Potsmortem Study and Attributable Mortality Due to Candidiasis in Non-Neutropenic Critically Patient
- 1. Department of Medicine, Hospital Sant Rafael, Spain
- 2. Department of Pathology, Hospital Quirón, Spain
- 3. Department of Pathology, Hospital General de Catalunya-Quirón, Sant Cugat del Valles, Spain
- 4. Unió Catalana d’Hospitals, Spain
Abstract
The relationship between death attributable to Candida spp and histopathological results is studied in an ICU population where Candida spp is isolated. Prospective study in non-neutropenic ICU patients in whom Candida spp. has been Candida spp has been detected. The following are defined: invasive candidiasis based on dissemination and multifocality, the therapeutic protocol, and the methodology of the postmortem study. Attributable mortality is distinguished according to statistical, histopathological and clinical criteria. The presence of Candida spp was observed in 145 cases of 3389 ICU discharges (4.3%). There are 120/145 cases (83%) classified as invasive candidiasis and 25/145 colonizations (17%). ICU mortality is 35% (51/145) and hospital mortality is 46% (67/145). The postmortem study has been carried out in 71% of those who died in the ICU (36/51). Candida albicans is the most frequently isolated species (87%), followed by Candida glabrata (18%). 24 candidemias and 3 endophthalmitis have been demonstrated. Isolation in bronchial secretions is the most frequent (86%), followed by pharynx (83%) and gastric secretions (78%); positive urine culture has been obtained in 36% of cases. The attributable mortality has been 25% by statistical methods, 28% by the postmortem study and 23% by clinical data. Abdominal surgery and non-Candida albicans are risk factors for attributable mortality, and correct antifungal treatment is a protective factor against said attributable mortality. In conclusion: histopathological attributable mortality is like that defined by both statistics and clinical data; the simplification of the definition of invasive candidiasis, in non-neutropenic critically ill patients, allows for faster and more targeted therapeutic action. The early antifungal treatment may improve therapeutic results in this population.
KEYWORDS
- Candidiasis; Attributable motality; Non-neutropenic; ICU; and Postmortem study
CITATION
Ibañez-Nolla J, Garcia F, Kaur R, Nolla-Salas M (2024) Potsmortem Study and Attributable Mortality Due to Candidiasis in Non-Neutropenic Critically Patient. Ann Clin Pathol 11(2): 1173.
INTRODUCTION
The evaluation of post-mortem cultures has been controversial since the studies of Carpenter et al., [1]. Microbial invasion during the period of agony, contamination of tissues during the post- mortem study and the preservation of the cadaver [2,3] are only some of the factors involved. Leaving these issues to one side, we decided to analyse the mortality attributable to Candida spp in non-neutropenic critical patients, taking as a starting point the conclusions of Martín Álvarez et al. [4], about the establishment of a correct clinical-pathological-microbiological correlation in order to obtain valid results with respect to attributable mortality. The objective was to discover whether a simple definition of untreated or insufficiently treated multifocal or disseminated candidiasis was related to mortality attributable to Candida spp according to the post-mortem study. Infections caused by Candida spp. in non- neutropenic critically ill patients have high prevalence [5] and morbi-mortality rates [6-10] . Early diagnosis and treatment of these patients can decrease the severity and incidence of these infections. Attributable mortality is defined in the majority of reports as the difference between crude mortality and group study mortality [11-13]; in some diseases, specifically infections, the analysis of microbiologic and histopathologic data, both pre and postmortem, may be a better guide and may be considered as a good method due to the evaluation ofdiagnosis. There are no specific studies on the classification of etiologic process of mortality in critically ill patients with candidiasis comparing patients with and without postmortem study. In 1869 Robin Parrot identified the first cases of visceral localization of Candida, but it was not until the antibiotic era when Folly-Warter (1949) gave pathogenic value to said fungus. In humans, Candida spp is a saprophytic fungus, which usual location is the digestive tract. For invasion and dissemination to occur, apart from the use of broad-spectrum antibiotics that modify the intestinal ecosystem, significant changes are required in the body’s defenses: decrease in the bactericidal function of leukocytes and the immune response cellular, generally associated with a breakdown of some muco-cutaneous barrier [14]. In the ICU, non-neutropenic patients who present with multiple organ failure and who will require prolonged ventilation (> 1 week) will be candidates for high risk of invasive or disseminated candidiasis, by the existence of an immunodeficiency secondary to multiorganic failure itself [6]. The diagnosis of this type of infection becomes complex. Frequently candidemia may appear for the first time only a few days before death. Candidemia occurs in 10-20% [6-8] and endophthalmitis in 3.7-25% of patients [15,16]. It is usually very difficult to identify deep-seated candidiasis in these patients. Based onprevious studies of diagnosis and treatment [14,17-20], we elaborated a diagnostic algorythmn to classify the cause of mortality due to candidiasis in non-neutropenic critically ill patients, in an attempt to establish a standardized method. The study presented was carried out to answer these questions related to the postmortem studies: identify the mortality attributable to Candida spp and that this is significantly reduced with antifungal treatment (adequate and early); and verify that Multifocal Candidiasis is a good indicator of early diagnosis of Invasive Candidiasis.
METHODS
Patients
A prospective study was carried out over 7 years in a 10- bed general ICU. Inclusion criteria was ICU patients in whom the presence of Candida spp. was detected in any sample throughout their treatment or in the postmortem study. Exclusion criteria: Isolation of Candida spp. in cultures prior to admission to the ICU; severe neutropenia (< 500/mm3); patients for whom a study of the presence of yeast in different foci could not be carried out during their stay in the ICU, once it was detected in a sample. Samples were taken from bronchial secretions, urine, throat smear, stool, wound, drainage fluids, fluids from sterile areas (cerebrospinal fluid, pleura, peritoneum, biliary, pericardium), hystologic samples, blood cultures and cultures from catheter-tips. Cutaneous tests were made to evaluate cellular immunity [21,22] using Multitest IMC® (Rhône Poulenc Pharma SAE, Alcorcón, Spain). An ophthalmologic evaluation was made in all patients in order to discard endophthalmitis [15]. All clinical, microbiological and demographic data were collected from the study group. A postmortem study with microbiological and hystological analyses was carried out when family consent was given, in line with the hospital Ethics and Mortality Committees guidelines.
Microbiology
Biopsy samples were obtained using the method of De Jongh modified by Dolan [2], and were sent to the microbiology laboratory for fungal and bacterial isolation and identification. All pre and postmortem specimens were cultured on the following agar media: Columbia 5% sheep blood, chocolate PolyVitex, McConkey, and Sabouraud agar with gentamycin and chloramphenicol for fungal and bacterial detection. All cultures were incubated at 370 C under aerobic and anaerobic conditions and in a CO2-enriched atmosphere. Identification of yeasts was made by morphology (germ tube production) and biochemical tests using the ID 32C® system (bioMèrieux SA, 69280 Mercy-l’Etoile, France). All microorganisms isolated were identified by standard laboratory methods [23]. The congruence or non-congruence of pre and postmortem positive cultures was analyzed in order to validate postmortem cultures. Identification of the same microorganisms in postmortem cultures as in premortem cultures or negative postmortem cultures in a correctly treated patient was considered congruent; identification of microorganisms in postmortem cultures not identified premortem was considered non-congruent.
Definition of multifocal candidiasis and disseminated candidiasis
- Multifocal candidiasis: Simultaneous isolation of Candida spp. in 2 or more of the following foci [20]:
- Respiratory focus: Isolation of tracheal aspirate samples.
- Digestive focus: Simultaneous isolation of gastric aspirate and pharyngeal swab.
- Urinary focus: Positive urine cultures.
- Drains: Positive culture of samples obtained from different drains.
- Disseminated candidiasis: Presence of endophthalmitis due to Candida spp or isolation from biopsies, usually sterile organic fluids or blood culture with a negative catheter tip.
- Invasive candidiasis: Patients who present both multifocal and disseminated candidiasis are included in this group.
- Colonization by Candida spp: When the isolation has occurred in a single focus not suggestive of dissemination or at the skin level, feces or vaginal smear.
Antifungal treatment
Antifungal treatment was performed in patients classified as having multifocal or disseminated candidiasis (Invasive Candidiasis). No antifungal treatments were performed in cases considered colonization. The initial treatment indicated was fluconazole at a dose of 200 mg/24 h. As a second choice, in cases of poor clinical evolution with persistence of Candida spp in the cultures, or in case of identification of Candida glabrata or Candida krusei, amphotericin-B was used at a dose of 50 mg/24 h. In cases of invasive candidiasis, if the treatment has not lasted more than 5 days or has not been carried out, it has been considered insufficient. Thus, correct treatment is defined when it is indicated and has been maintained for a minimum of 5 days, and incorrect treatment if it is indicated and has not been carried out or if 5 days have not been completed.
Postmortem study
Informed study has been requested from family members to perform the autopsy on all those who died in the ICU. All autopsies carried out with this consent were performed according to the M. Lefulle technique [24], assessing the causes of death and secondary injuries. Histological study to search for microorganisms in the autopsy: The usual stains (hematoxylin-eosin, periodic acid- schiff (PAS), gomori silver-methamine) were systematically performed. With this, the presence of yeast infection was diagnosed when filamentous forms (pseudohyphae) and spores associated with inflammatory infiltrates were found, considering their anatomical location [25,26]. Post-mortem microbiological study: In all autopsies, a microbiological study of different organs (lung, spleen) and other locations (peritoneal fluid, pleural fluid, cerebrospinal fluid, abscesses, purulent urine, etc...) was carried out according to the patient’s previous data and the macroscopic findings of the autopsy. The microbiological study of the samples was carried out according to the traditional method [2-4,27]. In some cases, the De Jongh method modified by Dolan [2] has also been used. The results of the cultures are reported, stating the type of microorganism and its location. Assessment of yeasts according to the affected organs: the findings of the postmortem study, both by histology and cultures, have been interpreted according to the organ of origin of the sample [26-29]. Histologies and culture samples have been considered significant in the following cases:
a. Heart, liver, spleen and brain: Presence of yeast in histology associated with polymorphonuclear inflammatory foci in different areas of the parenchyma studied.
b. Blood and spleen: Cultures obtained by puncture of cardiac cavities or splenic parenchyma with a sterile needle and prior cauterization of the puncture point.
c. Intestine: Presence in histology of pseudohyphae or spores that extend to the muscle and/or serosa, together with the presence of an important polymorphonuclear infiltrate. The culture is assessed from samples obtained from the peritoneal cavity or bile fluid.
d. Lung: Histology is evaluated if pseudohyphae and spores are found in the parenchyma, alveoli and vessels, along with polymorphonuclear infiltrates. The culture has been obtained from areas of the parenchyma distant from the main bronchi and trachea.
e. Kidneys: Presence of yeast with a polymorphonuclear inflammatory component in the kidney parenchyma. The culture is obtained from areas of the parenchyma and not from the urinary tract.
Positive results were not considered significant when they were obtained only in any of the following samples: Trachea, culture of the tracheal region and main bronchi in purulent mucus, it is collected with a swab; and urinary tract, culture of urinary bladder contents obtained by aspiration with a sterile syringe, in case of cloudy urine and with macroscopic inflammation of the lower urinary tract.
Interpretation of postmortem results in relation to the patient’s cause of death:
- Negative histology and postmortem cultures in significant organs positive for yeast without identification of other microorganisms: These cultures have been considered significant in relating yeast to the cause of death.
- Negative histology and postmortem cultures in significant organs positive for yeast with identification of other microorganisms: Yeast has not been considered as a cause of death.
- Negative cultures and positive histology in a patient who has received correct antifungal treatment: Death is attributed to yeast.
- Negative cultures and positive histology in more than one organ, in a patient who has received incorrect antifungal treatment: Death is attributed to yeast.
- Negative cultures and positive histology in a single organ, in a patient who has received incorrect antifungal treatment: Yeasts have not been considered as the cause of death.
- Cultures and histology positive for yeast: These have been considered the cause of death.
Definition of attributable mortality
1. Statistical definition of attributable mortality [11,13,30]: It is the difference between the mortality of the group studied and overall mortality-.
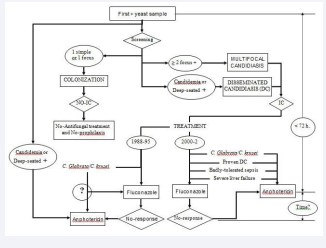
2. Definition of attributable mortality based on the postmortem study: It refers to patients who die because of a certain factor that is being analyzed. In a certain way, the “gold standard” for the diagnosis of invasive candidiasis will be biopsy [31] or the postmortem study [26]. In this study, the factor to be analyzed was candidiasis acquired in the ICU in non-neutropenic patients, as the main cause or factor that could influence the patient’s death. Following this work methodology, a classification algorithm for high probability of death attributable to yeast is defined [Figure 1].
Figure 1: Diagnostic-therapeutic algorythmn applied.
DC (Disseminated candidiasis), IC (Invasive candidiasis).
Foci: Respiratory (bronchial secretions), Digestive (gastric secretions
and throat smear), Urinary (urine) and Drainages.
Changes in the therapeutic plan related on the results of the application of the algorithm defined in 1988, which included Multifocal Candidiasis within the concept of Invasive Candidiasis.
3. Definition of attributable mortality based on the clinical study: The cases in which the postmortem study was not performed have been used as a reference group. The group of patients with a moderate probability of death attributable to yeast is defined based on the clinical cause of death, the existence of a multifocality of yeast isolations and the performance of incorrect antifungal therapy before death.
4. Definition of global attributable mortality: Global mortality attributable to yeast has been considered the sum of cases that have been defined as attributable mortality based on the postmortem study and those defined as attributable mortality based on the clinical study, in relation to the total of the deceased population
Statistical analyses
Categorical variables were compared between two groups with Chi-square or Fisher exact test as appropiate. Continous variables were analyzed with Student’s t test or Mann-Whitney U test when the distribution departed from normality, and described as mean (standard deviation) or as median (range of values), respectively. To estimate the odds ratio of attributable mortality for variables of interest, we used a logistic regression model adjusted for potentially confounding variables [12]. The statistical significance was established at p value < 0.05. Data were analyzed using the SPSS statistical program.
RESULTS
During the 7-year period, 3389 patients were treated in the ICU, with an average stay of 5.8 days (1-112) and a mortality of 9.6% (324). Of the total number of deaths, the postmortem study was carried out in 42% of cases (136). During the study period, there were 149 patients (4.4%) in whom yeasts were isolated throughout their stay in the ICU. Following the exclusion criteria set forth, 8 cases were rejected. Two of them for having isolated Candida spp in cultures prior to admission to the ICU, both died. The other 6 were excluded because they did not perform the multifocality study. Candida spp was identified in the urine culture prior to the patient’s discharge from the ICU. All of them survived without carrying out any antifungal treatment. Data from 141 patients were collected, observing that there were 4 patients (3%) who were subsequently readmitted, and that yeast was detected again during readmission. In total, it has been considered that there have been 145 cases meeting the inclusion criteria in the study. ICU mortality was 35% (51/145) and hospital mortality was 46% (67/145). The postmortem study was performed in 36 of the 51 cases who died before hospital discharge (71%).
Characteristics of the population studied
The population is made up of 77% men (111/145), with an average age of 53 (22) years. The average stay in the ICU is 28 days (2-93). The reason for admission was medical in 63% (91) and surgical in 37% (54). APACHE III score is 76 (18-138).
Comorbidity has been found to be 70% (102). In 34% (49) of them, the presence of more than 2 associated pathological processes was confirmed. The most frequent processes were:
Chronic obstructive pulmonary disease in 23% (34), diabetes mellitus in 22% (32), arterial hypertension in 22% (32), solid neoplasia in 13% (19), malignant hematological disease in 5%. (7), leukopenia less than 3000/mm3 upon admission to the ICU without criteria for neutropenia (> 500 neutrophils/mm3) in 4% (6) and acquired immunodeficiency syndrome in 3% (4). Risk factors for fungal infection were detected in 94% (139) of the patients upon admission to the ICU and in 100% at the time of the first yeast isolation [Table 1].
Table 1: Presence of risk factors for candidiasis in the study group
|
Risk factors for candidiasis |
Number of cases (%) |
|
Antibiotics |
144 (99) |
|
Central venous catheter |
143 (99) |
|
Urinary catheter |
140 (97) |
|
Antacid therapy |
141 (97) |
|
Naso-orogastric catheter |
131 (90) |
|
Arterial catheter |
125 (86) |
|
Oro-nasotracheal tube and / or tracheostomy |
124 (85) |
|
Surgical procedures |
85 (59) |
|
Vasoactive drugs |
72 (50) |
|
Total parenteral nutrition |
68 (47) |
|
Drainages |
65 (45) |
|
Blood derivatives |
62 (43) |
|
Corticoids |
56 (39) |
|
Hemodyalisis |
8 (5) |
|
Splenectomy |
5 (3) |
In 58% (84) of the cases there were a minimum of 10 associated risk factors when the first yeast isolation was obtained. Causes of admission of the study group are shown in Table 2.
Characteristics of fungal infection
1. Positive samples: The samples that were most frequently positive, both in the first positive culture and in screening, were the bronchial secretion cultures (66% and 86% respectively) [Table 2].
Table 2: Causes of admission of the study group
|
CAUSES OF ADMISSION |
Number of cases (%) |
|
Respiratory failure |
17 (12) |
|
Cardiovascular |
23 (16) |
|
Sepsis |
9 (6) |
|
Respiratory infection |
19 (13) |
|
Multiple trauma |
52 (36) |
|
Neurologic (non-cranioencephalic trauma) |
9 (6) |
|
Digestive and others |
16 (11) |
2. Blood cultures: The percentage of patients with candidemia, in relation to the number of patients treated during said period, has been 0.5% (18/3389). In total there were 24 positive blood cultures in 18 patients. In 4 of them the isolation was in the first sample, in 14 in the screening and in 6 in the follow-up blood cultures. 27 catheters with colonized tips were detected. Four in the first sample, 16 in the screening and 7 in the follow-up cultures. In 8 patients the association of a positive catheter with a positive blood culture was verified (44% of patients with a positive blood culture).
3. Identified yeast species: The most frequently isolated yeast has been Candida albicans, both in the first positive culture and in the screening (80% and 87% respectively), followed by Candida glabrata, also in both cases (11% and 18% respectively).
4. Classification according to location of the yeasts: The patients were grouped into multifocal candidiasis in 89 cases (61%), disseminated candidiasis 31 cases (22%) and colonization by Candida spp in 25 cases (17%). In total, 120 invasive candidiasis have been detected, representing 3.5% of the population treated during the period studied (120/3389):
a) Multifocal candidiasis: The respiratory focus was identified in 96% (85), the digestive focus in 93% (83), the urinary focus in 42% (37) and the drainage in 2% (2). In 67% (60) 2 simultaneous foci were demonstrated, in 33% (29) 3 foci and there were no cases with 4 foci.
b) Disseminated candidiasis: They were classified as such because they demonstrated the presence of endophthalmitis in 3 cases (one of them with a positive blood culture). In 7 cases the evidence was positive blood cultures and negative catheter tip. There were 12 cases in which the yeast was identified in samples obtained from puncture of abscesses, in 2 cases from cerebrospinal fluid and in another 2 from tissue biopsies. In 5 cases evidence of dissemination was demonstrated in the postmortem study.
c) Colonization: Yeasts were identified in bronchial secretions 15 cases, pharynx 15 cases, stool culture 10 cases, gastric aspirate 8 cases, urine culture 5 cases, vagina 2 cases, wounds 2 cases, catheters 2 cases, catheter together with positive blood culture 1 case, skin 1 case and nasal 1 case.
Antifungal treatment: Antifungal treatment was carried out in 109 cases (75%). In 95 of them, azole derivatives were used for 10 days (1-30) and in 42 amphotericin B was used for 15 days (4-39). In 4 cases, the administration of amphotericin-B had to be stopped due to nephrotoxicity. Antifungal treatment could be performed in 15 of the 18 cases that had a positive blood culture (83%). In 26 cases, the antifungal was changed due to poor clinical or microbiological response (isolation of Candida glabrata or Candida krusei).
Mortality: Mortality in cases of disseminated candidiasis was 48% (15/31), in the case of multifocal candidiasis 46% (46/89) and in colonization 24% (6/25). The mortality of cases with positive blood culture was 39% (7/18). 5 of the 15 patients (33%) in whom antifungal treatment was started and 2 of the 3 (67%) in whom such treatment was not carried out died. In the group of deceased, 54% (36/67) of the patients received correct treatment, a postmortem study was carried out in 50% of cases (18/36), and the treatment was not correct in 46% of cases (31 /67), with the postmortem study being carried out in 58% of them (18/31).
Postmortem study: Of the 38 postmortem studies carried out, 2 cases have been excluded because the autopsy study was not carried out following the described protocol. In total, 36 autopsies have been considered valid (71% of those who died in the ICU). The cause of death according to the autopsy study was septic shock/multiple organ failure in 18 cases (50%), respiratory failure in 8 cases (22%), brain death in 4 cases (11%), shock nonseptic in 3 cases (8%), failure in 3 cases (8%). The causes of death in the study group are shown in the Table3.
Table 3: Causes of death in the study group
|
Causes Of Death |
Number of cases (%) |
|
Septic shock – Multiorganic dysfunction syndrome |
18 (50) |
|
Respiratory failure (hypoxemia) |
8 (22) |
|
Brain death |
4 (11) |
|
Non-septic shock |
3 (8) |
|
Hepatic failure |
3 (8) |
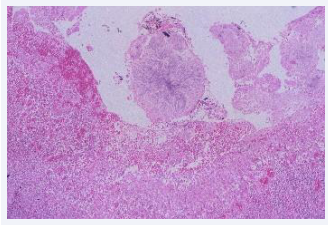
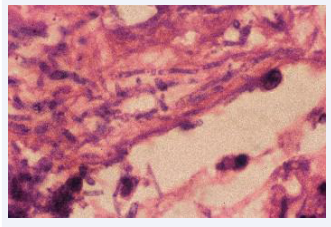
The postmortem microbiological study has demonstrated the presence of yeast in 16/36 autopsies. In 8 of them only positive cultures for yeast were obtained, in 4 cases the presence of yeast was only demonstrated in histology and in another 4 yeasts were present in both the cultures and in the histology [Figures 2,3].
Figure 2: Pseudohyphae and budding yeasts in a rechannelled pulmonary arteria trombus (HE 100x).
Figure 3: Inflammatory infiltrate with budding yeasts in a Vater ampulla. (HE 400x).
The organs affected according to the culture, histology or both simultaneously are detailed in Table 4.
Table 4: Postmortem evidence of yeasts in the study group.
|
Location |
Positive cultures only |
Positive histology only |
Positive cultures and histology |
All positive cultures (n=24) (%) |
All positive histology (n=36) (%) |
|
Lung |
5 |
3 |
3 |
8 (33) |
6 (17) |
|
Trachea |
4 |
|
|
4 (17) |
|
|
Bowel |
2 |
1 |
1 |
3 (12) |
2 (6) |
|
Heart |
1 |
2 |
|
1 (4) |
2 (6) |
|
Kidney |
1 |
2 |
|
1 (4) |
2 (6) |
|
Urinary tract |
3 |
|
|
3 (12) |
|
|
Liver |
2 |
|
|
2 (8) |
|
|
Spleen |
2 |
|
|
2 (8) |
|
|
CNS |
|
1 |
|
|
1 (3) |
|
Positive samples |
20 |
9 |
4 |
24 |
13 |
CNS: Central nervous system.
The species isolated in the postmortem cultures were Candida albicans in 9 cases, Candida glabrata in 2 cases and Candida tropicalis in 1 case. There were 3 patients in whom the result of the postmortem study did not coincide with the premortem study. In two cases it was because the diagnosis was made postmortem and in the other case because the species isolated in the postmortem study was different from that identified in the patient in life. In the remaining 9, the species identified in the postmortem study coincided with the identification in the live follow-up.
Attributable mortality
- Attributable mortaity according to statistical analysis, if the ICU mortality of the studied population was 35% and that of the population treated in the ICU during the study carried out was 9.6%, the attributable mortality according to statistical analysis is 25%.
- Attributable mortality according to postmortem study, of the 16 patients in whom yeast was identified in the postmortem study, there were 6 who died from respiratory failure, 5 from septic shock or MODS, 2 from non-septic shock, 2 from brain death and 1 case from hepatocellular insufficiency. According to the criteria based on the postmortem study, death was attributed to yeast in 10 cases (28%) [Table 5].
Table 5: Attributable mortality due to yeasts from patients with postmortem study.
|
Patients |
Cause of death |
CD Risk |
Histology |
Cultures |
Antifungal treatment |
|
1. JGB |
Non-septic shock |
Multifocality |
> 1 S.O. |
Negative |
Not given* |
|
2. ACG |
Septic shock |
Multifocality |
S.O. |
S.S. |
Given |
|
3. JLS |
Respiratory failure |
Multifocality |
Non-S.O. |
S.S. |
Given |
|
4. MTN |
Respiratory failure |
Multifocality |
S.O. |
S.S. |
Not given |
|
5. APS |
Septic shock |
Multifocality |
S.O. |
S.S. |
Not given |
|
6. JFC |
Respiratory failure |
Multifocality |
S.O. |
S.S. |
Given |
|
7. VLG |
Septic shock |
Multifocality |
> 1 S.O. |
Negative |
Not given * |
|
8. AMG |
Respiratory failure |
Multifocality |
S.O. |
Negative |
Given * |
|
9. BCP |
Septic shock |
Multifocality |
S.O. |
Negative |
Given * |
|
10.AMM |
Septic shock |
Multifocality |
S.O. |
S.S. |
Not given |
S.O.: Significant organs for histopathology. S.S.: Significant samples for microbiology.
* Definitive as attributable mortality.
3. Attributable death based on the clinical study, of the 31 cases in which no postmortem study was performed, 7 cases (23%) were considered to meet the criteria for death attributable to yeast [Table 6].
Table 6: Attributable mortality due to yeasts from patients without postmortem study.
|
Patients |
Cause of death |
CD Risk |
Candida spp. |
Antifungal treatment |
FUC |
|
1. JMS |
Septic shock |
Multifocality |
C. glabrata |
Incorrect |
Not made |
|
2. ECA |
Septic shock |
Multifocality |
C. glabrata C. albicans |
Incorrect |
Positive |
|
3. FVP |
Respiratory failure |
Multifocality |
C. glabrata C. albicans C. tropicalis |
Incorrect |
Positive |
|
4. BGB |
MODS |
Multifocality |
C. albicans |
Correct |
Positive |
|
5. TLC |
Septic shock |
Multifocality |
C. glabrata C. albicans C. krusei |
Incorrect |
Not made |
|
6. GMM |
MODS |
Multifocality |
C. albicans |
Incorrect |
Positive |
|
7. JG |
MODS |
Multifocality |
C. glabrata C. albicans C. tropicalis |
Incorrect |
Not made |
FUC: Follow-up cultures. MODS: Multiorganic dysfunction syndrome
4. Overall attributable mortality, assessing the data on attributable death according to the postmortem study together with that based on the clinical study, it can be considered that there were 17 cases out of 67 (25%) in which death could be attributed to yeast. This number of patients represents a mortality related to Candida spp. of 12% in the 145 patients studied.
The Predictive factors of overall mortality attributable to yeasts are shown in [Table 7]:
Table 7: Characteristics of patients by attributable mortality.
|
VARIABLES |
NAM |
AM |
P |
|
Age |
51 (22) |
69 (14) |
0.001* |
|
Previous diseases |
2 (0-4) |
3 (1-4) |
0.009† |
|
Apache III |
72 (24-136) |
90 (58-183) |
0.040† |
|
Abdominal surgery on admission |
24/128 (19) |
9/17 (53) |
0.001‡ |
|
More than one foci (risk classification) |
100/128 (78) |
17/17 (100) |
0.043§ |
|
Candida glabrata at screening |
20/128 (16) |
6/15 (40) |
0.020‡ |
|
Candida tropicalis at screening |
6/128 (5) |
3/15 (20) |
0.036§ |
|
Urine cultures at FUC |
20/128 (16) |
13/75 (75) |
<0.001§ |
* Student’s T test. Mean (SD)
† U Man-Whitney test. Median (range of values)
‡ Chi-square test. Number of positive cases / total (%)
§ Fisher Exact test. Number of positive cases / total (%)
NAM: Non-attributable mortality. AM: Attributable mortality. FUC: follow-up cultures.
age, previous disease, APACHE III, abdominal surgery as a risk factor, culture positive urine test at follow-up and identification of Candida glabrata and Candida tropicalis at screening. The multivariate analysis has selected
3 variables with statistical significance: abdominal surgery, Candida glabrata at screening and correct antifungal treatment as a protective factor [Table 8].
Table 8: Adjusted Odds Ratio of attributable mortality for variables of interest.
|
Variables |
Attributable Mortality |
||
|
Odds Ratio |
95% Confidence Interval |
P |
|
|
Abdominal surgery |
9.17 |
1.77-47.39 |
0.008 |
|
Antifungal treatment |
<0.01 |
<0.01-0.10 |
<0.001 |
|
Candida glabrata at screening |
7.38 |
1.24-43.98 |
0.028 |
|
Previous diseases |
1.82 |
0.98-3.37 |
0.055 |
DISCUSSION
It is now accepted that Candida spp infection is due to endogenous colonization and is facilitated using broad-spectrum antibiotics. Despite everything, we must not forget the possibility of an exogenously transmitted nosocomial infection that may occasionally occur [12,32-34]. Like other authors [6,10,35,36], this study confirms that Candida albicans is the most frequently isolated yeast in our environment, followed by Candida glabrata. When the study presented was designed, it was decided to define multifocality without incorporating the concept of colonization density or the colonization index by Candida spp. proposed by Pittet et al. [32], among other things because their results had not yet been published. However, our previous works [14], which coincide with those of other authors [37-41], had shown that the number of locations involved in colonization, together with the alterations in host defenses, characteristics of non- neutropenic critically ill patients treated in the ICU, they are key factors in the development of fungal infection. One piece of information that reveals an uncontrolled spread of mucosal colonization is the isolation of Candida spp simultaneously in samples of tracheal aspirate, pharyngeal swab and gastric aspirate. This concurrence, in the context of a patient under broad-spectrum antibiotic therapy and with a defense deficit secondary to his critical condition maintained for more than 10 days, leads to the assumption of a very high risk of translocation and consequently the development of a Candida spp. infection. In the study presented, more than 90% of the patients considered to have invasive candidiasis had positive cultures simultaneously in bronchial secretions, pharyngeal swab, and gastric aspirate. Based on the pre-established definitions in the work methodology, it can be observed that the incidence of invasive candidiasis in ICU patients is 3.5%, very similar to that observed by other authors (3.6 and 4.7%) [42,43]. However, it is striking: a high incidence of candidemia in ICU patients (0.5%) compared to other published studies (0.2%) [37]. The incidence of endophthalmitis in patients with Candida spp. (2%) is lower than the prevalence of this clinical manifestation. According to the studies consulted, it ranges between 3 and 30% [7,8,15,16,37,44,45].
The methodology applied for the early detection of yeasts in a risk population in the ICU has surely played an important role in obtaining positive blood cultures and in carrying out early antifungal therapy. It is likely that this treatment has significantly reduced ocular septic metastases and mortality in cases of candidemia (39% in this study compared to 58-80% in other reports) [37,47-57]. This study shows that the mortality related to invasive candidiasis, in a population considered high risk due to having detected mucosal colonization, was 12% and the mortality not related to said infection was 34%. The bibliographic review carried out shows that the mortality related to Candida spp. infection ranges between 8% according to Guiot et al. [58] and 70% according to Merz et al. [59]. The attributable mortality according to the statistical analysis (25%) has been very similar to the mortality based on the postmortem study (28%) and that based on clinical data (23%). These results on attributable mortality based on the postmortem study have been compared with those of other studies in which a postmortem study was performed on more than 50% of the deceased population. In these works [10,36,58,59] the attributable mortality ranges between 26 and 57%. A comparison of attributable mortality has also been carried out with studies in which clinical criteria were considered along with histopathological criteria [7-9,60,61]. In this group of articles, none have been found with several postmortem studies greater than 50% of the deceased. It is striking that in the works consulted the attributable mortality ranges between 47 and 75%. Other reports have been examined separately [11,37,62-66] in which there is no record of the number of postmortem studies carried out. These authors propose an attributable mortality that ranges between 38 and 52%. All these data indicate that there is no uniformity of criteria when evaluating and interpreting the results obtained in the event of a fungal infection. However, the methodology used in this study allows us to ensure that attributable mortality according to a simple statistical analysis is superimposable both with attributable mortality based on postmortem data and clinical data. At the same time, the results obtained allow us to agree with other authors [6,25,26,66-68] on the relevance of the lung in invasive candidiasis. It is confirmed that the lung is the most frequent location of Candida spp., both in living patients (in bronchial secretions) and in deceased patients (in lung tissue).
When the univariate statistical analysis has been carried out, it has been seen that chronic diseases, age, APACHE III [69] and abdominal surgery are risk factors in relation to attributable mortality. The existence of more than one focus and a positive urine culture for yeast in the follow-up study are also risk factors in relation to attributable mortality. The multivariate analysis shows that abdominal surgery is a risk factor for attributable mortalit, isolation of non-albicans Candida (Candida glabrata) is another risk factor for attributable mortality and correct antifungal treatment is a protective factor on attributable mortality.
FINDINGS
It can be concluded that the study presented confirms that the stated definition of untreated or insufficiently treated invasive candidiasis is related to a death attributable to Candida spp both according to the postmortem study and through clinical data. We believe this shows the importance of microbiological postmortem cultures as a complementary and sometimes principal method of diagnosing attributable mortality, taking into account the possibility of false-negative histology. Our study of candidiasis using multifocal classification combined with early diagnosis and antifungal treatment [18,20], has resulted in a reduction in the attributable mortality rate. The method described may be a good way of standardizing attributable mortality, not only when postmortem study is performed but also when autopsies are not carried out.
CONCLUSIONS, LIMITATIONS AND RECOMMENDATIONS
Theneedforacorrectclassificationofinvasivecandidiasisinnon- neutropenic critical ICU patients is demonstrated. This classification will require adequate therapeutic action, without having to propose prophylaxis concepts that may lead to insufficient treatments or the selection of resistant flora. A late start of treatment or inadequate treatment, when there is data of invasive candidiasis according to the proposed proposal, has a direct relationship with the mortality attributable to Candida spp according to the data obtained from the postmortem study. The mortality attributable from postmortem studies is very similar to that deduced from statistical criteria and from clinical data. Antifungal therapeutic alternatives, when solid foundations have been established for an early diagnosis of invasive candidiasis, allow the minimization of mortality attributable to Candida spp in this population.
REFERENCES
- Carpenter H, Wilkins RM. Autopsy bacteriology: Review of 2033 cases. Arch Pathol. 1964; 77: 73-81.
- Dolan CT, Brown AL, Ritts RE. Microbiological examination of postmortem tissues. Arch Pathol. 1971; 92: 206-211.
- DuMoulin GC, Paterson DG. Clinical relevance of postmortem microbiologic examination: A review. Hum Pathol. 1985; 16: 539-548.
- Martín Álvarez R, Pérez Sáenz JL. Microbiología postmortem. Med Clin (Barc). 1983; 81: 667-669.
- Vincent JL, Sakr Y, Singer M, Ignacio Martin-Loeches, Flavia R Machado, John C Marshall, et al. Prevalence and outcomes of infection among patients in Intensive Care Units in 2017. JAMA. 2020; 323: 1478-1487.
- Fraser VJ, Jones M, Dunkel J, Storfer S, Medoff G, and Dunagan WC. Candidemia in a tertiary care hospital: epidemiology, risk factors, and predictors of mortality. Clin Infect Dis. 1992; 15: 414-421.
- Harvey RL, Myers JP. Nosocomial fungemia in a large community teaching hospital. Arch Intern Med. 1987; 147: 2117-2120.
- Klein JJ, Watanakunakorn C. Hospital-Acquired fungemia. Its natural course and clinical significance. Am J Med .1979; 67: 51-58.
- Komshian SV, Uwaydah AK, Sobel JD, Crane LR. Fungemia caused by Candida species and Torulopsis glabrata in the hospitalized patient: frequency, characteristics, and evaluation of factors influencing outcome. Rev Infect Dis. 1989; 11: 379-390.
- Meunier-Carpentier F, Kiehn TE, Armstrong D. Fungemia in immunocompromissed host. Changing patterns, antigenemia, high mortality. Am J Med. 1981; 71: 363-370.
- Pittet D, Tarara D, Wenzel RP. Nosocomial bloodstream infection in critically ill patients. Excess length of stay, extra costs, and attributable mortality. JAMA. 1994; 271: 1598-1601.
- Wenzel RP. Nosocomial candidemia: risk factors and attributable mortality. Clin Infect Dis. 1995; 20: 1531-1534.
- Fagon JY, Chastre J, Hance AJ, Montravers P, Novara A, Gibert C. Nosocomial pneumonia in ventilated patients: a cohort study evaluating attributable mortality and hospital stay. Am J Med. 1993; 94: 281-288.
- Nolla M, Chanovas M, Torres JM, Nolla J, Garcés J. Cellular Immunity alterations and presence of Candida in patients admitted to the intensive care unit. In: Aochi O, Amaha K, Takeshita H (eds). Intensive and Critical Care medicine. Excerpta Medica. International Congress Series 885, Amsterdam 1990; 1135.
- Brooks RG. Prospective study of Candida endophthalmitis in hospitalized patients with candidemia. Arch Intern Med. 1989; 149: 2226-2228.
- Donahue SP, Greven CM, Zuravleff JJ, Eller AW, Nguyen MH, Peacock JE Jr, Wagener MM, Yu VL. Intraocular candidiasis in patients with candidemia. Clinical implications derived from a prospective multicenter study. Ophtalmology. 1994; 101: 1302-1309.
- Ibàñez-Nolla J, Torres-Rodríguez JM, Nolla M, et al. The utility of serology in diagnosing candidosis in non-neutropenic critically ill patients. Mycoses. 2001; 44: 47-53.
- Ibáñez-Nolla J, Nolla-Salas M, León MA, F García, J Marrugat, G Soria, et al. Early diagnosis of candidiasis in non-neutropenic critically ill patients. J Infect. 2004; 48: 181-92.
- Nolla-Salas M, Monmany J, Gich I, Ibàñez-Nolla J. Early Treatment of Candidiasis in Non-Neutropenic Critically Ill Patients. World Conference on Magic Bullets. Celebrating Paul Ehrlich’s 150th Birthday. Nürnberg, Germany, 2004; 347.
- Ibáñez-Nolla J, Nolla-Salas M. Multifocal Candidiasis can be considered a form of Invasive Candidiasis in critically non neutropenic patients. Int J Infect Dis. 2024; 147: 107171.
- Lefemine A, Acuff R, Vo N, Waycaster M. Delayed hypersentivity on a surgical service. J Am Coll Nutr. 1988; 7:355-359.
- Maxwell AP, McCluskey DR. Assessment of cell-mediated immunity in a British population using multiple skin test antigens. Clin Allerg. 1986; 16:365-369.
- Balows A, Harsler WJ. Manual of Clinical Microbiology. 5th ed., section III. American Society for Microbiology, Washington, 1991. DC. 209- 553.
- Ludwig J. Current methods of autopsy practice. Sunders Company WB, Philadelphia 1979.
- Bodey G, Bueltmann B, Duguid W, Gibbs D, Hanak H, Hotchi M, Mall G, Martino P, Meunier F, Milliken S, Naoe S, Okudaira M, Scevola D, van’t Wout J. Fungal infections in cancer patients. An International Autopsy Survey. Eur J Clin Microbiol Infect Dis. 1992; 11: 99-109.
- Hughes WT. Systemic candidiasis. A study of 109 fatal cases. Pediatr Infect Dis. 1982; 1: 11-18.
- Jandrlic M, Kalenic S, Labar B, Nemet D, Jakic-Razumovic J, Mrsic M, Plecko V, Bogdanic V. An autopsy study of systemic fungal infections in patients with hematologic malignancies. Eu J Clin Microbiol Infect Dis. 1995; 14: 768-774.
- DeGregorio MW, Lee WMF, Linker CA, Jacobs RA, Ries CA. Fungal infections in patients with acute leukemia. Am J Med. 1982; 73: 543- 548.
- Myerowitz RL, Pazin GJ, Allen CM. Disseminated candidiasis. Changes in incidence, underlying diseases and pathology. Am J Clin Path. 1977; 68: 29-38.
- Falagas ME, Snydman DR, George MJ, Werner B, Ruthazer R, Griffith J, Rohrer RH, Freeman R, Boston Center for Liver Transplantation CMVIG Study Group. Incidence and predictors of Cytomegalovirus pneumonia in orthotopic liver transplant recipients. Transplantation. 1996; 61: 1716-1720.
- Franklin C, Metry M. Life-threatening Candida infections in the intensive care unit. J Intensive Care Med. 1992; 7: 127-137.
- Pittet D, Monod M, Suter PM, Frenk E, Auckenthaler R. Candida colonization and subsequent infections in critically ill surgical patients. Ann Surg. 1994; 220: 751-758.
- Reagan DR, Pfaller MA, Hollis RJ, Wenzel RP. Evidence of nosocomial spread of Candida albicans causing bloodstream infection in a neonatal intensive care unit. Diagn Microbiol Infect Dis. 1995; 21: 191-194.
- Voss A, Hollis RJ, Pfaller MA, Wenzel RP, Doebbeling BN. Investigation of the sequence of colonization ans candidemia in nonneutropenic patients. J Clin Microbiol. 1994; 32: 975-980.
- Horn R, Wong B, Kiehn TE, Armstrong D. Fungemia in a cancer hospital: Changing frequency, earlier onset, and results of therapy. Rev Infect Dis. 1985; 7: 646-655.
- Meunier F, Aoun M, Bitar N. Candidemia in immunocompromised patients. Clin Infect Dis. 1992; 14; S120-S125.
- Nolla-Salas J, Sitges-Serra A, León-Gil C, Martínez-González J, León- Regidor MA, Ibáñez-Lucía P, Torres-Rodríguez JM. Candidemia in nonneutropenic critically ill patients: Analisys of prognostic factors and assessment of systemic antifungal therapy. Intensive Care Med. 1997; 23: 23-30.
- Anaissie E, Solomkin JS. Fungal infection. In: Wilmore DW, Cheung LY, Harken AH - American College of Surgeons (eds). FALL, Scientific American Surgery. Scientific American Inc., New York 1994; 1-19.
- Fung JC, Donta ST, Tilton RC. Candida detection system (Cand-Tec) to differentiate between Candida albicans colonization and disease. J Clin Microbiol. 1986; 24: 542-547.
- Kujath P, Lerch K, Kochendörfer P, Boos C. Comparative study of the efficacy of fluconazole versus amphotericin B/flucytosine in surgical patients with systemic mycoses. Infection. 1993; 21: 376-382.
- Pellinen TJ, Valtonen VV, Luomanmaki K, Sivonen A, Virtanen KS. The microbial colonization due to medical devices in intensive care patients with special emphasis on Candida albicans. Ann Clin Res. 1983; 15: 62-65.
- Trilla A. Epidemiology of nosocomial infections in adult intensive care units. Intensive Care Med. 1994; 20: S1-S4.
- Dlouhy P, Svejda J, Veselska A, Kalhousova V. Candida sepsis: Risk factors, pathogenesis and the clinical picture. Cas Lek Ces. 1993; 132: 393-396.
- Burchard KW, Minor LB, Slotman GJ, Gann DS. Fungal sepsis in surgical patients. Arch Surg. 1983; 118: 217-221.
- McQuillen DP, Zingman BS, Meunier F, Levitz SM. Invasive infections due to Candida krusei: Report of ten cases of fungemia that include three cases of endophthalmitis. Clin Infect Dis. 1992; 14: 472-478.
- Rex JH, Bennet JE, Sugar AM, Pappas PG, Van-der-Horst CM, Edwards JE, et al. A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. N Engl J Med. 1994; 331: 1325-1330.
- Caroll K, Jeppson K, Reading J, Reimer L. Factors influencing outcome in hospitalized patients with candidemia. Infect Dis Clin Pract. 1993; 2: 268-271.
- Develoux M, Gajdos P, Goulon M. Les candidoses disséminées. Vingt-trois cas observés dans un service de réanimation. Ann Méd Interne.1982; 133: 406-409.
- Dhahri MA, Jebali A, Lamine K, Labbene I, Ferjani M. Alerte aux septicémies à levures en réanimation polyvalente. Cahiers Anesthésiologie. 1995; 43: 13-19.
- Eubanks PJ, de Virgilio C, Klein S, Bongard F. Candida sepsis in surgical patients. Am J Surg. 1993; 166: 617-620.
- Neumann PR, Rakower SR. The risk of positive cultures for Candida in the critically ill patient. Crit Care Med. 1978; 6: 73-76.
- Sánchez-Rodríguez C, León-Regidor MA, Capell-Font S, Pérez-Campos A, Planes-Reig A, León-Gil C. Funguemia 1973-1983: Análisis de 67 pacientes. Med Clin (Barc). 1985; 84: 549-553.
- Slotman GJ, Burchard KW. Ketoconazole prevents Candida sepsis in critically ill surgical patients. Arch Surg. 1987; 122: 147-151.
- Soutter DI, Todd TR. Systemic candidiasis in surgical intensive care unit. Can J Surg. 1986; 29: 197-199.
- Bassetti M, Azoulay E, Kullberg BJ, et al. Definitions of Invasive Fungal Diseases: Summary of Activities of the Intensive Care Unit Working Group. Clin Infect Dis. 2021; 72:S121-7.
- Bassetti M, Scudeller L, Giacobbe DR, et al. Developing definitions for invasive fungal diseases in critically ill adult patients in intensive care units. Protocol of the Fungal Infections Definitions in ICU patients (FUNDICU) project. Mycoses. 2019; 62: 310-9.
- Bassetti M, Giacobbe DR, Agvald-Ohman C, Akove M, Alastruey- Izquierdo A, Arikan-Akdagli S, et al. Invasive Fungal Diseases in Adult Patients in Intensive care Unit (FUNDICU): 2024 consensus definitions from ESGCIP, EFISG, ESICM, ECMM, MSGERC, ISAC and ISHAM. Intensive care Med. 2024.
- Guiot HFL, Fibbe WE, Van’t Wout JW. Risk factors for fungal infections in patients with malignant hematologic disorders: Implications for empirical therapy and prophylaxis. Clin Infect Dis. 1994; 18: 525-532.
- Merz WG, Karp JE, Schron D, Saral R. Increased incidence of fungemia caused by Candida krusei. J Clin Microbiol. 1986; 24: 581-584.
- Calandra T, Bille J, Schneider R, Mosimann F, Francioli P. Clinical significance of Candida isolated from peritoneum in surgical patients. Lancet. 1989; 11: 1437-1440.
- Marsh PK, Tally FP, Kellum J, Callow A, Gorbach SL. Candida infections in surgical patients. Ann Surg. 1983; 198: 42-47.
- Aisner J, Schimpff SC, Sutherland JC, Young VM, Wiernik PH. Torulopsis glabrata infections in patients with cancer: Increasing incidence and relationship to colonization. Am J Med. 1976; 61: 23-28.
- Bryce EA, Roberts FJ, Sekhon AS, Coldman AJ. Yeast in blood cultures. Evaluation of factors influencing outcome. Diagn Microbiol Infect Dis. 1992; 15: 233-237.
- Wey SB, Mori M, Pfaller MA, Woolson RF, Wenzel RP. Hospital- acquired candidemia. The atributable mortality and excess length of stay. Arch Intern Med. 1988; 148: 2642-2645.
- Bross J, Talbot GH, Maislin G, Hurwitz S, Strom BL. Risk factors for nosocomial candidemia. A case-control study in adults without leukemia. Am J Med. 1989; 87: 614-620.
- Graninger W, Presteril E, Schneeweiss B, Teleky B, Georgopoulos A. Treatment of Candida albicans fungaemia with fluconazole. J Infect Dis. 1993; 26: 133-146.
- Meunier F. Candidiasis. Eur J Clin Microbiol Infect Dis. 1989; 8: 438-447.
- Sobel JD. Candida infections in the ICU. Crit Care Clin. 1988; 4: 325-344.
- Knaus WA, Wagner DP, Draper EA, Zimmerman JE, Bergner M, Bastos PG, et al. The APACHE III prognostic system. Risck prediction of hospital mortality for critically ill hospital adults. Chest. 1992; 100: 1619-36.