Surveillance of Populations of Aedes (Stegomyia) Mosquitoes at the Autonomous Port of Abidjan (C
- 1. Vector Control Department, National Institute of Public Hygiene, Côte d’Ivoire
- 2. Ministry of Health and Public Hygiene, Côte d’Ivoire
- 3. Expanded Program of Immunization, Ministry of Health and Public Hygiene, Côte d’Ivoire
ABSTRACT
Aedes mosquitoes are highly invasive and can survive in temperate as well as tropical climates. They transmit a number of major world’s deadly diseases. A monitoring of potential vectors of arboviruses initiated in Abidjan in 2009-2010 allowed noting the presence of Aedes albopictus at the autonomous port of Abidjan. Thus, this study was undertaken to determine the prevalence of Aedes populations and assess the potential health risks.
All emerging adults from eggs collected by ovitraps were Aedes aegypti. The analysis of environmental factors reveals that the presence of vegetation around ovitraps significantly influences its use by mosquitoes. Furthermore, human activity also promotes the use of ovitraps installed nearby. Eight different potential breeding habitats were found. Tarpaulins (36.7%) were the predominant potential breeding habitats followed tires (30.4%). Aedes aegypti has infested 88.7% of total positive habitats. Larvae of Culex and Anopheles have colonized respectively 7.5% and 3.8% of positive habitats. The most productive Aedes aegypti larval habitats were found to be tarpaulins and cavities of concrete electricity posts producing 47.6 and 30.7% of pupae respectively.
Port health authorities should develop an appropriate action plan to control the density of mosquitoes and minimize potential risks to global health with emphasis on vector surveillance.
KEYWORDS
• Aedes mosquitoes
• Entomological surveillance
• Port
• Côte d’Ivoire
CITATION
Yao LK, Atioumounan BK, Kouadio DE (2017) Surveillance of Populations of Aedes (Stegomyia) Mosquitoes at the Autonomous Port of Abidjan (Côte d’Ivoire). Ann Community Med Pract 3(2): 1021.
INTRODUCTION
With the expansion of transport, the area of distribution of Aedes mosquitoes, particularly Aedes albopictus is constantly changing with worrying risks for public health [1-2]. In areas where this invasive mosquito has established itself, local transmission of arbovirus infections has been observed [3-4]. Additionally, despite efforts to contain the disease, yellow fever continues to be a threat in tropical areas of Africa and Latin America. The number of cases has increased the last two decades due to the decreased immunity of population, deforestation, urbanization, population movements and climate change [5]. In 2013, Africa recorded 84,000 to 170,000 severe cases of yellow fever with 29,000 to 60,000 deaths [5]. This disease is also an important risk for travelers who go in endemic areas. Exposure to infective mosquito bites is the only significant mode of transmission of yellow fever.
Côte d’Ivoire has a history profoundly marked by yellow fever [6-9]. However, the country experienced a long period without outbreaks, for about two decades after the introduction of yellow fever vaccine into the Expanded Program of Immunization in 1983 [10]. In 2001, an outbreak occurred in Abidjan, the economic capital of the country. Despite immunization of 2.6 million people raising the vaccine coverage to 92.2% [11], the city of Abidjan was faced again with the co-circulation of yellow fever virus and DENV-3 in 2008 [12]. The surveillance of potential vectors of arboviruses, initiated in certain points of the city of Abidjan to strengthen monitoring of potential epidemic diseases, allowed the detection of the presence of Ae. albopictus at the container terminal of the autonomous Port of Abidjan [13]. Originally considered as rural vector [14], this mosquito has adapted well to urban environments with larvae now breeding in artificial containers and has become an important and sometimes sole vector in urban areas [15-17]. The establishment of this species could increase the transmission of arboviruses, hence the necessity to see if it had become established after its discovery at the Port of Abidjan. Moreover, as per the International Health Regulations Act [18], all international airports/seaports and peripheral areas up to 400 meters should be kept free from Aedes mosquito breeding or indices should be kept less than one to eliminate the chance of spreading of disease or vectors to any part of the world. Therefore, intensified surveillance was undertaken to determine the prevalence of Aedes mosquitoes at the seaport of Abidjan and the risk according to areas.
MATERIALS AND METHODS
Study site
The city of Abidjan, economic capital of Côte d’Ivoire with a population of over six million, is located in the southern part of the country (5°19’N, 4°01’W). The city comprises 10 municipalities and has an international airport and the largest seaport of the subregion after that of Lagos. At the heart of the city lies Banco Forest, a national park of 3474 hectares of rain forest. The climate is tropical which records temperatures of low amplitude (25°C to 30°C), a high humidity (80 to 90%) and abundant rainfall (1300 to 1600 mm). The major rainy season is from April to July and the shorter season is from October to November. The major dry season starts from December to March and a shorter one occurs from August to September [19-20].
The Autonomous Port of Abidjan is located in three areas of Abidjan: Plateau, Treichville and Port Bouet. The Port operates three docks, oil stations at sea, and several terminals including a container terminal, a fruit terminal, a timber terminal and a fishing port.
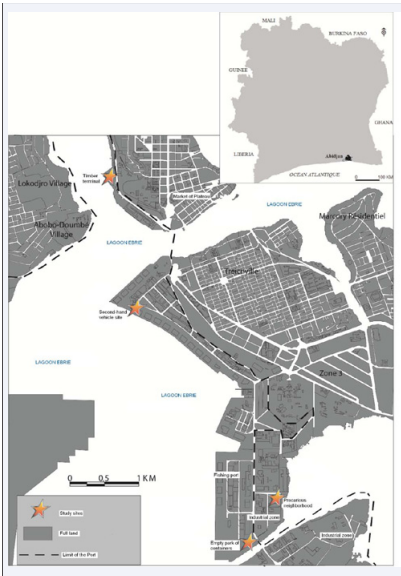
In this study, the timber terminal, the parking lot where second-hand vehicles are parked, the empty container lot and an adjacent undeveloped neighborhood were selected to monitor of populations of Aedes at the Port of Abidjan (Figure 1).
Figure 1: Location of collection sites at the autonomous port of Abidjan (Côte d’Ivoire).
Method
Entomological monitoring of Aedes mosquitoes was undertaken at the seaport of Abidjan from April to December 2014 using the ovitraps method and larval surveys. Ten ovitraps were installed in each site in order to collect Aedes eggsand their location recorded. The environment around each ovitrap was described by the presence or absence of vegetation, of breeding site, of human activity and of shade. The nature of the drop shadow (wall/tree) and the type of human activity were also noted.
Wooden paddles of each ovitrap were collected every 10 days and packed in individual labeled plastic bag and taken to the laboratory. At each collection, the water of the ovitrap was transferred and renewed. At the laboratory, paddles were dried on a table covered with mosquito netting to prevent additional egg laying by wild mosquitoes. After drying, paddles were individually immerged for 2 days in bowls labeled with the name of the site and location, containing 200 ml of water, 3 times successively separated by 5 days of drying. The larvae hatched in the laboratory and those found in ovitraps were counted and reared to adults. All mosquitoes that emerged were identified using the morphological identification keys [21] and morphological descriptions of African Aedes species [22]. The number of adults was recorded for each collection point. A larval survey that consisted of a search for the presence of immature populations of Aedes in breeding containers was conducted once a month. Immature mosquitoes were collected from each positive breeding site by using dipping and pipetting methods, preserved in identified jars and brought to the insectary. Larvae and pupae were counted, reared, and treated in the same way as above. The productivity of each type of container was estimated for those which harbored pupae of Aedes [23-24].
Statistical analyses
Data were analyzed using the software XLSTAT 2014.4.06. Shapiro-Wilk, Anderson-Darling, Lilliefors and Jarque-Bera normality test have been performed to see data distribution. As the distribution of data was not normal, Kruskal-Wallis test followed by multiple pair wise comparison was employed to compare the productivity of collected sites and points. The Steel-Dwass-Critchlow-Fligner procedure was used to compare the collection areas as well as specific collection points. A generalized additive model was used to identify the environmental factors related to the effctiveness of ovitraps using binomial distribution with Statistica Software version 10.
RESULTS
A total of 2460of emerging adults was obtained of which 79.1% by ovitraps and 20.9%by larval surveys.
OVITRAP COLLECTIONS
All emerging adults from eggs collected by ovitraps were Aedes aegypti with 48.2% of females of which 19.4% were collected at the undeveloped neighborhood, 21.4% at the empty container lot, 28, 5% at the timber terminal and 30.7% at the parking lot of second-hand vehicles. Considering the non-significant mortality of larvae during their rearing, the number of adult obtained by site was compared. The average number of adult mosquitoes obtained per site varied from 2.22 to 3.51 mosquitoes/ovitrap with a non-significant difference from one site to another (Table 1).
Table 1: Comparison of collection sites at the Autonomous Port of Abidjan (Côte d’Ivoire) according to the average number of adult mosquitoes obtained by ovitrap from April to December 2014.
|
Collection site |
Observation |
Average number of adult mosquitoes |
Standard deviation |
Sum of ranks |
Mean of ranks |
Groups |
|
Underdeveloped neighborhood |
170 |
2.218 |
4.903 |
55470,500 |
326,297 |
A |
|
Empty park of containers |
170 |
2.453 |
6.295 |
57326,500 |
337,215 |
A |
|
Second-hand vehicle |
170 |
3.512 |
9.066 |
59233,500 |
348,432 |
A |
|
Timber terminal |
170 |
3.265 |
9.254 |
59509,500 |
350,056 |
A |
|
Multiple pairwise comparisons using the Steel-Dwass-Critchlow-Fligner procedure / Two-tailed test. |
||||||
However, the average number of adults recorded per collection point varied according to the site. It has been statistically identical to all collection points of the underdeveloped neighborhood and second-hand vehicles unlike the two other collection sites (Table 2).
Table 2: Average number of adult mosquitoes by ovitrap at the Autonomous Port of Abidjan (Côte d’Ivoire) from April to December 2014.
|
Collection Points |
Underdeveloped neighborhood |
Park toempty containers |
Park of second-hand vehicles |
Timber terminal |
||||||
|
Mean ± Standard deviation |
Groups |
Mean ± Standard deviation |
Groups |
Mean ± Standard deviation |
Groups |
Mean ± Standard deviation |
Groups |
|||
|
Ovitrap 1 |
2.05 ± 3.99 |
A |
1.17 ± 2.53 |
A |
B |
2.52 ± 5.80 |
A |
1.93 ± 2.69 |
A |
B |
|
Ovitrap 2 |
4.05 ± 7.05 |
A |
0.23 ± 0.56 |
A |
|
5.11 ± 8.38 |
A |
3.62 ±7.18 |
A |
B |
|
Ovitrap 3 |
3.47 ± 7.06 |
A |
2.35 ± 5.87 |
A |
B |
1.52 ± 2.89 |
A |
4.06 ±7.24 |
A |
B |
|
Ovitrap 4 |
0.64 ± 1.11 |
A |
0.23 ± 0.97 |
A |
|
4.11 ± 9.98 |
A |
16.62 ± 22.79 |
|
B |
|
Ovitrap 5 |
2.76 ± 5.25 |
A |
0.41 ± 1.69 |
A |
|
0.29 ± 0.98 |
A |
4.93 ± 8.45 |
A |
B |
|
Ovitrap 6 |
0.00 ± 0.0 |
A |
0.05 ± 0.24 |
A |
|
0.52 ± 0.94 |
A |
1.93 ± 3.47 |
A |
B |
|
Ovitrap 7 |
3.58 ± 5.64 |
A |
3.52 ± 7.77 |
A |
B |
2.94 ± 5.43 |
A |
0.56 ±1.75 |
A |
|
|
Ovitrap 8 |
0.70 ± 1.75 |
A |
2.94 ± 6.62 |
A |
B |
3.05 ± 6.43 |
A |
0.12 ± 0.50 |
A |
|
|
Ovitrap 9 |
3.76 ± 6.54 |
A |
8.00 ± 10.44 |
|
B |
3.64 ± 8.69 |
A |
0.18 ± 0.54 |
A |
|
|
Ovitrap 10 |
1.11 ± 3.16 |
A |
5.58 ± 9.62 |
A |
B |
11.35 ± 20.31 |
A |
0.06 ± 0.25 |
A |
|
The analysis of environmental factors reveals that the presence of vegetation around ovitraps significantly influences its use by mosquitoes unlike breeding sites and shadow. Furthermore, human activity also promotes the use of ovitraps installed nearby (Table 3).
Table 3: Environmental factors associated with the effectiveness of ovitraps for Aedes aegypti femalesat the Autonomous Port of Abidjan (Côte d’Ivoire) from April to December 2014.
|
|
Sum of Squares |
Degree of Freedom |
Mean Square |
F |
P |
|
Intercept |
1462,58 |
1 |
1462,582 |
25,65095 |
0,000001 |
|
Vegetation |
319,92 |
1 |
319,917 |
5,61074 |
0,018131 |
|
Breeding Sites |
42,91 |
1 |
42,908 |
0,75252 |
0,385986 |
|
Shadow |
111,64 |
1 |
111,642 |
1,95799 |
0,162188 |
|
Activity |
555,09 |
1 |
555,092 |
9,73527 |
0,001885 |
|
Error |
38487,59 |
675 |
57,019 |
- |
- |
|
Univariate Tests of Significance for Number of mosquitoes. Sigma-restricted parameterization Effective hypothesis decomposition; Std. Error of Estimate: 7,551069 |
|||||
In this study, places of food sales, of handling or erected into mosque and housing have a positive influence on the ovitraps surrounding.
Seasonal variation of populations of Aedes aegypti
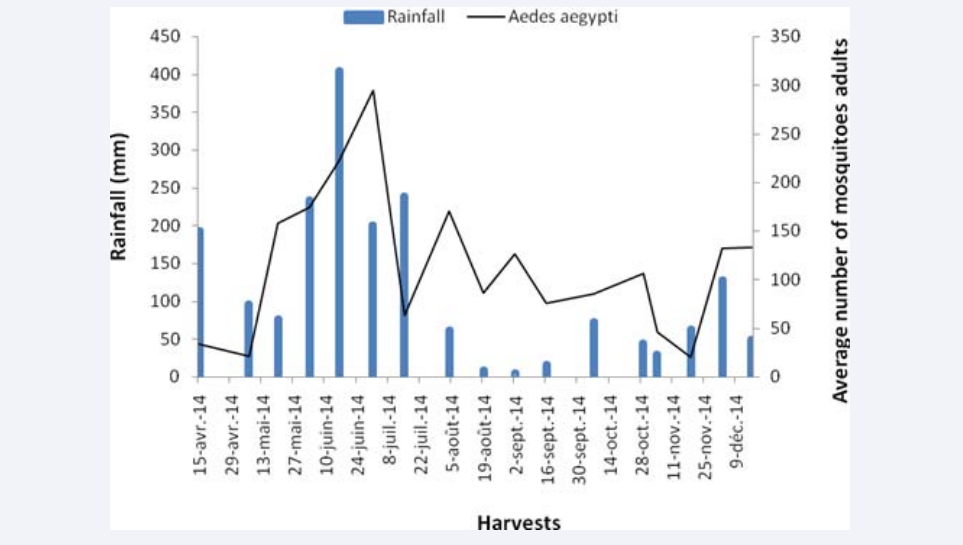
The average number of Aedes aegypti by harvest was 2.86 ± 0.29 specimens per paddle (S/Pl). The highest number (7.4 S/ Pl) was obtained at in June 2014 and the lowest (0.5 S/Pl) at in November 2014 (Figure 2).
Figure 2: Seasonal dynamics of the average number of Aedes aegypticollected by ovitraps at the autonomous Port of Abidjan (Côte d’Ivoire) from April to December 2014.
The collections can be divided into 3 groups. The first one with an average of between 0.5 and 1.5 S/ Pl corresponds to the harvests carried out at the end of the short rainy season and the beginning of the long rainy. The second includes the collections made during the rainy season with an average of between 1.9 and 5.6 S/Pl and the third corresponds to collections performed after the peak of rainfall with 7.4 S/Pl.
Larval surveys
Eight types of breeding habitats were found, half of which were found at the park of second-hand vehicles. Tarpaulins (36.7%) were the predominant potential breeding habitats followed tires (30.4%) (Table 4).
Table 4: Breeding habitats of mosquitoes found on the Port area of Abidjan from April to December 2014.
|
Types of container |
Underdeveloped neighborhood |
Park to empty containers |
Timber terminal |
Park of second-hand vehicles |
TOTAL |
||||||||||
|
|
N |
Mosquito larvae |
Aedes larvae |
N |
Mosquito larvae |
Aedes larvae |
N |
Mosquito larvae |
Aedes larvae |
N |
Mosquito larvae |
Aedes larvae |
N |
Mosquito larvae |
Aedes larvae |
|
Accessoires of vehicle |
0 |
0 |
0 |
1 |
1 |
1 |
0 |
0 |
0 |
1 |
1 |
1 |
2 |
2 |
2 |
|
Tarpaulin |
0 |
0 |
0 |
0 |
0 |
0 |
2 |
1 |
1 |
27 |
24 |
20 |
29 |
25 |
21 |
|
Security helmets abandoned |
0 |
0 |
0 |
2 |
1 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
2 |
1 |
1 |
|
Bucket of machine |
0 |
0 |
0 |
4 |
2 |
2 |
0 |
0 |
0 |
0 |
0 |
0 |
4 |
2 |
1 |
|
Tires |
2 |
2 |
1 |
10 |
6 |
6 |
0 |
0 |
0 |
12 |
8 |
7 |
24 |
16 |
14 |
|
Concrete electricity posts |
0 |
0 |
0 |
8 |
5 |
5 |
0 |
0 |
0 |
0 |
0 |
0 |
8 |
5 |
5 |
|
Abandoned containers (<2L) |
1 |
0 |
0 |
2 |
0 |
0 |
5 |
2 |
2 |
0 |
0 |
0 |
8 |
2 |
2 |
|
Abandoned containers (>5L) |
0 |
0 |
0 |
2 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
2 |
0 |
0 |
|
TOTAL |
3 |
2 |
1 |
29 |
15 |
15 |
7 |
3 |
3 |
40 |
33 |
28 |
79 |
53 |
47 |
|
N: Number of Potential Breeding Habitats |
|||||||||||||||
Overall, 67.1% (n = 79) of breeding habitats were found positive for mosquito larvae with 59.5% for Aedes aegypti. The park of second-hand vehicles had the highest percentage of positive breeding habitats. Among the containers, the most colonized by Aedes aegypti were respectively the accessories of vehicle (100%, n = 2), tarpaulins (72.4%, n = 29), concrete electricity posts with water in cavities (62.5%, n = 8) and tires (58.3%, n = 24). Larvae of three species of mosquitoes were collected. All different breeding habitats were found to have breeding of Aedes aegypti. However, larvae of Culex were found in tires and tarpaulins and those of Anopheles in tarpaulins. The most productive Aedes aegypti larval habitats were found to be tarpaulins and cavities of concrete electricity posts producing 47.6 and 30.7% of pupae respectively (Table 5).
Table 5: Productivity Aedes aegypti breeding sites on the port area of Abidjan (Côte d’Ivoire) from April to December 2014.
|
Type of containers |
Number of containers |
Positive containers for Ae aegypti |
Number of pupae |
Productivity |
|
Accessoires of vehicle |
02 (2.5%) |
02 (4.3%) |
00 |
00 |
|
Tarpaulin |
29 (36.7%) |
21 (44.6%) |
79 |
47.6 |
|
Security helmets abandoned |
02 (2.5%° |
01 (2.1%) |
00 |
00 |
|
Bucket of machine |
04 (5.2%° |
02 (4.3%) |
02 |
1.2 |
|
Tires |
24 (30.1%) |
14 (29.8%) |
31 |
18.7 |
|
Cavity of concrete spots |
08 (10.1%) |
05 (10.6%) |
51 |
30.7 |
|
Abandoned containers (<2L) |
08 (10.1%) |
02 (4.3%) |
03 |
1.8 |
|
Abandoned containers (>5L) |
02 (2.5%) |
00 |
00 |
00 |
|
Total |
79 (100%) |
47 |
166 |
|
|
Percentage in parenthesis |
||||
Four adult species of mosquito were obtained from larval rearing (Table 6). Ae. aegypti was the most abundant species with more than 83% of the emerged mosquitoes.
Table 6: Number of given species (and percentage) of mosquitoes adult from larvae collected at the port of Abidjan (Côte d’Ivoire) from April to December 2014.
|
Species |
Underdevelopedneighborhood |
Park toempty containers |
Timber terminal |
Park of second-hand vehicles |
Total |
|
Aedes aegypti |
2 |
150 |
6 |
271 |
429 (83.5) |
|
Anopheles gambiae |
0 |
0 |
0 |
13 |
13 (12.5) |
|
Culex cinereus |
0 |
0 |
0 |
8 |
8 (1.6) |
|
Culex quinquefasciatus |
0 |
0 |
6 |
64 |
64 (2.5) |
|
Total |
2 (0.6%) |
150 (29.3) |
12 (2.3) |
349 (67.9) |
514 |
|
Percentage in parenthesis |
|||||
Cx quinquefasciatus, Cx. cinereus and An. gambiae s.l. were represented respectively 12.5%, 1.6% and 2.5% of the total of emerging adults. More than 68% of these mosquitoes come from larvae collected at the park of second-hand vehicle, 28.8% at the park of empty container, 2.3% at the timber terminal and 0.6% at the precarious neighborhood.
DISCUSSION
In this study, only Aedes aegypti was collected by ovitraps on the port area of Abidjan with an average number of adult comparable on the different collection sites. Results reveal that the risk bound to this vector is identical on all port area. The continued presence of workers at the port would provide opportunities to females to feed and lay egg in the surrounding breeding sites due to their low dispersion. This would explain the preference of ovitraps installed near places used as places of rest or as mosque. According to Reiter et al. [25], Rodhain [26], and Edman et al. [27], the flight distance of Aedes aegypti in urban areas depends on the availability of hosts and breeding sites. The potential breeding habitats mainly consisting of discarded materials (tarpaulins, tires, helmet) and working equipment stored (loader buckets, concrete electricity posts) were more numerous at the park of second-hand vehicles then at the park to empty containers. Tarpaulins have been the dominant breeding habitats. Vijayakumar et al. [29], also found tarpaulins among breeding habitats of mosquitoes identified in Thiruvananthapuram, India. Nearly two-thirds of the identified breeding habitats were colonized by mosquitoes with the predominance of Ae. aegypti. Similar observations were made by Getachew et al., at Dire Dawa (East Ethiopia) [30]. Two other species, Cx. quinquefasciatus (16%) and An. gambiae (6%) were encountered, the first in the tires and tarpaulins and the second in tarpaulins, unusual breeding site for this species. This latter species was found in sunny breeding sites at the park of second-hand vehicles.
The productivity of breeding sites revealed that tarpaulins, concrete electricity posts, and discarded tires are respectively the most important breeding sites because they have been source of production of 47.8%, 30.7% and 18.7% of the populations of Aedes aegypti respectively. Their poor management is the main cause of their used by mosquito. In the study of Vijayakumar et al. [29], the most efficient containers in terms of breeding of Aedes were tires, followed by grinding stone, tarpaulin and metal containers. Port authorities should pay particular attention to this mosquito in managing the risk of transmission of diseases with epidemic potential. Mosquito breeding at the seaport is not just a simple local health problem but also a serious threat to global health security. Moreover the possible of introduction of invasive species and the permanent presence of workers in the port area are a significant risk for the transmission of arbovirus infections or their spread. Management measures are therefore essential to control the density of this vector below the level of disease-transmission level, which could be achieved by removal of the major breeding habitats in order to reduce not only the risk of autochthonous transmission of arboviruses but also the international spread of epidemic diseases. However, it is necessary to destroy also minor breeding habitats in order to avoid any rein festation. Future research priorities are regular monitoring of mosquitoes vectors of the port area with an aim to improve knowledge of the spatial distribution data of vectors, identify of origin and phylogenetic of these vectors.
ACKNOWLEDGEMENTS
We thank the Management of Autonomous Port of Abidjan and all staff for their cooperation. Our sincere thanks also to Ziogba T Jean Claude, Adou Koffi and Koffi Konan Patrice, technicians, for their help in the completion of this work. Dr Irish, Seth (CDC/ CGH/DPDM) for proof-reading this article.
REFERENCES
5. OMS. Fièvre jaune : aide-mémoire N°100. 2015.
6. Wondji CH. La fièvre jaune à Grand Bassam (1899-1903). Rev Fr Hist Outre Mer. 1972; 59: 205- 239.
14. Higa Y. Dengue Vectors and their Spatial Distribution. Trop Med Health. 2011; 39: 17-27.
18. WHO. International Health Regulations 2005. 2nd ed. Geneva: WHO. 2008.
26. Rodhain F. L’écologie d’Aedesaegypti en Afrique et en Asie. Bull Soc Pathol Exot. 1996; 89: 103-106.