Straightforward Detection of Reactive Compound Binding to Multiple Proteins in Cancer Cells: Towards a Better Understanding of Electrophilic Stress
- 1. Department of Pharmacy, University of Nantes, France
- 2. Department of Science and Technology, University of Nantes, France
- 3. ProtNeteomix, 29 rue de Provence, France
- 4. UMR 8601, Paris Descartes University, France
- 5. Protim, Inserm U1085 - Irset, Campus de Beaulieu, France
ABSTRACT
The straightforward assessment of fluorescent compound interactions with proteins in living cells is an important issue in biomedical research, clinical diagnostics and drug development. In-gel detection of compound-protein–bound complexes is often impossible due to the extinguishing of fluorescence in aqueous environments. This study demonstrates a way to assess authentic interactions of fluorescent nitro-benzoxadiazole (NBD) derivatives with proteins in cancer cells by blotting electrophoretically separated proteins onto a nitrocellulose membrane and scanning at 488-nm excitation and 520- nm emission wavelengths with an imaging system. Many protein bands were found to exhibit moderate to strong fluorescent signal in breast cancer and lung cancer cells after short exposure to NBD compounds. Only lipophilic NBD compounds were able to penetrate cancer cells and generate reactive oxygen species (ROS) and concomitant electrophilic NBD species. We hypothesized that ROS and electrophiles, which are generated during the redox cycling of NBD compounds in cytoplasm, behave as independent reactive entities that target multiple proteins. New results along with our recent findings argue in favour of the dual action of reactive species on proteins involved in signal transduction, DNA repair, and proteostasis. Impaired essential function (s) leads rapidly and simultaneously to oxidative and electrophilic stress in cells. This study shows that a commonly used blotting technique represents a simple alternative for in-gel detection. This tool can be used to rapidly assess the binding of fluorescent compounds to the human proteome and has potential for use in monitoring suitable biomarkers of electrophilic stress in diseased cells.
KEYWORDS
Protein blotting ; Fluorescence detection ;Nitro-benzoxadiazole ;Electrophilic stress ;Cancer.
CITATION
Sakanyan V, Benaiteau F, Alves de Sousa R, Pineau C, Artaud I (2016) Straightforward Detection of Reactive Compound Binding to Multiple Proteins in Cancer Cells: Towards a Better Understanding of Electrophilic Stress. Ann Clin Exp Metabol 1(1): 1006.
ABBREVIATIONS
BCA: Bicinchoninic Acid; c-Myc: Myelocytomatosis Cellular Oncogene; DMSO: Dimethyl Sulfoxide; EGFR: Epidermal Growth Factor Receptor; H2 O2 : Peroxide Hydrogen; λem: Emission Wavelength; λex: Excitation Wavelength; NBD:4-nitro-2,1,3- Benzoxadiazole; NC: Nitrocellulose; O-NBD: NBD Compound in Which a Side Chain (R) is Connected to NBD via Oxygen; PVDF: Polyvinylidene Difluoride; ROS: Reactive Oxygen Species; S-NBD:
NBD Compound in Which a Side Chain (R) is Connected to NBD via Sulphur; SOD1: Superoxide Dismutase 1.
INTRODUCTION
The straightforward and cost-effective assessment of small compound interactions with proteins in living cells is a challenge in developing biomolecular imaging tools and target-specific drugs. Proteins bound to fluorescent chemicals can be visualized in gel (in-gel detection) by electrophoretic separation and scanning at appropriate wavelengths [1]. However, a water environment affects photophysical properties such as emission spectra, extinction coefficients and quantum yields, and reduces if not completely abolishes the fluorescent signal emitted by many and different compounds in gel. Blotting of proteins onto a membrane support, described independently by Towbin and co-workers [2], and Burnette [3], drastically simplifies the detection of proteins with fluorescent-labelled probes. Surprisingly, this advantageous approach has not been applied to analyse fluorescent small compound interactions with proteins in living cells to date. Here we address blotting of proteins onto membranes to demonstrate that reactive nitro-benzoxadiazole derivatives bind to multiple protein targets in cancer cells.
Notably, 4-nitro-2, 1, 3-benzoxadiazole (known also as nitro-benzofurazan and referred to as NBD in this study) is characterized by an excitation wavelength (λex) of 440 nm and an emission wavelength (λem) of 480 nm. This compound has been widely used in developing fluorescent reagents for chemical and biological applications [4-7]. NBD is also a popular scaffold in drug development and has been used to target proteins of therapeutic interest such as glutathione transferase [8], Myc oncoprotein [9], and HIV-1 integrase [10]. However, recent studies have shown that NBD derivatives undergo redox cycling in cells through the reduction of the nitro group by generating reactive oxygen species (ROS) and electrophilic NBD species [11]. Noteworthy, lipophilic NBD compounds rapidly bind to and dimerize superoxide dismutase 1 (SOD1) in cytoplasm by forming a highly stable and active enzyme which, in the absence of adequate activity of downstream redox reactions, leads to further accumulation of ROS and NBD electrophiles [12], suggesting that both types of reactive molecules might attack proteins consistent with their ability to lead to oxidative and electrophilic stress in cells. Unlike oxidative stress, studying proteins involved in electrophilic stress is an emerging field in proteomics. Therefore, we turned to the blotting technique with a hope to override the bottlenecks of ingel detection, and assess, in the first approximation, the number of proteins targeted by NBD compounds within cells.
MATERIALS AND METHODS
Chemicals
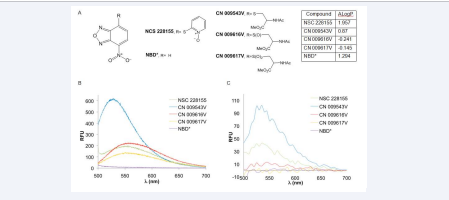
NBD was purchased from Santa Cruz Biotechnology; NSC 228155 was obtained from the Drug Synthesis and Chemistry Branch of the National Cancer Institute. The structures and fluorescent spectra of NBD and S-NBD derivatives are shown in Figure (1).
Figure 1 Structure, lipophilicity and fluorescence spectrum of S-NBD compounds
Synthetic route of CN 009543V, CN 009616V, and CN 009617 was described in previous studies [12,13].
Cell culture
MDA MB breast cancer cells and A549 lung cancer cells were grown in DMEM and RPMI 1640 media, respectively, to a density of 5 x 105 cells/ml at 37°C in a humidified atmosphere of 5% CO2 . Cells were exposed to S-NBD compounds or DMSO (0, 4%) at 37°C and then lysed with Pierce IP lysis buffer containing protease inhibitor cocktail (Thermo Scientific). The cell lysates were centrifuged at 13.000 g for 10 min and supernatant fractions were collected for further assays as described previously [12]. Protein concentration was determined by the BCA assay (Thermo Scientific).
Protein blotting
Protein samples of cancer cell cultures were separated by electrophoresis on 4-15% gradient TGX gels (Bio-Rad) in SDS-Tris-Glycine buffer, then transferred onto polyvinylidene difluoride (PVDF) or nitrocellulose (NC) membranes (Bio-Rad) using a Trans-Blot Turbo system (Bio-Rad). Other conditions were described in a previous study [14].
Fluorescence detection
Gels and membranes were scanned over a wide range of wavelengths, from 440 nm to 700 nm, with a Typhoon 9410 imaging system (Molecular Devices). The system is equipped with different filters, and the choice of an appropriate filter, conditions of monitoring fluorescence, including detection sensitivity and resolution are described in the manufacturer’s manual. Western blotting analysis was performed by using anti-EGFR and anti-αtubulin primary antibodies, and secondary antibody conjugated to DyLight-800 by scanning membranes at near-infrared wavelength with Odyssey imaging system (Li-COR).
RESULTS AND DISCUSSION
First, we attempted to assess proteins bound to S-NBD compounds in cells by in-gel detection. MDA MB468 and A549 cells were incubated with compound NSC 228155, and lysed in a moderate-strength buffer to maintain the integrity of putative proteins reacted with the S-NBD compound. The soluble fraction of cell lysates was subjected to electrophoresis under nonreducing conditions, and gels were analysed by staining with Coomassie Brilliant Blue or by scanning at different wavelengths with a Typhoon 9410 imaging system. In contrast to the stained gel, no protein bands were detected by scanning with the Typhoon imaging system (data not shown), indicating that the aqueous environment extinguished the fluorescence emitted by compound-bound proteins.
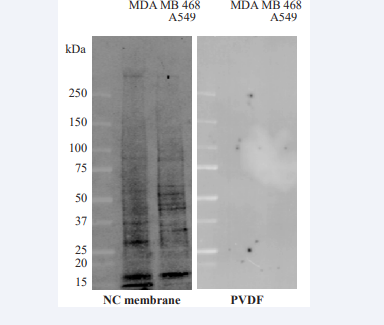
Next, to enhance fluorescent signal intensity, the protein extracts isolated from cancer cells after exposure to NSC 228155 were transferred from gel onto NC and PVDF membranes with a Turbo blotting system, and then scanned at different wavelengths. Florescent bands were not detected on PVDF membrane but appeared on NC membrane, with the greatest intensity at λex 488 nm and λem 520 nm (Figure 2).
Figure 2 Comparison of fluorescence detection of proteins bound to compound NSC 228155 in cells on PVDF or NC membranes.
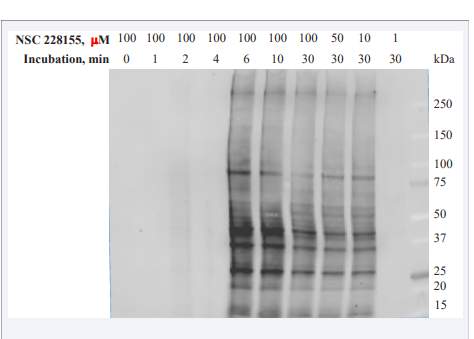
To determine the time required for the binding of NSC 228155 to proteins within cells, exposures to the compound varying from 1 min to 30 min were tested. Fluorescent bands were not yet visible in MDA MB468 cells at a 4-min exposure to NSC 228155 whereas many bands of proteins illuminated at 6-min exposure (Figure 3),
Figure 3 S-NBD compounds rapidly bind to multiple protein targets in breast cancer cells.
conforming to 5-min duration of the compound saturation in MDAMB468 cells observed previously with fluorescent microscopy [12]. The profiles of nearly 20 proteins emitting strong and moderate signal were similar in cells exposed to the NSC 228155 compound with concentrations from 10 μM to 100 μM. Exposure of cells to lower concentrations indicated that the limit of detection sensitivity is within 1 μM to 10 μM of NSC 228155 in analysed samples under the conditions used.
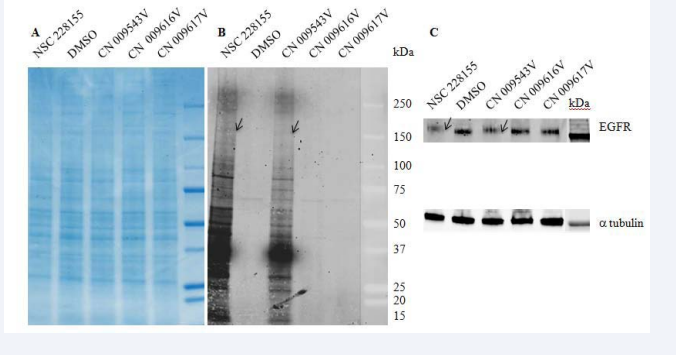
To confirm that the fluorescence was emitted by the proteins targeted within cells, we studied lung cancer A549cells treated with lipophilic and non-lipophilic S-NBD compounds for 10 min. No difference was observed between coloured protein samples in lysates of cells treated by four compounds after staining with Coomassie (Figure 4A).
Figure 4 Only lipophilic S-NBD compounds bind to proteins in lung cancer cells
On the contrary, fluorescent bands were detected after exposure of A549 cells only to lipophilic NSC 228155 or CN 009543V (Figure 4B),consistent with the ability of these S-NBD compounds to penetrate cells as previously revealed in cellulo with fluorescent microscopy [12]. It is noteworthy that profiles of fluorescent proteins were found to be similar but not identical in lung and breast cancer cells suggesting some difference in protein targets or different expression levels of the same proteins in two cell lines
Thus, S-NBD electrophiles, generated within 5-6 minutes in cytoplasm, bind to a large but limited number of proteins, ranging from 15 kDa to more than 250 kDa, which exhibit strong and moderate fluorescence on the NC membrane. A 180-kDa band close to the molecular mass of epidermal growth factor receptor (EGFR) attracted our attention as this transmembrane protein was previously used as a suitable probe in screening compound libraries that allowed us to select small molecule binders of the receptor, including the compound NSC 228155 [14]. Immunological analysis with anti-EGFR antibody revealed that fluorescent bands, detected at 520 nm and 800 nm wavelengths on the same membrane, perfectly fitted as indication that a 180-kDa protein belongs to EGFR (Figure 4C). Further characterization of fluorescent bands by mass spectrometrybased approaches will help to identify other proteins to which S-NBD compounds bind in living cells.
Recently, O-NBD compounds connected to biotin have been designed as binding probes to enhance the in-gel detection sensitivity of fluorescent signals emitted by mitochondrial proteins after enrichment by immune precipitation [15]. Our study highlights the possibility of an alternative straightforward method for assessing the authentic binding ability of S-NBD compounds to proteins by utilizing the blotting technique and appropriate scanning system. This approach is rather sensitive and does not require connecting detection tags to the compound structure or performing chemical tricks to enhance the sensitivity of in-gel detection of bound proteins. Such an advantage could be especially important at discovery stages in order to assess the reactivity and binding specificity of a bulk number of intermediate fluorescent constructions, without having to resort to more expensive methods that require more time.
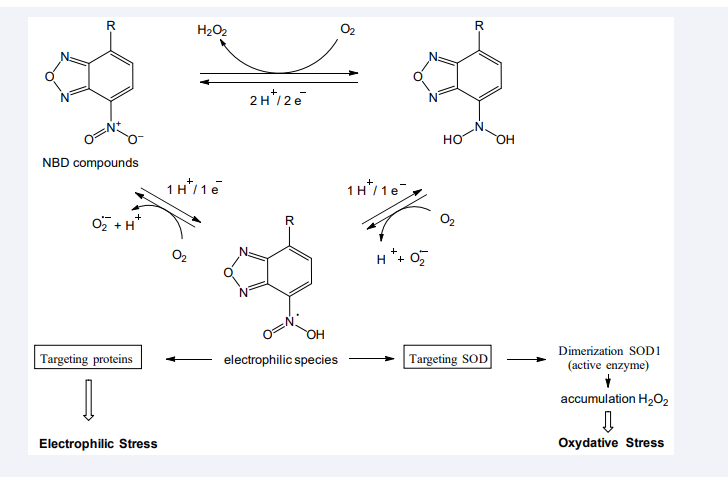
Recent studies indicate that S-NBD electrophilic species, having accumulated to critical concentration levels, impair the function of key proteins, leading to cell death [11,12,14,16,17]. On the basis of these results and new finding that only lipophilic NBD compounds were able to bind to multiple proteins shortly after exposure of cells, we propose a plausible explanation of a role of electrophilic species in the induction and progression of oxidative and electrophilic stress in cells (Figure 5).
Figure 5 Dual action of reactive NBD compounds on proteins and the progression of electrophilic and oxidative stress in cancer cells.
S-NBD compounds undergo redox cycling through the reduction of a nitro group; this is accompanied by the generation of ROS and electrophilic NBD species in cultured cells, especially if they grow under high concentrations of oxygen [11]. The electrophiles could target and bind to nucleophilic amino acids in proteins. Notably, NBD electrophiles generated already at first steps of redox cycling bind to and dimerize SOD1, a key enzyme in the reduction of spontaneously formed superoxide ions into peroxide hydrogen in cytoplasm [12]. Protein dimerization was prevented in SOD1 knockdown cells and in cells pre-incubated with antioxidants, especially with thioglycerol. The aberrantly formed SOD1 dimer is stable and active enzyme, therefore it provokes the accumulation of peroxide hydrogen in cytoplasm in the absence of adequate activity of other enzymes involved in the reduction of this rather stable reactive molecule (no alterations were observed in the expression levels of catalase and glutathione peroxidase). Hence, the binding of S-NBD electrophiles to SOD1resulting in the dimerization of the enzyme can be considered a crucial and indispensable event for rapid and significant increase in toxic concentrations of H2 O2 in cells.
At low concentrations, H2 O2 plays the role of a second messenger in cell signalling; at high concentrations, this reactive molecule attacks proteins, DNA and lipids, leading to oxidative stress and further cell death [18]. In this regard, the accumulation of H2 O2 has been shown to promote remarkable enhancing tyrosine phosphorylation of EGFR and aberrant triggering downstream signalling pathways in cells detectable within 5-min exposure to S-NBD compounds14 . Besides, the increased level of DNA-dependant protein kinase dimers has been detected in cancer cells shortly after exposure to compound NSC 224185, and this is associated with the degradation of the DNA repair protein through protein ubiquitination and probably other degradation pathways [17]. Furthermore, rapid blebbing was observed in cancer cells incubated with S-NBD compounds [11,12], as an indication of membrane detachment[19].
Thus, two types of reactive species, S-NBD electrophiles and H2 O2 , target multiple proteins in living cells, and the dual action of reactive species aggravates deleterious effects, leading ultimately to cell death. However, the gravity of oxidative stress first depends on the rate of impairing functions of SOD1, and then apparently of other proteins to be targeted. Given that the nucleophilicity of amino acids in cell depends on various factors, including redox status, it can be assumed that the number of bound proteins, including non-specific interactions might be variable under different culture conditions. Even at low doses, electrophilic species might irreversibly bind to proteins, and thereby, unpredictably contribute to deregulation of cellular metabolism. Therefore, the mass spectrometrybased identification of proteins that covalently react with NBD compounds in living cells will help to establish a list of suitable diagnostic and therapeutic biomarkers of electrophilic stress as a prerequisite to developing new strategies in fighting cancer and neurodegenerative diseases.
CONCLUSION
The commonly used blotting technique [2,3] offers up an alternative to in-gel detection for straightforward evaluating the selectivity of imaging probes for diagnostic purposes and the binding specificity of fluorescent intermediate compounds during the course of drug development. Furthermore, use of the nitrocellulose membrane with blotted proteins is a promising format for developing simplified and cost-effective approaches with which to assess the impact of fluorescent organic chemicals and potential pollutants on the human proteome.
ACKNOWLEDGMENTS
The authors acknowledge the Drug Synthesis and Chemistry Branch of the NCI for providing the NSC 228155 compounds and the French National Chemical Library for initially providing the CN 009543V compound. ProtNeteomix and CNRS supported this work.