Cross-Protection Against Homo and Heterologous Influenza Viruses via Intranasal Administration of an HA Chimeric Multiepitope Nanoparticle Vaccine
- 1. College of Veterinary Medicine, Northwest A&F University, China
- 2. Yangling Carey Biotechnology Co., Ltd., China
Citation
Zhao Y, Yang D, Du E (2025) Cross-Protection Against Homo and Heterologous Influenza Viruses via Intranasal Administration of an HA Chimeric Multiepitope Nanoparticle Vaccine. Ann Clin Pathol 12(1): 1176
EDITORIAL
Influenza viruses, notably Influenza A viruses (IAVs), persist as a formidable global health challenge through recurrent epidemics and intermittent pandemics, imposing substantial disease burdens worldwide [1,2]. While vaccination remains the cornerstone of influenza prevention, the clinical effectiveness of current vaccines is intrinsically limited by the antigenic congruence between vaccine strains and circulating variants - a critical vulnerability exacerbated by continuous viral evolution through antigenic drift [3]. This fundamental constraint has driven urgent investigations into novel vaccine platforms capable of eliciting broad-spectrum protection. The hemagglutinin (HA) surface glycoprotein, serving as the principal target for neutralizing antibodies, maintains its status as the dominant antigenic focus for subunit vaccine development. However, its pronounced genetic plasticity substantially compromises vaccine efficacy against heterologous strains. Complementary strategies targeting conserved viral components have emerged, including matrix protein 1 (M1) and the extracellular domain of matrix protein 2 (M2e), though these epitopes typically demonstrate suboptimal immunogenicity [4,5]. An innovative approach involves the rational design of chimeric HA antigens incorporating conserved influenza epitopes. This paradigm-shifting strategy addresses the dual challenges of HA variability and conserved antigen immunogenicity through three synergistic mechanisms: (1) preservation of HA’s inherent immunodominance, (2) immune focusing on conserved regions via epitope scaffolding, and (3) activation of cross-protective immunity through conserved domain presentation. Such engineered vaccines can potentially overcome the limitations of conventional approaches by inducing durable, broad spectrum immune responses, thereby representing a transformative advance in influenza prophylaxis.
On the other hand, the emergence of nanotechnology has revolutionized vaccine design through innovative antigen delivery systems, with self-assembling nanoparticles establishing themselves as a transformative platform. Among these, ferritin - an evolutionarily conserved iron storage protein - has emerged as a particularly promising candidate due to its unique biological properties. This naturally immunogenic protein not only activates antigen presenting cells through pattern recognition receptor engagement, but also potentiates antibody responses via enhanced lymphocyte activation [6]. Structurally, the ferritin nanocage comprises 24 symmetrically arranged subunits forming a hollow dodecahedral architecture (10-12 nm diameter). This configuration enables three critical functional advantages: (1) high-density antigen multimerization through precise surface conjugation, (2) exceptional biophysical stability under thermal stress (up to 80 °C) and across wide pH ranges (2-12), and (3) optimal lymphatic trafficking facilitated by size-dependent drainage (8-100 nm preferred range) [7]. Mechanistically, the nanocage’s dimensions permit efficient uptake by macrophages and dendritic cells via macropinocytosis, followed by rapid transport to lymph nodes where it stimulates coordinated cellular (Th1/CTL) and humoral (neutralizing IgG/IgA) immune responses. These inherent advantages have propelled ferritin-based platforms to the forefront of vaccinology, with demonstrated efficacy across diverse therapeutic domains: (1) pandemic preparedness through universal influenza vaccine development, (2) precision oncology via tumor-associated antigen presentation, and (3) immune tolerance modulation in autoimmune conditions. The platform’s modular design allows for combinatorial antigen display while maintaining structural integrity, positioning itself as a versatile scaffold for next-generation vaccine development [8-13].
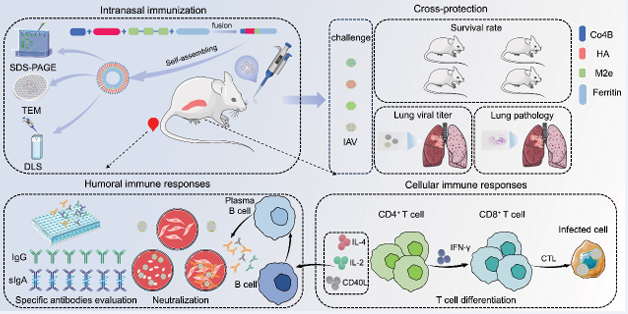
In a groundbreaking study recently published in the Journal of Nanobiotechnology, Zhao et al., reported the development and immunological evaluation of a novel nanoparticle vaccine, CHM-f (Figure 1).
Figure 1 Graphical abstract
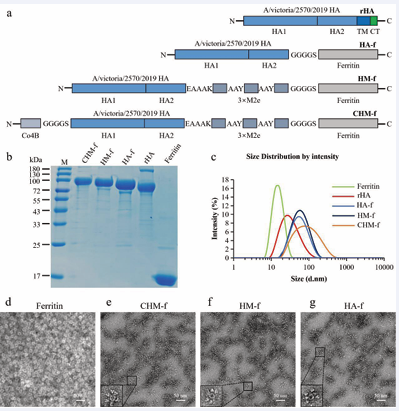
Using a baculovirus-insect cell expression system, the researchers successfully produced an HA-ferritin nanoparticle vaccine. This innovative vaccine consists of self-assembling ferritin nanoparticles (f) that serve as an antigen delivery platform, displaying the extracellular domain of Hemagglutinin (HA), three sequential repeats of highly conserved M2e epitopes, and an M-cell targeting ligand (Co4B) on its surface (Figure 2).
Figure 2 Antigen design and nanoparticle characterization
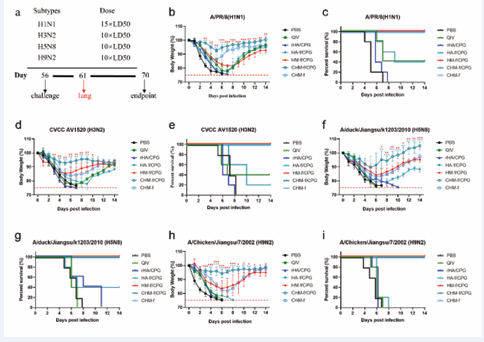
Following intranasal administration in mice, this ferritin nanoparticle vaccine induced significantly higher levels of HA-specific IgG and hemagglutination inhibition (HAI) antibodies when compared to the quadrivalent inactivated influenza vaccine (QIV). Additionally, it triggered robust secretory IgA responses at mucosal sites, a key factor in mucosal immunity. In challenge studies, the vaccine provided broad protection against multiple
influenza A virus (IAV) subtypes, including H1N1, H3N2, H5N8, and H9N2. Notably, co-administration of CHM-f with a novel adjuvant, CpG IAMA-002, further enhanced immune responses, leading to increased antibody titers, improved cell-mediated immunity, and higher mucosal IgA production. When compared to the rHA and QIV groups, the CHM-f-immunized mice showed significant improvements in body weight recovery, reduced lung viral loads, and less severe histopathological changes (Figure 3).
Figure 3 Cross-protection of CHM-f nanoparticles against different IAV subtypes
This study represents a significant advance in influenza vaccine research. The innovative aspect of this work lies in the integration of M-cell targeting strategies with multi-epitope antigen display technologies. Together, they enhance mucosal immunity while addressing the challenges posed by HA antigenic variation. Moreover, the study highlights the unique advantages of the intranasal route for vaccine administration, which not only induces systemic immunity but also elicits robust mucosal immune responses. When compared to traditional influenza vaccines, the CHM-f nanoparticle vaccine offers several notable advantages, including stronger immunogenicity, broader protective coverage, and a more convenient route of administration. Most importantly, the CHM-f vaccine demonstrated superior immune responses to those of QIV, even without adjuvants, while maintaining a favorable safety profile in murine models. These attributes suggest that the CHM-f nanoparticle could be a promising candidate of influenza vaccines, particularly for pediatric use.
REFERENCES
- Iuliano AD, Roguski KM, Chang HH, Muscatello DJ, Palekar R, Tempia S, Cohen C, et al. Global Seasonal Influenza-associated Mortality Collaborator Network. Estimates of global seasonal influenza- associated respiratory mortality: a modelling study. Lancet. 2018; 391: 1285-1300.
- Monto AS, Fukuda K. Lessons from Influenza Pandemics of the Last 100 Years. Clin Infect Dis. 2020; 70: 951-957.
- Wei CJ, Crank MC, Shiver J, Graham BS, Mascola JR, Nabel GJ. Next- generation influenza vaccines: opportunities and challenges. Nat Rev Drug Discov. 2020; 19: 239-252.
- Sei CJ, Rao M, Schuman RF, Daum LT, Matyas GR, Rikhi N, et al. Conserved Influenza Hemagglutinin, Neuraminidase and Matrix Peptides Adjuvanted with ALFQ Induce Broadly Neutralizing Antibodies. Vaccines (Basel). 2021; 9: 698.
- Zharikova D, Mozdzanowska K, Feng J, Zhang M, Gerhard W. Influenza type A virus escape mutants emerge in vivo in the presence of antibodies to the ectodomain of matrix protein 2. J Virol. 2005; 79: 6644-6654.
- Yamashita I, Iwahori K, Kumagai S. Ferritin in the field of nanodevices. Biochim Biophys Acta. 2010; 1800: 846-857.
- Lee NK, Cho S, Kim IS. Ferritin - a multifaceted protein scaffold for biotherapeutics. Exp Mol Med. 2022; 54: 1652-1657
- He J, Fan K, Yan X. Ferritin drug carrier (FDC) for tumor targeting therapy. J Control Release. 2019; 311-312: 288-300.
- Hong S, Choi DW, Kim HN, Park CG, Lee W, Park HH. Protein-Based Nanoparticles as Drug Delivery Systems. Pharmaceutics. 2020; 12: 604.
- Kanekiyo M, Wei CJ, Yassine HM, McTamney PM, Boyington JC, Whittle JR, et al. Self-assembling influenza nanoparticle vaccines elicit broadly neutralizing H1N1 antibodies. Nature. 2013; 499: 102-106.
- Karch CP, Matyas GR, Burkhard P, Beck Z. Self-Assembling Protein Nanoparticles: implications for HIV-1 vaccine development. Nanomedicine (Lond). 2018; 13: 2121-2125.
- Sun W, He L, Zhang H, Tian X, Bai Z, Sun L, et al. The self-assembled nanoparticle-based trimeric RBD mRNA vaccine elicits robust and durable protective immunity against SARS-CoV-2 in mice. Signal Transduct Target Ther. 2021; 6: 340.
- Yan Y, Wang X, Lou P, Hu Z, Qu P, Li D, et al. A Nanoparticle-Based Hepatitis C Virus Vaccine with enhanced Potency. J Infect Dis. 2020; 221: 1304-1314.