Environment Influencing Serotonin Syndrome Induced by Ecstasy Abuse
- 1. Charles E. Schmidt College of Medicine, Florida Atlantic University, USA
- 2. Ross University School of Veterinary Medicine, West Indies
Abstract
Ecstasy is a recreational drug containing 3,4-methylenedioxymethamphetamine (MDMA). In the U.S., there are several millions of lifetime users, and millions each year added to the list as new users. Only several thousand every year show signs of severe toxicity and require emergency intervention. The illness is known as serotonin (5-HT) syndrome, which can be mild, moderate or severe. The relationship between mild, moderate and severe syndromes appears to be interchangeable, but the severe syndrome is life-threatening. The serotonergic mechanisms of how the mild or moderate syndrome becomes severe and life-threatening have attracted considerable attention in the last few years as an effort to explore new treatments potentially to manage illness and prevent death of patients. High levels of extracellular 5-HT in the brain produced by large doses of MDMA are not always necessary to cause a severe serotonin syndrome. Additional mechanisms may be more important. Recent research has demonstrated that environmental conditions (i.e., non-drug factors) are more critical in determining the severity of MDMA-induced serotonin syndrome than the drug dose. The purpose of the current article was to review available evidence regarding the effect of non-drug factors on serotonergic extrasynaptic receptor responsivity and the severity of MDMA-induced serotonin syndrome.
Keywords
Ecstasy; Serotonin syndrome; MDMA toxicity; Extrasynaptic receptor; 5-HT1A; 5-HT2A; Hyperthermia.
CITATION
Tao R, Shokry IM, Callanan JJ (2017) Environment Influencing Serotonin Syndrome Induced by Ecstasy Abuse. Ann Forensic Res Anal 4(1): 1039.
ABBREVIATIONS
5-HT: 5-Hydroxytryptamine; SERT: Serotonin Reuptake Transporter; MDMA: 3,4-Methylenedioxymethamphetamine; 5-HTP: 5-Hydroxytryptophan; MAOI: Monoamine Oxidase Inhibitor; 8-OH-DPAT: 8-Hydroxy-2-(Dipropylamino)Tetralin; Pki : Negative Logarithm Of The Equilibrium Dissociation Constant; nM: Nanomolar; µM: Micromolar; SC: Subcutaneous
INTRODUCTION
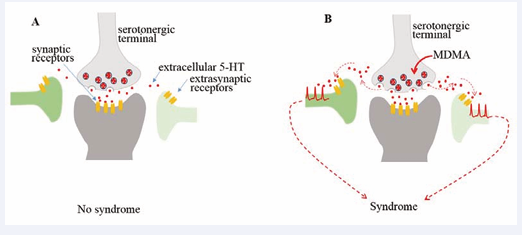
3,4-Methylenedioxymethamphetamine (MDMA) is the major constituent in Ecstasy tablets, which can cause an excessive release of serotonin (5-HT) in the brain. Under a normal condition, 5-HT as a neurotransmitter is released to the synaptic cleft, exerting physiological effects on synaptic receptors (Figure 1A), then undergoes reuptake by serotonin reuptake transporters (SERTs). Some 5-HT molecules that diffuse away from the cleft space become a spill over and act as a volume transmitter for extrasynaptic receptors. It is generally believed that extrasynaptic receptors are functionally different from synaptic receptors [1,2]. The role of synaptic receptors is mainly to exert physiological functions while activation of extrasynaptic receptors is attributed to pathological changes and diseases [1,2]. In this review, we will discuss the relationship between extracellular 5-HT concentrations and activation of extrasynaptic receptors in a serotonin syndrome caused by MDMA (Figure 1B).
Figure 1: Serotonin syndrome initiated by volume transmission of excessive 5-HT to extrasynaptic receptors.
5-HT receptors are distributed in both synaptic and extrasynaptic compartments. The role of extrasynaptic receptors may be different from that of synaptic receptors. A: Synaptic receptors are activated by increasing 5-HT in the synaptic cleft where it typically contains high 5-HT concentrations [19]. In contrast, extrasynaptic receptors are not activated due to a low concentration of extracellular 5-HT (normally at sub-nanomolar levels [49,50]). B: MDMA increases 5-HT, resulting in excessive 5-HT in the extracellular space. High extracellular 5-HTcan reach extrasynaptic receptors (volume neurotransmission), initiating serotonin syndrome.
Serotonin syndrome is an acute symptomatic response mainly to excessive 5-HT in the brain. Other neurotransmitters, including dopamine, noradrenaline, GABA or glutamate, which may be changed in the syndrome development [3,4], are beyond the scope of this review. The syndrome can be mild, moderate or severe [5]. The relationship between mild, moderate and severe syndromes appears to be interchangeable, but the severe syndrome is life-threatening. Considering the vast amounts of symptomatic studies, we can only cite some representative publications. Additionally, MDMA abuse can also cause delayed and reversible architectural and neurochemical damages in serotonergic neurons. The delayed neurotoxic effect of MDMA abuse has been excellently reviewed somewhere else [6,7], and publications relevant for this topic will not be cited in this review.
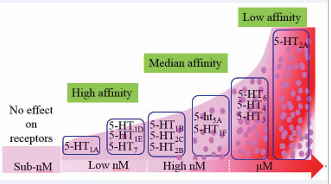
There are at least 14 subtypes of 5-HT receptors and topographically distributed all over the brain [8,9]. In this review, the term ‘receptors’ here describe extrasynaptic receptors unless otherwise mentioned. These receptors would remain quiescent until surrounding 5-HT molecules reach a concentration high enough to bind the receptors. The relationship between 5-HT concentrations and receptor binding can be expressed by a pKi value that has been reviewed elsewhere [10]. Briefly, a receptor pKi value at 6.0 indicates a number of 5-HT molecules that can bind 50% of the receptors will be in micromolar (µM) concentrations. The pKi value at 9.0 represents 5-HT in nanomolar (nM) concentrations that bind 50% of the receptors. Since 1980s, 5-HT1A and 5-HT2A receptors have been known to be the two major receptors responsible for serotonin syndrome [11-14]. The pKi value of 5-HT1A receptors was found in vitro to be at 9.0, suggesting that 50% of 5-HT1A receptors can bind 5-HT at concentrations as low as nM level [15]. Many drugs, including MDMA, can easily increase 5-HT to the nanomolar concentrations that activates both synaptic and extrasynaptic 5-HT1A receptors [16]. Since 5-HT2A receptor affinity is at pKi value of 6.0, µM concentrations are required for binding and activating the receptors (Figure 2).
Figure 2: Illustrating the relationship between 5-HT concentrations and activation of 5-HT receptor subtypes.
When5-HTis at a low nanomolar (nM) level, only high-affinity receptor subtypes (5-HT1A, 5-HT1D, 5-HT1E and 5-HT7) are activated; when increasing to high nM levels, both high-affinity and median-affinity receptor subtypes (5-HT1B, 5-HT2C, 5-HT2B, 5-ht5A, 5-HT1F) are activated; at mM concentrations, all receptor subtypes are activated. Thus, thousand times 5-HT concentrations are required to activate5-HT1A and 5-HT2A receptors. Given that difference in 5HT concentrations between synaptic cleft and extrasynaptic compartments [19,41], extrasynaptic 5HT receptor subtypes are not activated until extracellular 5HT becomes excessive. It is likely that MDMA at recreational doses could elevate extracellular 5-HT to low or high nM levels. However, there is no evidencein vivo indicating that the concentration can reach an mM level.
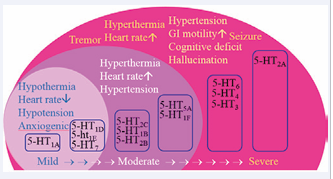
These findings led to the proposal that 5-HT2A receptors would require a thousand times greater 5-HT concentration than that for binding 5-HT1A receptors [17,18]. Synaptic 5-HT2A receptors may be activated since 5-HT concentrations in the synaptic cleft are extraordinarily high, for instance, 6 mM [19] also see a review [20]. However, there is no evidence in vivo indicating that the concentration in the extrasynaptic space can reach such a high level. This is partly relevant to etiological mechanisms that serotonin syndrome can never be idiopathic, but only iatrogenic. Although serotonin syndrome is caused by excessive 5-HT, it is important to recognize that the amount of 5-HT is not necessarily proportional to the syndrome severity [21]. The syndrome severity is strongly dependent on the responsivity of extrasynaptic 5-HT receptors, especially the 5-HT1A and 5-HT2A subtypes [22,23]. It has been reported that the activity of 5-HT1A and 5-HT2A subtypes can be significantly modulated by environment conditions as examined in vitro test tubes [24] and also in live animals as well [22,23]. We proposed that there is a strong but comprehensive relationship between 5-HT receptors and syndrome severity (Figure 3).
Figure 3: Difference of 5-HT receptor subtypes in mediating the mild, moderate, or severe syndromes.
It has been proposed that only high-affinity 5-HT receptors (i.e., 5-HT1A, 5-HT1D, 5-ht1E and 5-HT7) are involved in the mild syndrome and as the syndrome progresses to be moderate, both high-affinity and median-affinity receptors are activated. In the severe syndrome, all of high-affinity, median-affinity and low-affinity subtypes may be involved.
Therefore, serotonin syndrome is collectively determined by two different but interrelated mechanisms; the syndrome initiation and syndrome development [25]. The syndrome is initiated primarily by excessive 5-HT, but how the syndrome can progress is determined ultimately by respective receptor subtypes. Apparently, the syndrome associated with 5-HT1A receptors [26,27] would be markedly different from that mediated by 5-HT2A receptors [28]. In this review, we will discuss the syndrome severity from two aspects: 1) 5-HT receptor’s subtypes are different in mediating mild, moderate, and severe serotonin syndrome; 2) Changes in responsivity of 5-HT receptors due to ambient temperature and physical activity (non-drug factors) are the key mechanisms underlying severe serotonin syndrome.
5-HT receptor subtypes are different in mediating the mild, moderate and severe serotonin syndrome
It has been reported that patients in the emergency departments have a wide spectrum of symptoms of serotonin syndrome. Common symptoms include hyperthermia, hypertension, confusion, agitation, and seizures. In some cases, patients could have nausea, sweating, and headache. Halpern et al. (2016) conducted a case review on 52 patients, and described 45 different symptoms associated with Ecstasy toxicity. Of those, although hyperthermia is considered to be a common sign of serotonin syndrome, there were only 6% of patients having it. Psychomotor agitation appears to have the highest percentile in those symptoms, which was presented only in 38% of 52 cases. Clearly, no single symptom is presented in every case reported in the medical literature [29]. The cause of symptomatic discrepancy between patients is not well understood, but likely attributed to at least 5-HT receptor subtypes that can be activated differently between patients. Theoretically, activation would occur first in 5-HT1A receptors when extracellular 5-HT concentrations are at nM, then probably in 5-HT1D , 5-ht1E or 5-HT7 receptors when 5-HT concentrations are further elevated [15,30] as shown in Figure 2. 5-HT1A receptors are located on somatodendritic sites of serotonergic neurons in the raphe nuclei as autoreceptors, or on postsynaptic neurons as heteroreceptors. 5-HT1A autoreceptors are coupled with G protein-activated inwardly rectifying potassium (GIRK) channels that inhibit serotonergic neuronal activity [31] and 5-HT efflux [32]. These autoreceptors should not be considered as receptors that induce serotonin syndrome. On the contrary, they protect against excessive 5-HT. Therefore, 5-HT1A receptor antagonists are not a choice of drugs for therapeutic treatment of serotonin syndrome [33]. The heteroreceptors are widely distributed in the forebrain (i.e., hypothalamus) and cortices (i.e., prefrontal cortex). The receptors on the hypothalamic neurons mediate autonomic activity and hypothalamic-pituitary-adrenal axis [34]. In rodents, activation of hypothalamic 5-HT1A receptors resulted in hypothermia [35]. It has been recognized recently that hypothermia is considered as a neuroprotective response against neurotoxicity relevant to excessive 5-HT, and thus the syndrome is always mild and benign [5,36]. However, whether hypothermia could occur in human patients is not known, but worthwhile to be investigated. Studies reported that Ecstasy caused hypotension [37] and positive mood [38] and suggested that they could be also attributed to 5-HT1A receptors. This hypothesis is consistent with preclinical observation that injection of 5-HT1A receptor agonists can cause hypotensive [39] and anxiogenic effects in rodents [40]. It is known that there is a large amount of 5-HT1A receptor reserve (spare receptors) in the brain. It is likely that the receptor reserve is critical for the syndrome severity. The role of 5-HT1A receptor reserve in the syndrome can be examined by administering different doses of the 5-HT1A receptor agonist 8-OH-DPAT. For instance, a small dose of 8-OH-DPAT (< 0.05 mg/ kg, SC) was sufficient to cause hypothermia associated with the mild syndrome. Neuromuscular hyperactivity such as myotonia and tremor related to the severe syndrome was not elicited until a high dose was injected (> 1.0 mg/kg; [26,28,41]). Interestingly, the high dose still causes the hypothermic response, implicating that receptor reserve that is normally not activated in the benign syndrome can be abnormally activated in the severe syndrome. Nevertheless, 5-HT1A receptors mediate symptoms ranging from mild to severe syndromes. In addition to 5-HT1A receptors, other high-affinity receptors such as 5-HT1D , 5-ht1E and 5-HT7 receptors may also be activated in the mild syndrome. However, the role of 5-HT1D , 5-ht1E or 5-HT7 in the serotonin syndrome is not known.
It has been suggested that 5-HT7 receptors mediate hypothermia [35], which may be neuroprotective against excessive 5-HT in the brain.
5-HT2C , 5-HT1B , 5-HT2B , 5-HT5A and 5-HT1F are the group of receptor subtypes with modest binding affinity, requiring moderate to high nanomolar 5-HT concentrations [42-44] as shown in Figure 2. It is possible that this group of subtypes would drive the syndrome from mild to moderate level. In animal research, an effective approach to reveal the moderate syndrome is to measure body core temperature, showing characteristic normothermia [5]. 5-HT2C receptors participate in thermoregulation [45], functionally opposite to those of 5-HT1A and 5-HT7 receptors. Furthermore, activation of 5-HT2C receptors causes tachycardia and hypertension [46], which are also functionally opposite to those of 5-HT1A receptors. After summation of those receptor subtypes, few symptoms are expressed in the moderate syndrome as examined in an animal model [5]. Activation of 5-HT1B autoreceptors reduces the 5-HT release [47]. In this regard, 5-HT1B receptors may help protect against excessive 5-HT elicited by MDMA. Interestingly, it appears that 5-HT2B receptors would attenuate the syndrome severity [48]. However, how 5-HT5A or 5-HT1F receptors attribute to serotonin syndrome is not known.
It is likely that the severe and life-threatening syndrome is a collective activation of most, or all of 14 subtypes, including low affinity receptors such as 5-HT6 , 5-HT4 , 5-HT3 , and 5-HT2A (Figure 2). Theoretically, the binding to those low-affinity receptors requires micromolar concentrations of extracellular 5-HT, or at least > 1000-fold increases from a basal 5-HT at low nanomolar concentrations [49,50]. It has been reported that microdialysis in animal studies suggests that MDMA has a limited effect on eliciting 5-HT, demonstrating that there are only < 100-fold increases even though drug doses have been increased up to a neurotoxic level (> 7 mg/kg) [3,51,52]. This suggests that MDMA would not be able to elicit micromolar 5-HT concentrations for directly activating extrasynaptic receptors. Therefore, the severe syndrome is very rare relative to the mild or moderate syndromes. The severe syndrome does, however, occur due to additional mechanisms that can modulate responsiveness of 5-HT receptors, including those low-affinity 5-HT receptors (see discussion further below). In the severe syndrome, patients typically have high incidence of hyperthermia, tachycardia, hypertension, diarrhea, disorientation and seizure [53,54]. Activation of 5-HT2A together with 5-HT2C receptors leads to hyperthermia, tachycardia and hypertension as examined in animals using selective receptor agonists [23,46,55]. The shaking behavior that typically occurs in severe serotonin syndrome caused by Ecstasy [56] has been replicated with 5-HT2A receptor agonists administered to animals [57]. Neuropsychiatric disorders such as confusion, disorientation, and hallucination are also obvious in the severe syndrome [56]. Many 5-HT receptor subtypes, particularly 5-HT1A , 5-HT6 , 5-HT2A , are involved in neuropsychiatric behavior. 5-HT2A receptors are considered to be the major target mediating drug hallucination [58]. Nausea in patients can be interpreted as a result of activation of 5-HT3 receptors in the medulla, and diarrhea may be due to activation of 5-HT4 receptors in the gastrointestinal cells.
Changes in responsivity of 5-HT receptors due to ambient temperature and physical activity (non-drug factors) are the key mechanisms underlying severe serotonin syndrome
In the emergency room, serotonin syndrome is always described as drug overdose [37,59]. The overdose hypothesis is simply based on that higher doses would produce higher extracellular 5-HT concentrations within the brain, and activation of more receptor subtypes resulting in severe syndrome. However, the relationship between extracellular 5-HT concentrations and receptor subtype activation in vivo is more complicated than it is in vitro. In rodents, extracellular 5-HT concentration in healthy brains is very low, about a sub-nanomolar level [49,50]. Although elevation in extracellular 5-HT is normally considered to be beneficial for brain function, behavioral tests demonstrated that 5-HT potentially causes serotonin syndrome when the extracellular level goes beyond 10-fold above baseline [60]. This raises a question as to whether a recreational dose of MDMA can cause excessive 5-HT in the brain. In rodents (typically rats), a dose equivalent to human recreational dose is approximately 2 - 5 mg/kg [61,62]. In 5-HT microdialysis tests, this dose range increases extracellular 5-HT by at least 5-15 fold above baseline in rats [3,36]. If the result is translated to people, this suggests that a recreational human dose of MDMA (75-150 mg; see details in [6]) is sufficient to cause excessive 5-HT in the brain. Under this condition, extracellular 5-HT concentration values at a low nanomolar level, which likely binds only those high-affinity extrasynaptic subtypes such as 5-HT1A , 5-HT1D , 5-HT1E or 5-HT7 receptors, causing the mild syndrome (Figure 3).
In rats, greater than twice or three times the recreational dose (> 7.5 mg/kg) would elevate extracellular 5-HT in the brain by 50 100 fold above baseline [63,64]. This elevation likely increases extracellular 5-HT to high nanomolar, but not micromolar concentrations. As illustrated in Figure (2 and 3), 5-HT at the high nanomolar concentration can bind median-affinity receptors (e.g., 5-HT2C , 5-HT1B , 5-HT2B , 5-HT5A and 5-HT1F ) in addition to the high-affinity receptors (5-HT1A , 5-HT1D , 5-HT1E and 5-HT7 ) located in the extrasynaptic compartments. The syndrome is expected to be moderate, but not severe or life-threatening. In literature, except for 5-HTP combined with MAOI [4], there is no drug that could promote extracellular 5-HT up to the micromolar level at which the low-affinity receptors (e.g., 5-HT2A ) are activated, resulting in severe or life-threatening serotonin syndrome. It appears that additional mechanisms are involved in the severe syndrome caused by Ecstasy use.
Ambient temperature and physical activity are two environmental factors (non-drug factors) commonly associated with Ecstasy abuse at rave parties. It has been recognized recently that non-drug factors influence the responsivity of extrasynaptic 5-HT receptors, significantly altering neural consequence involved in thermoregulation [22,23]. Interestingly, as ambient temperature increases, 5-HT1A receptors gradually relinquish its primary role in heat loss for hypothermic regulation; instead, these receptors can stimulate heat gain. Pathological mechanisms for functional change in extrasynaptic 5-HT1A receptors are not understood, but certainly impacting development of hyperthermia in the syndrome. In addition, responsivity of 5-HT2A receptors is also altered. For instance, 5-HT2A receptors are known to stimulate heat gain, resulting in hyperthermia. Increasing ambient temperature dramatically promotes 5-HT2A receptors to produce more heat resulting in hyperthermia. Altogether, both 5-HT1A and 5-HT2A receptors will favor the mechanisms for hyperthermia as ambient temperature becomes hot. This may explain the preclinical observation that MDMA in cold environment cause hypothermia while in hot environment elicits hyperthermia [65].
Interestingly, although increasing ambient temperature alone has effects on MDMA-elicited serotonin syndrome, the illness is not life-threatening, particularly at the recreationally relevant doses [36,66]. Considering that Ecstasy at recreational doses causes deaths in humans, it suggests other non-drug factors are involved in addition to increased ambient temperature. Physical activity is another important non-drug factor as patients with Ecstasy usually have dancing activity. In rodents, the role of physical activity can be examined by placing animals on treadmills or running wheels. It appears that physical activity alone has a small impact on serotonin syndrome elicited by MDMA [67]. However, the syndrome becomes severe and life threatening as both physical activity and warm temperature are applied together [36]. Despite this, extracellular 5-HT is still within the range of a nanomolar concentration, but not the micromolar level, supporting the suggestion that there is a limited role of extracellular 5-HT in increasing the syndrome from mild to severe. In addition, changes in responsivity of extrasynaptic receptors, particularly 5-HT1A and 5-HT2A subtypes, may be critical in producing a severe syndrome in MDMA users [36].
Cold temperature is considered to be an effective approach to alleviate serotonin syndrome. Ecstasy users in the nightclubs are advised to take cold showers by which severe syndrome can be avoided. In the emergency department, patients with high hyperthermia caused by Ecstasy are often treated with surface cold blankets or/and intravenous administration of cold saline [37,68]. Animal studies suggest that 5-HT2A receptor activity is significantly reduced at cold ambient temperature [21]. Interestingly, since experimental animals such as rats and mice are homothermous, it suggests that changes in 5-HT receptor activity cannot be ascribed simply to changes in body-core temperature. In this regard, mechanisms of how environmental or non-drug factors modulate activity of 5-HT receptors are not known. Understanding the interaction between non-drug factors and serotonin syndrome may provide valuable insights into the underlying mechanisms.
CONCLUSION
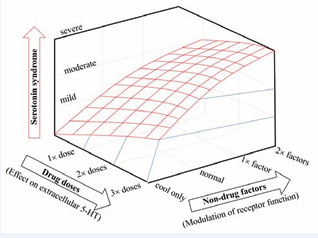
Ecstasy use at common recreational doses causes an excessive increase in extracellular 5-HT in the brain at nanomolar concentrations that bind to high-affinity and 5-HT receptors in the extrasynaptic compartments, resulting in a mild serotonin syndrome. Drug overdose could elevate extracellular 5-HT up to the high nanomolar concentrations for activating both the high- and median-affinity receptors for the moderate syndrome. However, it is unlikely to reach to micromolar concentrations that act on all the 14 5-HT receptor subtypes, especially low-affinity receptors such as 5-HT2A receptors. It seems that other mechanisms are involved in the severe and life-threatening serotonin syndrome. Strong evidence indicates that the responsivity of extrasynaptic 5-HT receptors can be altered by ambient temperature and physical activity [22,36]. Therefore, the severe syndrome should be attributed to two factors: drug factor that elevates extracellular 5-HT, and non drug factor that modulates responsivity of extrasynaptic 5-HT receptors. As elucidated in Figure 4, although MDMA can cause dose-dependent increases in extracellular 5-HT, decreasing ambient temperature that diminishes the responsivity of 5-HT receptors, especially 5-HT2A receptors to the high 5-HT, resulting in only mild or moderate syndrome.
Figure 4: Serotonin syndrome is determined not only by drug doses, but also non-drug factors. MDMA causes a dose-dependent increase in 5-HT, activating extrasynaptic 5-HT receptors producing serotonin syndrome. However, what receptor subtypes (especially low-affinity 5-HT2A receptors) activated will depend mainly on the receptor responsivity influenced by non-drug factors (ambient temperature and physical activity). Cold ambient temperature diminishes responsivity of 5-HT receptors to high 5-HT, resulting in a mild syndrome, while, hot ambient temperature alone (1× factor), or in combination with physical activity (2× factors) increases responsivity of 5-HT2A receptors to high 5-HT, resulting in moderate, or severe and life-threatening syndromes, respectively [36].
Hot ambient temperature alone or in combination with physical activity sensitizes5-HT2A receptors to respond to too much 5-HT, resulting in severe and life-threatening syndrome. Unraveling the relationship between 5-HT receptor responsivity and environmental (non-drug) factors may provide new insights into understanding MDMA toxicity in the brain, and potentially new therapeutic targets.
ACKNOWLEDGEMENTS
This study was supported by the NIH grant (R15DA029863) and the Ross University School of Veterinary Medicine research grant.