Gastric Carcinoid Tumors
- 1. Department of Gastroenterology, Kocaeli University, Turkey
- 2. Department of Gastroenterology, Mardin State Hospital, Turkey
ABSTRACT
With the widespread use of diagnostic endoscopy, gastric carcinoids are increasingly encountered in clinical practice. World Health Organization (WHO) decided to use the term neuroendocrine tumors (NET) instead of carcinoid tumor in 2010. Type I and type II NETs associated with hypergastrinemia are more common, while sporadic type III is rare. Type I NETs constitute 70-80% of gastric nets; type II 5-8%, type III 15-20%. These tumors are usually asymptomatic and have been diagnosed by gastroscopy for other reasons and rarely for bleeding or anemia. Gastric NETs are highly heterogeneous in their clinical behavior. Type I and type II gastric NETs can be frequently followed and treated endoscopically in sizes smaller than 1 cm. sporadic types may require oncologic surgery or systemic treatment.
KEYWORDS
Carcinoid tumors ; Stomach ;Gastric neuroendocrine tumors ; Diagnosis ; Therapy.
CITATION
Hülagü S, Y?lmaz H (2017) Gastric Carcinoid Tumors. Ann Clin Exp Metabol 2(2): 1017.
ABBREVIATIONS
NET: Neuroendocrine Tumors; WHO: World Health Organization; ECL: Enterochromaffin-like Cells; SEER: Surveillance Epidemiology and End Results; IR: Incidence Rate, CCK-2: Cholecystokinin-2 Receptors; MENI: Multiple Endocrine Neoplasia type 1; ZES: Zollinger–Ellison Syndrome; GI: Gastro Intestinal; EUS: Endoscopic Ultrasonography; ESD: Endoscopic Submucosal Dissection; SSAs: Somatostatin Analogs
INTRODUCTION
The term “carcinoid” was first used by German pathologist Siegfried Oberndorfer, and is defined as a small submucosal tumor in the small intestine at a 48-year-old women’s autopsy [1]. Since then, a great deal of knowledge has been gained about the physiology, pathology, and management of these tumors. Italian pathologist Carlo Capella defended not to use the term “carcinoid”, instead he introduced the term “neuroendocrine tumors (NET)” in 1995. Later, “NET” was accepted by the World Health Organization (WHO) for common terminology in 2010 [2]. Recently the term “NET” is accepted worldwide.
NETs are a heterogeneous group of tumors regarding clinical behavior, histology and prognosis, which arise from enterochromaffin-like cells (ECL) spread to the whole gastrointestinal tract. In this review, we will scope to an overview of recent developments in gastric NETs and the latest approach to classification, diagnosis, and treatment.
EPIDEMIOLOGY
The incidence of neuroendocrine tumors seems to have increased globally considering the recent medical literature. Two major reasons of this raising up is firstly; common nomenclature was not used for NETs in the past years, and in many studies the incidence was low because of the absence of tumor anatomical localization, secondly the widespread use of imaging methods and surveillance endoscopy have increased the awareness of these tumors even if they are asymptomatic. Gastric NETs are representing 8% of the neuroendocrine tumors of the gastrointestinal tract [3]. In the USA, according to Surveillance Epidemiology and End Results (SEER) database, the gastric NETs incidence rate (IR) was 0.03 per 100000 populations per year between 1973-1977 and it increased to 0.33 between 2003-2007 [4]. IR of gastric NETs was 0.16 and 0.15 for male and female, respectively, in England between 2000-2006 and it was 0.08 at Austria between 2004-2005 [5,6]. There is also a remarkable increase in incidence from 39% to 88% in gastric NET in Norwegian population over a decade between the 1990s and 2000s [7].
PATHOPHYSIOLOGY AND CLASSIFICATION
Following food intake, gastrin is released physiologically from the G cells in the antrum and binds to the cholecystokinin-2 receptors (CCK-2) of ECL cells localized in the gastric corpus and fundus, leading to histamine release from these cells. Histamine leads to acid production from parietal cells. Gastrin is suppressed by somatostatin release from D cells in the antrum. If gastric acid secretion decreases (achlorhydria), hypergastrinemia will occur and lead to trophic effects on ECL cells which cause hyperplasia. Hypergastrinemia is inadequate for arising NET from ECL cells alone, whereby the long-term PPI use or vagotomy, does not seem to be associated with gastric NETs. Genetic (loss of heterozygosity of the MEN-I gene locus on 11q13 was identified of 17-73 %of type I NETs), epigenetic (LINE1 hypomethylation was identified 50% gastric NETs), bacterial (in an animal model it has shown H. pylori lipopolysaccharides and peptidoglycans has an influence on the proliferation of ECL cells) and dietary (smoking status) factors can contribute to tumor development [8-10].
Gastric NETs are solid lesions which are usually yellowish white color and could be present endoscopically as mucosal nodular, polypoid, ulcerous lesions or submucosal lesions, as well. Randi et al. described three subtypes of gastric NETs; type I and type II are associated with hypergastrinemia and type III are formed sporadically [11].
Type I gastric NETs constitute 70-80% of all gastric NETs. They are multiple carcinoid tumors that are smaller than 1 cm in diameter and tend to be localized in the corpus and antrum [12]. It is the type associated with atrophic gastric [13]. The metastasis rate is <5% [14]. Gastric acidity is low and serum gastrin level is high in type I patients.
Type II gastric NETs constitute 5-8 %of the gastric NETs. They are also multiple, smaller than 1cm and localized at the fundus, corpus, and antrum of the stomach. Gastric acidity and serum gastrin levels of type II patients are both high. Type II lesions are associated with Multiple Endocrine Neoplasia type 1(MENI) and Zollinger–Ellison Syndrome (ZES). These lesions are moderately malignant and metastasis rate is below 10% [15].
Type III Gastric NETs are about 15-20 %of the gastric NETs. This type is the most aggressive type of tumors and metastasis rate is over 50% [16]. This lesion is solitary, bigger than 2 cm and generally localized to antrum of the stomach. Patients are mostly male and it occurs sporadically.
WHO classified NETs into three groups based on cell proliferations, which is defined by the number of mitoses per 10 high-power microscopic fields (HPF), and on the percentage of tumor cells positively immune-labeled for Ki-67 antigen(Labeling Index, LI): grade 1; mitoses ≤ 2/10 HPF and Ki-67 LI ≤ 2% and grade 2 mitoses 2–20/10 HPF and Ki-67 LI 3–20% and grade 3 mitoses >20 HPF orKi-67 LI >20% [17].
Grade 1 and grade 2 tumors are well differentiated and show organoid architecture with trabecular, gyriform pattern, neoplastic cells are uniform and a plenty content of secretory granules responsible for intense and diffuse staining for general neuroendocrine markers (synaptophysin and chromogranins). The nuclear chromatin is regular and mitosis is not common. Grade 3 neoplasms are poorly differentiated and have highly pleomorphic, atypical nuclei, a solid growth pattern, and nonischemic necrosis areas, mitosis are always rich and often atypical [18].
Currently Chromogranin A (CgA) is the most widely used biomarker for the diagnosis of NETs. Chromogranin A is a watersoluble acidic glycoprotein of 68 kDa located in the matrix of large secretory granules of neural and endocrine cells. These protein levels, which can be measured in serum and plasma, correlate with the NET mass [19]. CgA may be found in healthy tissues, and serum levels may also increase in other diseases such as renal failure, cardiac diseases, prostate cancer and small cell lung cancer. Measurable values are affected by proton pump inhibitors and food consumption [20]. Sensitivity is 60-90% but its specificity is below 50% due to reasons listed above. There is also no standard in existing CgA kits and there is wide variability between laboratory measurements [21].
Pankreostatin is a chromogranin derivative and could be used as a tumor marker for carcinoids. Its advantage over he chromogranin A is not being affected by the proton pump inhibitor usage [20].
NSE) is a protein that occupies the cytosol of the neuron and neuroendocrine cells and enzymatically acts on the glycolytic cycle and can indicate the NET. It is not a better indicator than CgA and at the present time clinical use is not common [22].
Synaptophysinis a transmembrane glycoprotein of the presynaptic vesicles. Commonly expressed by the neuroendocrine cells, which reveals a good biomarker potential. However, it is not specific because it is also found in adrenal cortical cells and adenomas.
CD56 or neural cell adhesion molecule is a sialoglyco protein which interacts adhesion between two cells. CD56 is widely expressed at the cell membrane of neuroendocrine cells but also at the tumors of kidney, endomterium and, ovary that causes reduction of its specificity as a carcinoid biomarker [23,24].
From the aspect of immunohistochemical staining, good and poorly differentiated gastric neuroendocrine tumors are stained diffusely and strongly with chromogranin, synaptophysin, and NSE [25]. Chromogranin A and synaptophysin are potent markers of neuroendocrine differentiation and may show immunohistochemical staining up to 90% of gastric NET’s [26].
DIAGNOSIS
Type I NETs are non-functioning tumors so symptoms due to the tumors themselves are rare unless developing an ulcer or bleeding. B12 and iron deficiency symptoms can occur because type I gastric NETs are associated with atrophic gastritis and pernicious anemia. This lesion is generally detected by upper GI endoscopy performed for another reason. Gastroscopy is usually revealed multiple polypoid lesions with a background of the atrophic gastric mucosa. In case of gastric NETs, three biopsies from the mid part of the gastric-body mucosa border along the great curve and three biopsies from the antralmucosa should be obtained for the diagnosis.
It has been described earlier with more extensive biopsy sampling of 2 from antrum 4 from the body and 4 from fundus mucosa correlates with the rate of the neuroendocrine tumors diagnosis in patients with chronic atrophic gastritis [27,28]. The possibility of carcinoma should be considered and biopsies should be taken from the greatest polyp, for assessing ECL cell hyperplasia and atrophic gastritis biopsies also should be taken surrounding mucosa [29].
Most type II patients who have peptic ulcer complaints are unresponsive to treatment due to increased gastric acid secretion [30]. A large number of polypoid lesions are detected by endoscopy, while gastritis and ulcer are observed in the surrounding mucosa.
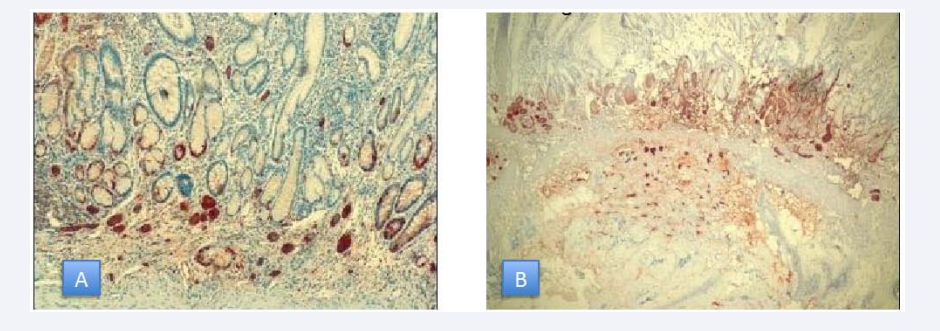
Type III gastric NET patients may present with abdominal pain, weight loss, anemia of iron deficiency, upper gastrointestinal bleeding. Symptoms of itching and bronchospasm secondary to excessive histamine release may be seen [31]. Chromogranin A- a glycoprotein which is released from the secretory granules of ECL cells- and serum gastrin levels can be used as biological markers for diagnostic purpose. Immunohistochemical staining with chromogranin A and synaptophysin is also used for diagnosis of the NETs (Figure 1).
Figure 1 A: Gastric NET limitedtomucosa, 40x magnification B: Gastric NET limitedtomucosa, 10x magnification.
Gastroscopy commonly detects a single ulcerovegetant lesion with a diameter greater than 2 cm. Narrow band imaging or magnified endoscopy may provide additional benefit in detecting type I lesions smaller than 1 cm which may have a micronodular appearance. Lesions larger than 1 cm should be evaluated by endoscopic ultrasonography (EUS) for submucosal invasion or lymph node metastasis [32]. Since type II lesions tend to be locally advanced, they must be visualized by EUS, scintigraphy, CT or MRI. If these diagnostic modalities are insufficient for diagnosis of NETs and gastrinomas, PET-CT with 68 Ga-DOTANOC may have an additional contribution and can help localize gastrinomas [33]. Type III lesions often require cross-sectional imaging like in adenocarcinomas which frequently undergo distant metastases.
Conventional imaging methods such as CT, MRI, and PET / CT have been frequently used for diagnosis of primary and metastatic NETs. FDG-PET / CT may have a limited power to identify these tumors because the rate of glucose degradation of well differentiated NETs is close to normal [34].
Positron emitting radionuclide gallium 68 added to the chelator DOTA and somatostatin analogs to obtain radio labeled tracer DOTA- NOC (68Ga-DOTA,1-Nal3]-octreotide) or DOTATATE (DOTA-DPhe,1Tyr3-octreotate) for better detection of somatostatin receptor expressing tumors. Kayani et al., found 68Ga-DOTATATE PET/CT sensitivity 82% where the 18F-FDG PET/CT sensitivity 66% in a cohort of 38 patients and detection of the low-grade NETs was significantly higher than FDG PET/CT with the 68Ga-DOTATATE PET/CT [35].
68Ga-DOTA-NOC PET/CT is significantly superior identifying both primer and metastatic NETs over contrast enhanced CT. In a study where 109 patients were screened, six patients (5.5%) had chance of surgical treatment for lesions which were not exposed by conventional imaging modalities, DOTA NOC revealed additional resectable lesions therefore, 8(6.4%) patients surgical planning changed, 4 (3.6%) patients had spared from unnecessary surgery with detection of advanced disease stage [36].
68 Ga-DOTATATE has an affinity for somatostatin receptor subtype 2 whereas 68 Ga-DOTA-NOC has a broader receptor affinity for subtype 2, 3, 5and is therefore superior to the 68GaDOTATATE aspect of imaging. In a single center study from London, the lesion based sensitivity of 68Ga-DOTANOC PET was 93.5%, compared with 85.5% for 68Ga-DOTATATE PET for detecting gastroenteropancreatic NETs. (P < 0.005). DOTA peptide imaging methods also give the information about the somatostatin receptor condition of the tumors, and patients may be candidates for peptide-based radionuclide therapy or the somatostatin analogs treatments [37].
MANAGEMENT
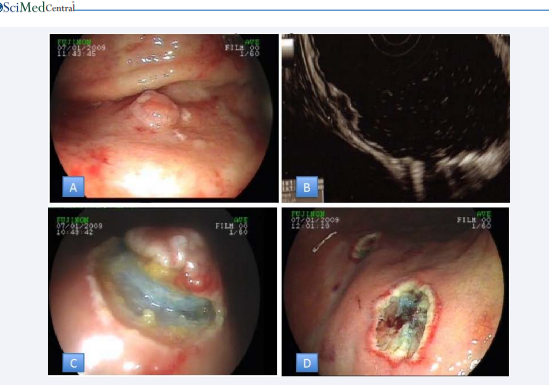
Clinical management and treatment for gastric NETs rely upon metastasis risk which is indicated by tumor size, the presence of muscular wall infiltration, increased tumor cell proliferation. The treatment of type I and type II Gastric NETs is quite similar and in this respect, they differ from type III. Sato at al., pointed that throughout 7 years of endoscopic surveillance tumors ≤ 10 mm in diameter did not display advancement.8 tumors smaller than 10 mm among 82 was capillary invasive but none of them revealed recurrence or distant metastasis after endoscopic treatment during follow-up.(38) Uygun et al., also reported a median 7 years of follow up of type I NETs after endoscopic treatment and denoted 100% rate of overall survival rate and disease?free survival [39]. Because of slow progression and low risk of metastasis type I and type II lesions, which are smaller than 1 cm, can be taken under endoscopic surveillance annually [40]. Follow up of the small type I and type II tumors should include gastroscopy and EUS where further modalities such as octreoscan and radionuclides imaging did not reveal additional pathology over conventional imaging [41]. Lesions that are 1-2cm size should be checked with EUS primarily for the invasion of muscularispropria or local lymph node metastasis (Figure 2).
Figure 2 A: Gastric NET B: NET confinedtosubmucosadetectedby EUS C: Sub mucosal view of NET D: Lesion basis after removal of lesion
Endoscopic treatments should be considered for lesion which is limited to the mucosa and submucosa. Endoscopic mucosal resection, endoscopic mucosal resection with a ligation device or endoscopic submucosal dissection (ESD) may be selected as an endoscopic procedure, but any of them should be performed with experienced endoscopists. ESD is based on the stepwise dissection of the lesion with special endoscopic knives following injection of fluids that will form a submucosal cushion below the lesion (Figure 2). Among our ESD cases, four gastric NETs were observed and total en bloc resection was achieved in all of the cases. Li and colleagues performed 100% en bloc and complete resection in a wider series of 23 patients. The endoscopic appearance of NETs is often followed by increased capillary on the lesions. These lesions tend to bleed during ESD because of their hypervascularity and even late bleeding can be observed after 1 week [42,43].
Medical treatment options include Somatostatin analogs and the newly developed oral CCK-2 receptor antagonist Netazepide, which are reported to prevent progression of type I and type II lesions by reducing the size and number [44,45]. In a study of 5 patients who were treated with 20 mg octreotide LAR every 28 daya for gastric NETs for a year had a reduction of tumor size and the number of visible tumors was decreased by more than 50 % [46]. Significant gastric carcinoid recurrence was not observed in 12 months after patients treated with octreotide LAR [47].
Treatment with SSAs (somatostatin analogs) in type I NETs leads to an actual tumor load reduction and the decrease of serum gastrin levels suggesting anti-proliferative effect of SSA on ECL cells. Complete disappearance of the tumors at 1 year of treatment was achieved 73% of the patients [48,49].
Surgical treatment is indicated in the presence of six or more lesions where three of them are larger than 1 cm or a single lesion larger than 2 cm when muscularispropria invasion or lymph node metastasis are present [50,51]. In cases of type II gastric NETs if gastrinoma can be located, it should be removed and proton pump inhibitors should be used to prevent peptic complications. Due to the high risk of metastasis, all type III lesions that are not at an advanced disease stage should be treated according to surgical oncologic principles which surgical intervention should aim complete resection of all detectable tumor manifestations [52]. Surgery should provide partial or total gastrectomy with lymph node dissection that surgical treatment not differs from gastric adenocarcinomas. The applicability of resection with no residual tumor depends on the localization of the primary lesion, the proportions of local metastasis, the number and localization of liver metastasis. In case of liver metastases, tumor localized to one lobe or located separately in two lobes but fewer than five metastases could be fully resectable [53,54].
Metastatic lesion of neuroendocrine tumor in the liver could be curable when they are operated, and patients who had the chance of metastasectomy have 5-year survival rates of 71–85% [55,56].
Tumor burden should be reduced by metastasectomy and cytoreductive surgical methods in appropriate patients with advanced disease [57]. Ablation treatments can be used alone or in conjunction with surgery [58]. Hepatic artery embolization techniques are useful in the widespread disease where surgery cannot be performed [59]. SSAs, oral target of rapamycin (mTOR) inhibitor Everolimus, Peptide Receptor Radionuclide Therapy are the treatment modalities for systemic treatment. Mammalian target of rapamycin (mTOR) is a serine-threonine kinase that regulates cell growth, proliferation, and survival signaling reaction to metabolic stress factors and inhibition of the mTOR pathway has in vitro antiproliferative effects on NET cells [60]. Everolimus is an oral inhibitor of mTOR which had assessed in phase III trial of RADIANT-4. 302 patients with advanced, progressive, well differentiated NETs of the lung or gastrointestinal tract received either everolimus 10 mg or placebo. Median progression free survival was 11 months in the Everolimus group while it was 3.9 months patients who received placebo. Reduction in the estimated risk of progression was 52% with Everolimus [61].
Peptide receptor radionuclide therapy (PRRT) targets somatostatin receptor expressing well differentiated NETs by radio labeled somatostatin analogs with yttrium-90 or lutetium-177. DOTA used as a chelator for somatostatin and yttrium to obtain radionuclide 177Lu-DOTA0-Tyr3-octreotate. Recently a phase III trial of NETTER-1 mention that PRRT has promising results of progression free survival in patients with neuroendocrine tumors with an overall response rate of 18% [62]. In conclusion, overall 5-year survival rate of the gastric NETs is 98% for type I, 100% for type II, 60% for type III and 39% for the poorly differentiated gastric NETs [63].
ACKNOWLEDGEMENTS
Sadettin Hülagü researched and wrote the review as the first author. Hasan Y?lmaz contributed and reviewed the article.
REFERENCES
1. Oberndorfer S. Carcinoid tumors of the dunndarms. Frankfurt Z Pathol. 1907; 1: 426-432.