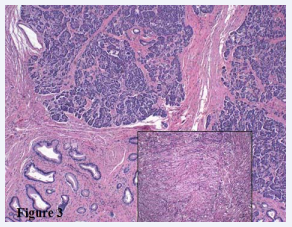
No evidence of vascular invasion was identified within the superior mesenteric vessels. Radiologic impression was concerning for malignancy, although pancreatic pseudocyst in the background of chronic pancreatitis could not be excluded. A CT-guided needle core biopsy was performed and showed actively inflamed glands and normal duodenal mucosa. The patient underwent a Whipple resection with curative intent. Gross examination revealed a poorly defined, white mass originating in the duodenal wall and extending through the muscularis propria into underlying pancreatic parenchyma (Figure 2b). The entire mass as well as the duodenum at the minor papilla was submitted for histologic review. No malignancy was identified. The mass was composed of benign ductal structures, periductal mucous glands, disorganized nerve fibers, pancreatic acini and a storiform proliferation of smooth muscle (Figure 3).
Figure 3: H&E 100X The mass consists of benign ductal structures admixed with pancreatic tissue and smooth muscle proliferation. Insert) H&E 400X High power view of storiform smooth muscle proliferation.
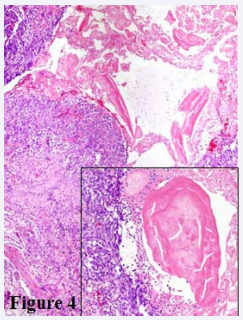
Figure 4: H&E 200X Focal ulceration with loss of epithelium and associated granulation tissue was noted at the minor ampulla. Insert) H&E 400X High power view of eosinophilic mucoprotein secretions present at ulcerated minor ampulla.
Focally, the minor ampulla was ulcerated with associated acute inflammation, granulation tissue and mucoprotein plugs (Figure 4). Together the clinical history, imaging and histologic features were consistent with the diagnosis of paraduodenal pancreatitis.
Paraduodenal pancreatitis has been documented under many names, including groove pancreatitis, myoepithelial hamartoma and paraduodenal wall cyst [23]. The current consensus term is paraduodenal pancreatitis [23]. A distinct form of chronic pancreatitis, this process occurs within the minor pancreatic duct (duct of Santorini) and associated duodenal wall, which contains various amounts of trapped pancreatic tissue secondary to the embryologic merging of the ventral and dorsal pancreatic buds. This disorder is generally seen in males >40 years of age with a history of chronic alcohol dependence. It is thought that the embryological entrapped pancreatic tissue and minor duct of Santorini are particularly susceptible to alcohol-induced injury resulting in acute on chronic changes, which induce an obstructive mass effect capable of mimicking a malignant process [23]. Characteristic magnetic resonance imaging findings include tubulocystic changes along the duct of Santorini path with abnormal or cystic enhancement of the duodenal wall.
Case 3
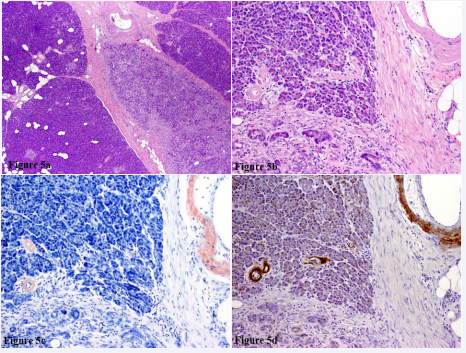
A 52-year old male with nephrolithiasis, obesity and hypertension presented with new onset mild jaundice. CT imaging revealed an ill-defined area of pancreatic duct stenosis near the ampulla of vater. CT-guided FNA was non-diagnostic and serum IgG4 was within the normal range. A Whipple resection was performed for diagnostic and therapeutic purposes. Gross examination revealed an atrophic-appearing, ill-defined fibrotic area surrounding the pancreatic duct. The remaining pancreatic parenchyma was normal in appearance. No definitive mass was identified within the pancreatic parenchyma, pancreatic duct or common bile duct. The entire common bile duct and the majority of the pancreas were submitted for histologic review. No malignancy was identified. There was however, a geographically defined region of chronic pancreatitis within the pancreatic head in direct association with the main pancreatic duct (Figure 5a).
Figure 5: A) H&E 100X Centrally localized chronic pancreatitis with normal pancreatic parenchyma on either side. B) H&E 200X High power view highlighting small intraparenchymal and medium sized extraparenchymal vessels, both with intramural deposition of homogenous eosinophilic material. C) Congo red stain. Vessels walls stain bright red on Congo red with apple green birefringence with polarized light (not shown). D) Immunohistochemical stain confirms the same vessels to be positive for Amyloid AA.
An eosinophilic, homogenous deposition was noted involving intra and interlobular vasculature, in both areas of chronic pancreatitis as well as unremarkable pancreatic parenchyma (Figure 5b). The vascular lumens were patent. Positive Congo red staining with apple-green birefringence under polarized light and positive AA amyloid by immunohistochemistry confirmed the presence of AA amyloid deposition (Figure 5c-d). Two days after surgery the patient suffered sudden cardiac arrest and died in the surgical intensive care unit. Autopsy revealed extensive amyloid vasculopathy involving the heart, kidneys, lungs, liver, spleen, adrenal gland and bone marrow.
Amyloidosis refers to a diverse group of diseases linked by a common end result. Due to various underlying pathologies, including acquired, inherited, degenerative, and infectious and paraneoplastic mechanisms, abnormally folded proteins are deposited extracellularly, displacing normal structures and ultimately disrupting organ function. Amyloid deposition can be mass-like or diffuse and is classically diagnosed microscopically by its characteristic staining with Congo red, exhibiting apple green birefringence when exposed to polarized light. When viewed by electron microscopy, these abnormal protein structures aggregate into characteristic Beta-pleated sheets. Systemic amyloidosis commonly affects multiple organs, but localized deposition has also been recognized [24]. The most severe cases are often a result of chronic inflammatory disorders, which result in AA proteins, and commonly affect the kidneys, liver, spleen, lymph nodes, adrenals and thyroid. Organ involvement is highly variable however and while any form of systemic amyloidosis can affect the heart, cardiac involvement is more commonly associated with plasma-cell dyscrasias and “senile amyloidosis” [24,25]. Amyloidosis can mimic a malignant process when there is mass forming deposition (amyloidoma) or by causing secondary changes, as in our case of localized chronic pancreatitis in the pancreatic head.
Presumed Malignancy or Recognized Benign Disease
In an era of advanced imaging and FNA techniques, the number of Whipple procedures performed for presumed malignancy which are ultimately diagnosed as benign is small, at approximately 7% [10]. Recognition of autoimmune IgG4-related pancreatitis prior to a Whipple resection may help to reduce this number even further. However, given the complex anatomy of this region, a subset of cases exist in which definitive diagnosis by imaging or FNA is limited, making a certain number of Whipple procedures resulting in benign underlying disease inevitable. It is also notable that some PDAC may show overlapping features of AIP, including frequent IgG4 cells, making definitive diagnosis on small biopsy specimens difficult [26]. Of note, the mortality in this subset of patients has been documented as very low [27]. In their series of pancreaticoduodenectomy performed in patients with benign disease, Barnes et al found a mortality rate of less than 1% [28]. Thus, although seemingly aggressive at first glance, a Whipple resection may be warranted in the context of benign disease for either symptomatic relief or in cases in which a thorough preoperative workup fails to definitively rule out malignancy
References
- Schnelldorfer T, Sarr MG. Alessandro Codivilla and the first pancreatoduodenectomy. See comment in PubMed Commons below Arch Surg. 2009; 144: 1179-1184.
- Halsted WS. (1899) Contributions to the surgery of the bile passages, especially of the common bile-duct. Boston Med Surg J 141: 645–654.
- Are C, Dhir M, Ravipati L. History of pancreaticoduodenectomy: early misconceptions, initial milestones and the pioneers. See comment in PubMed Commons below HPB (Oxford). 2011; 13: 377-384.
- WHIPPLE AO. An evaluation of radical surgery for carcinoma of the pancreas and ampullary region. See comment in PubMed Commons below Ann Intern Med. 1949; 31: 624-627.
- Schnelldorfer T, Adams DB, Warshaw AL, Lillemoe KD, Sarr MG. Forgotten pioneers of pancreatic surgery: beyond the favorite few. See comment in PubMed Commons below Ann Surg. 2008; 247: 191-202.
- Dragstedt LR, Dragstedt C, McClintock JT, Chase CS. (1918) Extirpation of the duodenum. Am J Physiolo 46: 584–590.
- Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. See comment in PubMed Commons below Ann Surg. 2006; 244: 10-15.
- Fernández-del Castillo C, Morales-Oyarvide V, McGrath D, Wargo JA, Ferrone CR, Thayer SP. Evolution of the Whipple procedure at the Massachusetts General Hospital. See comment in PubMed Commons below Surgery. 2012; 152: S56-63.
- Abraham SC, Wilentz RE, Yeo CJ, Sohn TA, Cameron JL, Boitnott JK. Pancreaticoduodenectomy (Whipple resections) in patients without malignancy: are they all ‘chronic pancreatitis’? See comment in PubMed Commons below Am J Surg Pathol. 2003; 27: 110-120.
- de la Fuente SG, Ceppa EP, Reddy SK, Clary BM, Tyler DS, Pappas TN. Incidence of benign disease in patients that underwent resection for presumed pancreatic cancer diagnosed by endoscopic ultrasonography (EUS) and fine-needle aspiration (FNA). J Gastrointest Surg. 2010; 14: 1139-1142.
- SARLES H, SARLES JC, MURATORE R, GUIEN C. Chronic inflammatory sclerosis of the pancreas--an autonomous pancreatic disease? Am J Dig Dis. 1961; 6: 688-698.
- Kamisawa T, Tu Y, Egawa N, Sakaki N, Inokuma S, Kamata N. Salivary gland involvement in chronic pancreatitis of various etiologies. See comment in PubMed Commons below Am J Gastroenterol. 2003; 98: 323-326.
- Külling D, Tresch S, Renner E. Triad of sclerosing cholangitis, chronic pancreatitis, and Sjögren’s syndrome: Case report and review. See comment in PubMed Commons below Gastrointest Endosc. 2003; 57: 118-120.
- Weber SM, Cubukcu-Dimopulo O, Palesty JA, Suriawinata A, Klimstra D, Brennan MF. Lymphoplasmacytic sclerosing pancreatitis: inflammatory mimic of pancreatic carcinoma. See comment in PubMed Commons below J Gastrointest Surg. 2003; 7: 129-137.
- Niemelä S, Lehtola J, Karttunen T, Lähde S. Pancreatitis in patients with chronic inflammatory bowel disease. See comment in PubMed Commons below Hepatogastroenterology. 1989; 36: 175-177.
- Epstein O, Chapman RW, Lake-Bakaar G, Foo AY, Rosalki SB, Sherlock S. The pancreas in primary biliary cirrhosis and primary sclerosing cholangitis. See comment in PubMed Commons below Gastroenterology. 1982; 83: 1177-1182.
- Ketwaroo, G.A, Sheth S., Autoimmune pancreatitis. Gastroenterology Report (2013) 1–7, doi:10.1093/gastro/got011.
- Shimosegawa T, Chari ST, Frulloni L, Kamisawa T, Kawa S, Mino- Kenudson M, Kim MH, Klöppel G, et al. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas 2011; 40: 352–58.
- Deshpande V, Zen Y, Chan JK, Yi EE, Sato Y, Yoshino T. Consensus statement on the pathology of IgG4-related disease. See comment in PubMed Commons below Mod Pathol. 2012; 25: 1181-1192.
- Sah RP, Chari ST. Serologic issues in IgG4-related systemic disease and autoimmune pancreatitis. See comment in PubMed Commons below Curr Opin Rheumatol. 2011; 23: 108-113.
- Ngwa TN, Law R, Murray D, Chari ST. Serum immunoglobulin G4 level is a poor predictor of immunoglobulin G4-related disease. See comment in PubMed Commons below Pancreas. 2014; 43: 704-707.
- van Heerde MJ, Biermann K, Zondervan PE, Kazemier G, van Eijck CH, Pek C. Prevalence of autoimmune pancreatitis and other benign disorders in pancreatoduodenectomy for presumed malignancy of the pancreatic head. See comment in PubMed Commons below Dig Dis Sci. 2012; 57: 2458-2465.
- Adsay NV, Zamboni G. Paraduodenal pancreatitis: a clinico- pathologically distinct entity unifying “cystic dystrophy of heterotopic pancreas”, “para-duodenal wall cyst”, and “groove pancreatitis”. Semin Diagn Pathol. 2004; 21: 24.
- Falk RH, Comenzo RL, Skinner M. The systemic amyloidoses. See comment in PubMed Commons below N Engl J Med. 1997; 337: 898- 909.
- Pepys MB. Amyloidosis. See comment in PubMed Commons below Annu Rev Med. 2006; 57: 223-241.
- Zhang X, Liu X2, Joseph L, Zhao L, Hart J, Xiao SY3. Pancreatic ductal adenocarcinoma with autoimmune pancreatitis-like histologic and immunohistochemical features. See comment in PubMed Commons below Hum Pathol. 2014; 45: 621-627.
- Cohen JR, Kuchta N, Geller N, Shires GT, Dineen P. Pancreaticoduodenectomy for benign disease. See comment in PubMed Commons below Ann Surg. 1983; 197: 68-71.
- Barens SA, Lillemoe KD, Kaufman HS, Sauter PK, Yeo CJ, Talamini MA. Pancreaticoduodenectomy for benign disease. See comment in PubMed Commons below Am J Surg. 1996; 171: 131-134.