Mucocutaneous Leishmaniasis with A Typical Clinical Manifestation: A Case Report
- 1. Department of Dermatovenereology, Addis Ababa University, Ethiopia
- 2. Department of Dermatology, University of California, USA
- 3. Armauer Hansen Research institute, Addis Ababa University, Ethiopia
- 4. Department of Communicable Disease, University of Gothenburg, Sweden
- 5. Department of Communicable Disease, University of Gothenburg, Sweden
Abstract
The usual clinical presentation of cutaneous leishmaniasis is easily diagnosed by clinicians in endemic regions, but the unusual forms may give rise to difficulties in diagnosis and appropriate management. In recent times, there has been an increase in the number of reports for new, rare and typical variants of cutaneous leishmaniasis. Here, we report a 14 year old male Ethiopian patient with an unusual clinical presentation of mucocutaneous leishmaniasis who presented with a punched out ulcer, extending to the lower lip with associated edema of the lower half of the face and oral mucosa, mimicking malignant neoplasm. The clinical differential diagnosis included, among other entities, Burkitt’s lymphoma, rhabdomyosarcoma and midline lethal granuloma. Accordingly, and we took an incisional biopsy specimen to exclude cancer. Indeed, leishmania amastigotes were detected by histopathology, and the patient was successfully treated with Amphotericin B.
Mucocutaneous leishmaniasis mimicking malignant neoplasm, as exemplified by our patient, is especially rare. To our knowledge this presentation has not been described in Ethiopia before and may present difficulties in making a definitive diagnosis.
Even in an endemic country it is worth reporting unusual and rare clinical forms and localizations of cutaneous leishmaniasis in order to avoid inappropriate diagnosis and management.
Keywords
• Cutaneous leishmaniasis; Mucocutaneous
leishmaniasis; Case report; Clinical diversity; Atypical
clinical forms; Amphotericin B
Citation
Yosef T, Harris RM, Girma S, Dotevall L, Issa A, et al. (2018) Mucocutaneous Leishmaniasis with A Typical Clinical Manifestation: A Case Report. Ann Clin Med Microbiol 3(1): 1014.
INTRODUCTION
Leishmaniasis is a widely dispersed parasitic vector-borne disease, caused by different species of the obligate intracellular protozoa of the genus Leishmania and transmitted through the bite of an infected females and fly [1,2]. Around 23 species of leishmania are responsible for one of the three clinical forms of the disease: cutaneous, mucosal and visceral leishmaniasis. It is endemic in 98 countries, with an estimated incidence of 2 million new cases per year (0.5 million of visceral leishmaniasis and l.5 million of cutaneous leishmaniasis) and more than 350 million people at risk [2,3]. Cutaneous leishmaniasis (CL) is an important disease with a wide spectrum of clinical manifestations posing a public health problem in many parts of the world. Approximately 75 present of CL is reported from 10 countries, including Ethiopia [1]. It occurs in a variety of clinical forms depending on the responsible subspecies of the Leishmania involved, and host-related factors [4]. The common clinical types present no diagnostic difficulties. At times, unusual presentations may cause diagnostic challenges and treatment delay. In addition to the classical clinical presentation, there are several infrequent and atypical features of the disease that have been reported in the last several years [4,5].
In this case report, we describe a rare variant and unusual form of mucocutaneous Leishmaniasis, resembling malignant neoplasm, which was successfully treated with Amphotericin B.
CASE REPORT
A 14-year old male presented with a three-year history of skin lesion on the chin. The initial lesion started as asymptomatic, painless papules, which slowly coalesced and formed an infiltrative plaque. Two years after the initial papule, ulceration developed on the lesion with associated edema. Over the ensuing months the lesion enlarged to cover the entire lower half of the face, causing much difficulty with eating.
There was no history of facial trauma or insect bite. He had no significant past medical history.
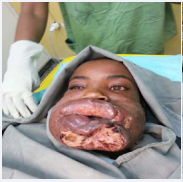
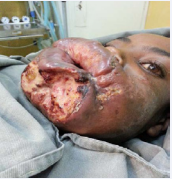
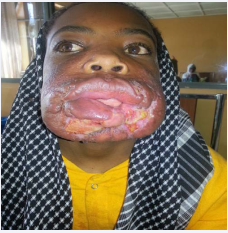
Clinical examination revealed an indurated plaque on the cheeks, including extensive punched out ulcer with a fibrinous base and necrotic border, extending to the lower lip. There was also associated edema of the lower half of the face including lip and oral mucosa (Figure 1A and B).
Figure 1a: Indurated plaque on the cheeks, punched out ulcer with dirty base and necrotic border, extending to the lower lip with associated edema of the lower half of the face and oral mucosa before treatment
Figure 1b: Indurated plaque on the cheeks, punched out ulcer with dirty base and necrotic border, extending to the lower lip with associated edema of the lower half of the face and oral mucosa before treatment
No regional lymph nodes were found. Systemic physical examination was normal.
The clinical features were consistent with malignancy. But, considering the asymptomatic nature of the initial lesion, the long period of clinical evolution and the endemic prevalence of cutaneous leishmania in Ethiopia, atypical mucocutaneous leishmaniasis mimicking neoplasm were also included in the differential diagnosis.
Routine laboratory tests were within normal limits and an HIV serology test was negative.
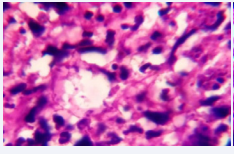
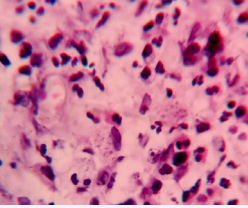
An incisional skin biopsy from the outer edge of the lesion was performed with the presumptive clinical diagnosis of malignant neoplasm, including lymphoma, sarcoma and lethal midline granuloma. Tissue impression smears, culture, Polymerase chain reaction (PCR), and histopathology examination were performed. A direct microscopic examination stained with Giemsa showed amastigotes with some visible kinetoplasts (Figure 2A and B).
Figure 2a: Giemsa staining (100X) Amastigotes with some visible kinetoplasts.
Figure 2b: Giemsa staining (100X) Amastigotes with some visible kinetoplasts.
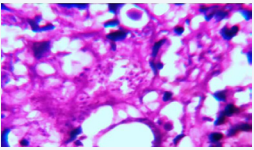
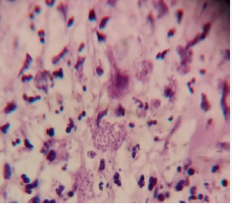
The histopathological examination showed a diffuse dermal inflammatory infiltrate composed of macrophages, lymphocytes, eosinophils and neutrophils. In most of macrophages amastigotes were seen (Figure 3A and B).
Figure 3a: Hematoxiline-Eosin (40X) Compact inflammatory infiltrate on deep dermis and numerous amastigotes in most of macrophages. (leishmania’s bodies in the cytoplasm ofhistiocytes infiltrating the dermis).
Figure 3b: Hematoxiline-Eosin (40X) Compact inflammatory infiltrate on deep dermis and numerous amastigotes in most of macrophages. (leishmania’s bodies in the cytoplasm ofhistiocytes infiltrating the dermis).
Culture and PCR were negative for Leishmania, however despite the smear and histologic findings.
The diagnosis of mucocutaneous leishmaniasis was confirmed by detection of numerous amastigotes and Leishmaniasis bodies in the cytoplasm of histiocytes in direct microscopy and histological sections, respectively.
The patient was started on treatment with N-methyl glutamine antimoniate after a baseline investigation was done at the dose of 30mg/kg by intramuscular injection for 45 consecutive days with local wound care. After receiving a total of 45 days of systemic meglumine antimoniate, there was no significant improvement.
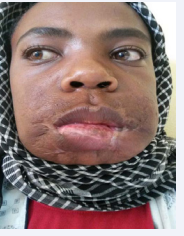
Four weeks after the end of systemic meglumine antimoniate treatment, a second course of treatment with amphotericin B liposomal formulation was started at a dose of 3mg/kg/day intravenous, with a total of 7 doses (5 consecutive days, on the 14th day and 21st day). After completion, there was significant improvement on the ulcerated lesion but there was some active satellite lesion (Figure 4A).
Figure 4a: While on the first cycle of Amphotericin B (A), After completion of the second cycle of Amphotericin B (B).
Due to this reason, a second cycle of amphotericin B with the same dose was administered. The treatment was well tolerated by the patient, except slight elevation in serum alkaline phosphates (ALP), which normalized within a month.
At the end of the two treatment cycle, clinical recovery was almost complete, only scarred tissue was observed in the place of the large disfiguring gulcerated lesion (Figure 4B).
Figure 4b: While on the first cycle of Amphotericin B (A), After completion of the second cycle of Amphotericin B (B).
Accordingly, response to treatment in this atypical presentation of mucocutaneous leishmaniasis was very satisfactory.
DISCUSSION
Cutaneous leishmaniasis (CL) is endemic disease in Ethiopia and widely distributed all over the country. It is a zoonotic disease, mainly occurring in the highland regions, involving rock hyraxes as reservoir. The disease predominantly affects children, adolescents and young adults. Within Ethiopia, the annual CL burden is estimated to be approximately 20.000 to 40.000 cases per year. A recent study estimated almost 30 million Ethiopians to be at risk for CL [6-11]. L. aethiopica causes more than 99.9% of the CL cases in Ethiopia [11]. L. major and L. tropica have been isolated from sand fly vectors and one case of CL due to L. tropica has been reported [12,13].
The clinical manifestations of L aethiopica are particularly diverse and ranges from a localized cutaneous form (LCL), diffuse cutaneous (DCL) forms to the more distractive and disfiguring mucocutaneous (MCL) variant [14, 15]. CL is known for its clinical diversity. In addition to the classical clinical picture, a number of rare and atypical variants of the disease mimicking both infectious and malignant conditions (e.g. tropical ulcers, sporotrichosis, leprosy, lupus vulgaris, deep mycosis, basal cell carcinoma and cutaneous lymphoma) have been reported in the literature [4,5,14]. A wide variety of clinical presentations can delay correct diagnosis and proper treatment. In such cases, a high index of suspicion and diagnostic laboratory techniques can facilitate the diagnosis [15,16].
Several cases reports of atypical clinical presentation of CL (both in old and new world disease) have been related to a range of possible causes including host immuno suppression, co-morbidities, and malnutrition [15-17]. However, some of the atypical cases do not seem to be related to obvious risk factors, as in our immuno competent patient with no other significant comorbities. Thus, other explanations must be sought to address the diversity of disease forms. Clinical and experimental evidence indicates that genetic diversity among strains may also be a partial determinant of the atypical CL phenotype [17].
Mucocutaneous leishmaniasis (MCL), is a serious and occasionally a life threatening form of leishmaniasis, which has been mainly reported in the New World in association with L.
braziliensis [17]. However, cases showing chronic destructive ulcerative lesions of the nasal cavity and mid face have been seen in the Old World in association with L. aethiopica [4,8].
In Ethiopia, MCL most frequently remains confined to the borders of the nose and the mouth. However, it can also spread deeply into the nose, the palate or the pharynx, where it may cause extensive and disfiguring destructive lesions due to either mucosal metastatic spread from a primary lesion of the skin or by direct inoculation of mucous membranes [11,14,15]. While most of the clinical presentations of CL in Ethiopia are characteristic and pose no diagnostic difficulties, patients with severe and atypical lesions are rarely encountered [11]. Our patient presented with an extensive and aggressive chronic ulcer, involving the mucosa, mimicking a malignant neoplasm. Therefore, it is recommended that MCL should be included in the differential diagnosis of dermatological neoplastic conditions, especially in endemic areas.
Misdiagnosis of this highly destructive and disfiguring variant of MCL may lead to an unfavorable outcome; therefore, prompt confirmation of the clinical diagnosis is desirable. Unfortunately, the parasite can only be identified in 50% of cases of MCL, even in experienced hands [18,19]. Overall, the combination of parasitological techniques is slightly more sensitive (100%) than the PCR assay (98.8%) in detection of Old World CL (47.4% to 83.3% in MCL) [18-20]. Inability to identify the causative subspecies in our patient could be due to the scarcity of parasites in chronic MCL [21]. Currently, Leishmania aethiopica is shown to be the only species causing CL in Ethiopia, and is endemic in the Ethiopian highlands (> 2,000 meters) [7-14]. Although, we were not able to identify the species, it seems highly likely that this atypical MCL mimicking malignant neoplasm in our patient was caused by L.aethiopica.
Despite the substantial burden, evidence-based treatment guidelines are lacking in Ethiopia. Currently, pentavalent antimonials are the first-line drugs in Ethiopia, although a high prevalence of resistance with frequent relapses is described with this regimen [14,8]. Other studies have shown that sodium stibogluconate demonstrated poor in vitro activity against L. aethiopica derived from an Ethiopian CL patient; additionally, clinical experience in treatment of patients with MCL have shown SSG to be ineffective [22], further suggesting that alternative first line treatments should be explored.
In view of the fact that leishmaniasis is a disease of neglected people belonging to low socio-economic strata of the population, other antileishmanial drugs such as liposomal amphotericin B or miltefosine were not included among the possible treatments of CL in Ethiopia due to the cost [11,14,22]. Studies have shown that atypical clinical presentations of CL have proven significantly more refractory to antimony than the classic CL, but fortunately, fully responsive to amphotericin B. Moreover, mucosal lesions have also been reported to responded well to amphotericin B [17,23]. Although, amphotericin B is active on OWL species in vitro, no controlled study confirms the efficiency of the drug in the treatment of the disease [22].
In our patient, the failed first line treatment in light of a potential destructive and severely disfiguring clinical presentation of chronic MCL on the face prompted our decision to initiate liposomal amphotericin B treatment. Analogous with the recommended dosage for treatment of MCL, we administered a total of 7 doses (3mg/kg). This treatment was well tolerated and rapid clinical improvement of the ulcer with striking reduction of mucosal edema was achieved within a few weeks of treatment. The patient returned for follow up after 3months and his ulcer had healed completely with no recurrence. He has been sent to plastic surgery for lip reconstruction.
CONCLUSION
In conclusion, we report a rare and unusual presentation of MCL mimicking malignant neoplasm in Ethiopia, extending the clinical spectrum of OWL. As this atypical clinical presentation was perplexing to even the most experienced physicians. Providers need to be familiar with this and other atypical clinical presentations of MCL to facilitate early detection and proper treatment, potentially cure the disease, as well as prevent negative psychological impact and prevent transmission.
This case report also contributes to the efficiency of liposomal amphotericin B in the treatment of antimony resistant chronic and severe MCL. Although, it is not possible to arrive at a definite conclusion from a single case report, considering the disease burden and poor response to pentavalent antimonials, clinical trials evaluating the pharmacotherapy of liposomal amphotericin Bare urgently needed to establish cost-effective and therapeutic protocols in Ethiopia.