Metformin
- 1. Consultant Endocrinologist, National Hospital of Sri Lanka, Sri Lanka
- 2. Senior Registrar in Endocrinology, National Hospital of Sri Lanka, Sri Lanka
ABSTRACT
Metformin, being one of the oldest drugs, is the only biguanide agent currently available for treatment of diabetes mellitus. With data for its central role in treatment of glycemic and metabolic abnormalities as well as established safety profile and emerging understanding of its non-glycemic benefits, it is considered as the first line agent for treatment of diabetes mellitus. After systemic absorption following oral administration, metformin exerts its effects through multiple mechanisms. Inhibition of mitochondrial respiration and activation of 5’AMP-activated protein Kinase (AMPK) leads to inhibition of gluconeogenesis; improved hepatic insulin sensitivity and increased cellular uptake contribute to the glycemic benefits of metformin. Apart from its systemic effects, there is emerging evidence on metformin action in gut in glycemic control. In addition it has shown to have multiple non-glycemic benefits: anticancer effects, weight reduction, favorable lipid profile, and neuro protective effects. Further it has been shown to be beneficial as a treatment modality in pre diabetes, polycystic ovarian syndrome and nonalcoholic fatty liver disease. Adverse effects of metformin are largely confined to gastrointestinal system and rarely lactic acidosis, which can be prevented by using caution when prescribing metformin in acute illnesses and in chronic renal impairment and cardiac failure.
CITATION
Somasundaram NP, Wijesinghe AM (2016) Metformin – A Mini Review. Ann Clin Exp Metabol 1(1): 1003.
INTRODUCTION
Metformin which is extracted from plant Galega officinalis, is the only biguanide agent that is currently used in treatment of diabetes mellitus. Biguanides are composed of two guanidine groups combined together with loss of ammonia. Other biguanide agents such as phenformin and buformin are currently not in use due to the increased adverse effect profile, especially lactic acidosis. Despite being one of the oldest drugs in use since 1957 for treatment of diabetes, metformin still remains the first line treatment for type 2 diabetes [1].
Pharmacokinetics and pharmacodynamics
Metformin is orally administered and is absorbed mainly in the duodenum and jejunum. The absolute bioavailability of a Metformin hydrochloride 500 mg tablet given under fasting conditions is approximately 50% to 60%.It is negligibly bound to plasma proteins. It is not metabolized in the liver and is renally excreted through active tubular secretion in the kidney. Metformin has a half-life of about 6 hours.
Metformin is available as immediate release tablets and extended release tablets. It is usually started at a dose of 500mg per day dose and increased in weekly increments until maximum tolerated dose or desired glycemic control is reached, usually to a maximum dose of 2g per day in two to three divided doses.
Mechanism of action and cellular effects
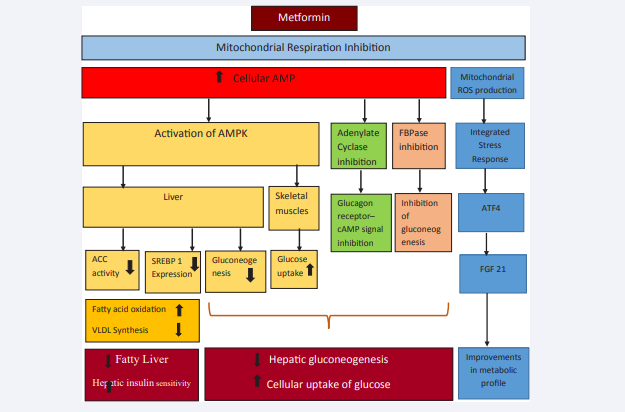
The mechanism of action of metformin is thought to be mediated via its systemic absorption leading to inhibition of hepatic gluconeogenesis, increased cellular glucose up take and improvement in hepatic insulin sensitivity (Figure 1).
Figure 1 Action of metformin. Abbreviations: AMP: Adenosine Mono Phosphate; AMPK5’: Adenosine Monophosphate –Activated Protein Kinase; FBPase: Fructose-1, 6-bisphosphatase; SREBP 1: Steroid Regulatory Element Binding Protein 1; ACC: Acetyl Co A Carboxylase Activity; VLDL: Very Low Density Lipoproteins; ROS: Reactive Oxygen Species; ATF4: Activating Transcription Factor 4; FGF-21: Fibroblast Growth factor 21 Metformin inhibits mitochondrial respiration through inhibition of complex I in the electron transport chain leading to elevation of cellular AMP and mitochondrial ROS production. Elevated AMP activates AMPK and inhibits adenylate cyclase and FBPase, resulting in increase in peripheral glucose uptake and inhibition of hepatic gluconeogenesis. AMPK suppress ACC activity and expression of SREBP 1, resulting in increased fatty acid oxidation and diminished VLDL production with reduction in fatty liver and improving hepatic insulin sensitivity. Mitochondrial ROS initiates an integrated stress response (ISR) through ATF4 to induce fibroblast growth factor 21, which improves the metabolic profile associated with obesity or lipotoxicity.
Following gut absorption, metformin reach liver in high concentrations in the portal vein and enter hepatocytes through Organic Cation Transporter 1 (OCT 1) to affect mitochondrial respiration through inhibition of complex I in the mitochondrial electron transport chain. The resultant cellular energy depletion lead to reduction in Adenosine Tri Phosphate (ATP) levels with concomitant rise in Adenosine Mono Phosphate (AMP) levels resulting in increased ADP:ATP and AMP: ATP ratios. The elevation of cellular AMP levels mediates three actions: activation of 5’AMP-activated protein Kinase (AMPK); inhibition of glucagon receptor - cAMP signaling through adenylate cyclase; inhibition of fructose-1, 6-bisphosphatase (FBPase) with inhibition of gluconeogenesis, all of which decreases hepatic glucose production.
Activation of AMPK leads to increased glucose uptake in skeletal muscles. In the liver, apart from inhibiting hepatic gluconeogenesis, AMPK modulates adipokine secretion and also suppress Acetyl Co A Carboxylase activity and also suppresses steroid regulatory element binding protein 1 (SREPB 1) leading to increased fatty acid oxidation and improving insulin sensitivity through inhibiting genetic expression of lipogenic enzymes. The resultant reduction in glucotoxicity and lipotoxicity attenuate insulin signaling defects and thereby improve glycemic control as well as cardiovascular risk reduction.
Metformin also exerts its effects through AMPK independent pathways. Inhibition of complex I in the mitochondrial electron transport chain lead to production of mitochondrial reactive oxygen species (ROS). This in turn has been shown to initiate an integrated stress response (ISR) through activating transcription factor 4 (ATF4) to induce fibroblast growth factor 21 (FGF21). Fibroblast growth factor 21 improves the metabolic profile associated with obesity or lipotoxicity [2-4]
However, there is emerging evidence that the primary glucose lowering effect of metformin may reside in the gut itself than in the systemic circulation. [5] This was demonstrated by John B. Buse and colleagues by the observation that higher blood sugar reductions in a new formulation of delayed release metformin (Met DR) having poor systemic absorption. Met DR targets ileum where absorption is much less compared to conventional metformin preparations (Metformin immediate release and extended release where absorption occurs in duodenum and jejunum.) Even though Met DR had a lower bioavailability and lower plasma metformin concentrations than the conventional preparations, it produced a more significant and sustained reduction in plasma glucose suggesting a role of unabsorbed metformin in lower gut. The action of metformin in the gut is postulated to be mediated through increase in GLP1, peptide YY, and action on Farnisoid X receptor (FXR) [6,7]. In addition, metformin has effects on gut micro biota where it increases the abundance of “Akkermania spp.”, a mucus-degrading Gram-negative bacterium in the gut, which is associated with restoration of reduced regulatory T (Treg) cells and amelioration of low-grade tissue inflammation in the adipose tissue of obese animals, leading to improved insulin sensitivity [8].
In addition to its beneficial effects on glycemic control, metformin has shown to have anti cancer effects. It had been widely accepted that metformin induced AMPK activation suppresses the cellular proliferation in malignant as well as non-malignant cells. This is thought to be mediated through regulation of cell cycle through inhibition of mammalian target of rapamycin complex 1 (mTORC1) and up regulation of p53 –p 21 axis. The mTOR pathway plays a pivotal role in metabolism, growth and proliferation of cancer cell. In addition, reduction of hyperinsulinemia by AMPK activation by metformin, results in amelioration of the insulin and IGF 1 induced stimulatory effects on cellular proliferation. Apart from regulation of glucose uptake, Insulin/IGF-1 is involved in carcinogenesis through up regulation of insulin/IGF receptor signaling pathway, through insulin receptor substrate (IRS), phosphoinositide 3-kinase (PI3K), and Akt/protein kinase B (PKB) that indirectly activates mTORC1.
There is emerging evidence challenging this protective role of AMPK as a tumor suppressor. AMPK has recently been demonstrated to promote cancer cell survival in the face of extrinsic and intrinsic stressors including bioenergetic, growth factor and oncogene stress, compatible with studies showing that AMPK is required for oncogenic transformation [9-12]. Therefore whether AMPK acts a tumor suppressor or contextual oncogene remains still controversial.
Apart from AMPK related effects, metformin is known to inhibit cancer cell proliferation by suppressing mitochondrialdependent biosynthetic activity. Metformin decreases the flow of glucose and glutamine derived metabolic intermediates into the Tricarboxylic Acid (TCA) cycle, leading to reduced citrate production and de novo lipid biosynthesis. Deficiency of these metabolic intermediates required for cell growth results in inhibition of proliferation of cancer cells [13].
METFORMIN USES
Glycemic benefits
Type 2 diabetes mellitus: The main indication for metformin is in treatment of type 2 diabetes. It is a drug with low cost and long known experience with well-established safety profile. Apart from its glucose lowering effects, it has multiple beneficial effects in diabetes patients, which includes: appetite suppression and weight loss; absence of hypoglycemia; cardiovascular benefits.
Cardiovascular benefits of metformin appears to be multifactorial; glycemic control, AMPK mediated increased free fatty acid oxidation; reduction of infarct size through preventing the opening of mitochondrial permeability transition pore (mPTP) by activation of phosphatidylinositol-3-kinase (PI3K) – Akt; improving peripheral circulation by inhibiting Plasminogen Activator inhibitor 1. In UKPDS trial [14] , metformin reduced the incidence of microvascular and macrovascular complications including cardiovascular complications in over weight type 2 diabetic patientsby 32% compared to patients on conventional therapy with insulin or sulfonylurea as (p=0.0023). Further analysis of the macrovascular benefits of metformin therapy showed that metformin has major benefit in diabetic cardiovascular complications with a 39% risk reduction in myocardial infarction and a 50% risk reduction in coronary deaths. In addition, metformin as primary therapy reduced the risk of diabetes related deaths by 42% (p=0.017) and reduced all-cause mortality by 36% (p=0.011).
Metformin can be combined with any class of oral hypoglycemic drugs (Sulfonylurea, Thiazolidinediones, DPP4 inhibitors, GLP 1 agonist or SGLT2 inhibitors) or insulin. Further, metformin has shown to increase GLP 1 levels after oral glucose load [15] and has shown to have synergistic action in combination with DPP4 inhibitors with good tolerability [16]. Combination of metformin and insulin has also shown to have improved glycemic control, insulin sparing effect with reduction of insulin requirement by about 15% - 29% and minimized weight gain. [17,18]. Another frequently used combination is metformin and sulfonylurea drugs. The combination therapy has shown to attain a greater reduction in HbA1c (0.8–1.5%) than using either drug alone [19].
Pre diabetes: In addition to its glycemic benefits in diabetes patients, metformin has shown to reduce diabetes incidence by 31% in the Diabetes Prevention Program (DPP) trial [20]. And this effect lasted even after two weeks drug wash out period suggesting that, at least in the short-term, metformin may help delay the onset of diabetes. The benefits of metformin were primarily observed in patients <60 years old (RR 0.66) and in patients with a body mass index greater than 35 kg/m2 (RR 0.47). Apart from this, similar studies conducted in Indian [21] and Chinese Asian[22] populations have shown similar outcome implying the benefit of metformin in pre diabetes patients.
Pregnancy: Metformin improves insulin sensitivity and glucose tolerance in pregnancy by reducing the physiological rise in insulin resistance that occurs during pregnancy. In Metformin in Gestational Diabetes (MiG) trial, there was no significant difference in the composite fetal outcome (neonatal hypoglycemia, respiratory distress, birth trauma, Apgar score at 5 minutes <7, need for phototherapy, or prematurity) between metformin and insulin treated groups [23]. Women who were treated with metformin had more preterm births and less weight gain compared to those in the insulin treated group. Few other systematic reviews and meta-analysis has shown beneficial effects of metformin over insulin in pregnancy [24,25]. Follow up of the children born to the participants in MiG trial (MiG TOFUMetformin in Gestational Diabetes: The Offspring Follow-Up), had larger measures of subcutaneous fat in those who were treated with metformin, but overall body fat was the same as in children whose mothers were treated with insulin [26]. The lack of long term follow up data of outcome of the children doesn’t make metformin a first line agent in treatment of diabetes. However, further large scale long term studies are required to establish the safety and fetal outcomes.
Non glycemic benefits of metformin
Weight Reduction: The mechanism of weight loss by metformin is thought to be mainly mediated through reduced food intake. Metformin acts on the central nervous system to reduce appetite by attenuating hypothalamic AMPK activity, which decreases NPY (orexigenic) and increases POMC (anorectic) expression. In addition, it improve insulin sensitivity, alters gut flora, reduce gut glucose absorption and increase GLP1 levels which act as an appetite suppressant [27].
Diabetes Prevention Program (DPP) trial is one of the largest studies which looked at the weight loss in patients having impaired glucose tolerance with metformin over 10 years. In this study, patients randomly assigned to receive lifestyle treatment initially lost weight but gradually regained the lost weight over the 10-year follow-up period. Patients who were assigned to receive metformin lost less weight at the beginning of the study (about 2.5 kg) and were able to sustain the weight loss over 10 years [20].
In nondiabetic patients with obesity, some of studies doesn’t show a significant weight loss with metformin [28] while few show significant weight loss [29].
Polycystic ovarian syndrome: Insulin resistance leading to elevated free testosterone levels with resultant hyper androgenemia is one of the key factors in pathogenesis of polycystic ovarian syndrome (PCOS). Metformin reduce the insulin levels by improving insulin sensitivity and thereby improve clinical manifestations of the disease. Metformin has shown to improve spontaneous & clomiphene/ FSH induced ovulation [30], regularity of menstruation and improve pregnancy rates in both lean and obese PCOS with hyperinsulinemia. It also has shown to have weight loss and improve lipid profiles [31]. And there is limited data that metformin may improve cutaneous manifestations such as hirsutism, but it is not recommended to be used as a first line treatment for cutaneous manifestations or menstrual irregularities.
A systematic review in ovulation induction in PCOS patients demonstrated that in clomiphene-resistant women, metformin plus clomiphene led to higher live birth rates than clomiphene alone and metformin also led to higher live birth rates than laparoscopic ovarian drilling [32]. In addition, metformin may prevent the development of OHSS in women with PCOS receiving gonadotropin therapy for IVF [33]. The routine use of metformin during pregnancy in women with PCOS is unwarranted, although it may be useful to treat gestational diabetes.
Non alcoholic fatty liver disease
Metformin is known to have favorable effects on NAFLD as it increases free fatty acid oxidation through activation of AMPK. In animal models, metformin has shown to improve fatty liver, resolution of hepatomegaly and reversal of steatosis in histology. However, human studies have shown favorable results in weight and liver enzymes, but not on histology. A systematic review and meta-analysis has shown that metformin improves liver function, HOMA-IR and Body Mass Index to some extent, but not histological response in NAFLD patients [34].
Effects on lipid profile
Metformin is known tocorrect of the lipid profile of bloodwith improvements in lipoprotein metabolism, decreases in LDLCholesterol (35, 36), Triglycerides, and free fatty acids [35,36]. Further, metformin treatment is known to lower inflammation marker levels in patients at high risk of cardiovascular disease and restore impaired HDL-mediated cholesterol efflux from macrophages due to glycation. These effects may contribute to the anti-atherosclerotic properties of metformin [37-40].
Neuroprotection
Even though human studies on neuro protective effects of metformin lacks, there is evidence from rodent studies that metformin does cross the blood-brain barrier and activates AMPK in CNS tissue. In central nervous system neuronal cell lines, exposure to metformin sensitizes neurons to insulin and also prevents Alzheimer’s disease pathology in neurons chronically exposed to a hyperinsulinemic environment [41].
Anti-cancer effects
Metformin has recently been shown to have anti-cancer effects. Mechanism of this anti-cancer effects may be mediated through either AMPK mediated regulation of cell cycle, AMPK independent suppression of mitochondrial-dependent biosynthetic activity (described in section mechanism of action and cellular effects) or through its pro senescent activity.
Some recent studies have evaluated and shown the beneficial effects of metformin in prevention of malignancies especially breast, colonic, pancreatic, prostate and liver cancers [42, 43- 46]. In one meta-analysis that included 47 independent studies with a total of more than 65,000 patients demonstrated that the overall cancer incidence was reduced by 31% and cancer mortality was reduced by 34% in metformin users [43]. In a study based on data from Danish Cancer Register, the odds ratio for breast cancer incidence was found to be 0.77 in metformin using type 2 diabetes patients [47]. Anticancer properties of metformin is not confined to diabetic population; in one of the studies where metformin was administered as a neoadjuvant therapy at a dose of 500 to 1000 mg daily for 2 to 4 weeks before surgery to breast cancer patients, it was shown that there is a decrease in the mean mitotic index and signaling pathway activity in breast tumor tissues, although serum insulin level was unchanged [48]. Further, daily low dose metformin in colorectal carcinoma had shown to decrease the number of aberrant crypt foci in the rectum and cell proliferation rate in colonic epithelium [49].
Aging
Metformin treatment has shown to delay aging in animal models. It has been shown to induce genetic profiles in mice similar to the state of “caloric restriction” [50] resulting in expansion of their lifespan by almost 40% [51]. Cabreiro and colleagues conducted a series of experiments with metformin in the roundworm Caenorhabditis elegans [52], which demonstrated that c. elegans treated with metformin showed limited size loss and no wrinkling as opposed to those who didn’t receive metformin who showed signs of aging such as gradual shortening, wrinkling and reduced mobility. Alterations in metformin-induced longevity by mutation of worm methionine synthase (metr-1) and S-adenosylmethionine synthase (sams1) imply metformin-induced methionine restriction in the host, consistent with action of this drug as a dietary restriction mimetic. There is a recent ongoing phase 4 trial - Metformin in Longevity Study (MILES study), where the primary objective is to assess whether treatment of metformin will restore gene expression profile of older glucose intolerant adults to a younger levels. The reversal of gene expression profile would be assessed by identifying changes in gene expression in muscle and adipose tissue with RNA sequencing. In addition, number and morphology of mitochondria and also content and activation of adipose tissue macrophage will be assessed in the study.
DELAYED CELLULAR SENESCENCE
Cellular senescence is a change in “cell state” where normal diploid cells cease to divide. However senescent cells remain metabolically active and commonly adopt an immunogenic phenotype consisting of a pro-inflammatory secretome, the up-regulation of immune ligands, a pro-survival response, promiscuous gene expression and stain positive for senescenceassociated β-galactosidase activity [53]. Moiseeva et al. has demonstrated that metformin delay cellular senescence in animal models by inhibiting the expression of genes coding for multiple inflammatory cytokines seen during cellular senescence. Bio informatic analysis of genes down regulated by metformin suggests that it blocks the activity of the transcription factor NFκB [54]. Another study by Hooten et al indicated that metformin up regulates DICER1 (a micro RNA-processing protein) through a post-transcriptional mechanism and resulted in decreased cellular senescence in several senescence models in a DICER1- dependent manner in both mice and in humans with diabetes mellitus [55].
However, some of the studies have demonstrated that metformin acts as a pro senescent agent in tumor genesis and contributes to its anti-cancer effects through apoptosis of tumor cells. Chen et al. has shown that combining p53 tumor suppressor stabilizers with metformin induces synergistic apoptosis through regulation of energy metabolism in castration-resistant prostate cancer. In addition, metformin has shown to manipulate the threshold for stress-induced senescence, thus accelerating the onset of cancer-protective cellular senescence in response to oncogenic stimuli [56]. Therefore, it is evident that metformin exerts its cellular metabolic effects through multiple mechanisms and there is necessity of further sophisticated and extensive research which would enable to utilize all beneficial metabolic effects of metformin
Adverse drug reactions
Commonest adverse effects of metformin are gastrointestinal disturbances in the form of recurrent abdominal pain, loss of appetite, flatulence, and diarrhea. These effects are usually transient and can be minimized by taking the drug with the meals and doing gradual dose increments rather than sudden dose escalations. In those who still find it difficult to tolerate, delayed release preparations may be more tolerable. About 5% may not tolerate metformin permanently.
There are some studies suggesting vitamin B12 deficiency with use of metformin, especially in those who are on higher dose for a longer duration and rarely, it can be associated with megaloblastic anemia [57].
Lactic acidosis used to be a dreaded complication of metformin use. However, multiple large scale studies including the Cochrane group [58], Comparative Outcomes Study of Metformin Intervention versus Conventional Approach (COSMIC) [59] study, and the United Kingdom Prospective Diabetes study [14] have not shown to increase incidence of lactic acidosis with metformin. Presence of renal impairment, liver failure or hypoxic conditions (recent myocardial infarction, cardiac failure, pulmonary disease) has shown to increase risk of lactic acidosis [60,61].
Cautions and contraindications
Metformin is contraindicated in patients with acute medical illnesses such as; acute renal failure, acute liver failure, diabetic ketoacidosis, severe infection and shock. It is also best avoided in acute or chronic disease which may lead to tissue hypoxia (cardiac or respiratory failure, recent myocardial infarction or shock and until 48 hours after intravascular administration of iodinated contrast agents.
Metformin should be used with caution in chronic renal impairment. Estimated glomerular filtration Rate (eGFR) is recommended to be used as a guidance in using metformin in renal impairment as opposed to serum creatinine alone [62]. Metformin may be continued or initiated with an eGFR of 60 mL/ min per 1.73 m2 but renal function should be monitored annually. It can also be used in those with eGFR 45 to 60 mL/minper 1.73 m2 with frequent monitoring of renal functions, at least 3 -6 monthly. The dose of metformin should be reviewed and reduced (e.g. by 50% or to half-maximal dose) in those with an eGFR 35 - 45 mL/ min per 1.73 m2 , and renal function should be monitored closely (every 3 months). Metformin should not be initiated in patients at this eGFR. The drug should be stopped once eGFR falls to 30 mL/ min per 1.73 m2 or lower.
Use of metformin in cardiac failure was thought to be associated with increased risk of metformin induced lactic acidosis. However, there is emerging evidence that use of metformin does not carry additional risk of lactic acidosis and additionally it is associated with low risk of mortality compared to use of insulin or sulfonylureas [63]. Current recommendation is that metformin may be used in patients with type 2 diabetes with stable congestive heart failure if renal function is normal but should be avoided in unstable or hospitalized patients with congestive heart failure [64].
CONCLUSION
Metformin, being in clinical use for more than fifty years with well-established safety profile, is a wonder drug with multiple glycemic as well as non-glycemic benefits and it still remains the first line agent for treatment of type 2 diabetes.
REFERENCES
1. Standards of medical care in diabetes. 2016.
42.Ruiter R, Visser LE, Herk-Sukel MP, Coebergh WW, Haak HR, Duijvestijn PH, et al. Lower Risk of Cancer in Patients on Metformin in Comparison With Those on Sulfonylurea Derivatives -Results from a large population-based follow-up study. Diabetes Care. 2012; 35: 119-124.
53.Campisi J. Aging, Cellular Senescence, and Cancer. Annual Rev Physiol. 2013; 75: 685-705.