Elemental Speciation, Bioavailability and Risk Assessment of Heavy Metals in the Intertidal Surface Sediments of Sungai Puloh Mangrove, Malaysia
- 1. Department of Biology, Alex Ekwueme Federal University Ndufu-Alike, Nigeria
- 2. Department of Biology, Universiti Putra Malaysia, Malaysia
- 3. Group Health, Malaysia
Abstract
The speciation profile of Cd, Ni, and Zn as well as the total organic carbon (TOC) and pH were determined in 42 surface sediment samples from Sungai Puloh intertidal mangrove ecosystem. The four stage sequential extraction technique (SET) (exchangeable, acid–reducible, oxidizable-organics, and residual fractions) was employed to investigate the heavy metal distribution pattern. The Risk assessment Criteria Code (RAC) was applied to estimate the metals bioavailability and implication to food chain. The result showed that the total mean metal concentrations for Cd, Ni, and Zn were (1.04±0.36, 37.76±7.65, and 1034.56±410.79)µg/g respectively. More than 80% of Zn in all sampling stations existed in the non-residual fraction and as high as 15% in exchangeable faction indicating that Zn may be easily remobilized. The non-residual fraction of Cd 53%, Ni 51%, showed that Cd and Ni are at the borderline for remobilization. Cadmium speciation, like Zn revealed elevated levels of 11.94% in exchangeable fraction. The possibility of remobilization of heavy metal in the study area is in the order of Zn>Cd>Ni. Based on the RAC, Ni poses low risk. However, Cd and Zn pose medium to high risk for the possibility to enter into the food chain indicating that the food web and aquaculture activities within Sg. Puloh mangrove may be impacted. The TOC values ranged from 2.31 to 4.12%, and the pH varied from 3.49 to 4.83. The relationship obtained for TOC, pH, and non-resistant fractions indicated that TOC has more significant effect on the distribution of elements among phases than pH.
Keywords
• Bioavailability
• Heavy metal
• Risk assessment
• Surface sediments
• TOC
Citation
Udechukwu BE, Ismail A, Zulkifli SZ (2024) Elemental Speciation, Bioavailability and Risk Assessment of Heavy Metals in the Intertidal Surface Sediments of Sungai Puloh Mangrove, Malaysia. Ann Mar Biol Res 8(1): 1034.
INTRODUCTION
The mangrove ecosystem is one of the world’s major productive ecosystems because they are key ecological habitats that link terrestrial and marine environments [1]. Mangroves are unique systems that have the potentials to stabilize shore lines, trap sediments, and improve shore line protection [2]. The intertidal mud flats of this ecosystem sustain a good diversity of marine organisms including mudskippers, gastropods, crabs, barnacles, rodong shell, mullet, mussels monitor lizard, migratory shorebirds, and aquaculture products (principally fish and prawn) which are commonly consumed in South-East Asia [3,4].
However, today’s mangrove ecosystem has been subjected to intense and continuous chemical anthropogenic inputs resulting from increased human activities [5], urbanization and industrialization [7,8]. Among the major chemical contaminants from anthropogenic inputs are heavy metals [8]. A greater percentage of these heavy metals, which are released into aquatic systems, are quickly taken up by plankton [9,10], and are adsorbed to suspended particulate matter, which eventually settles down and becomes incorporated into bottom sediments.
Chemical speciation can be defined as a process by which different forms, species or phases of chemical present in material are identified and quantified. These species can be described (a) functionally, for example those that can be concentrated by plants, (b) operationally, regarding to the procedures and reagents used in their isolation, and specifically (c) as a particular compound or oxidation states of the metals [11,12]. The sequential extraction technique (SET) which involves the use of sequential extrancts to release species associated with particular sediment phases [11] provides detailed information about identification of binding sites, the strength of metal binding to particulates and the phase of association of trace elements in sediment [13]. This will give us a better insight on the geochemical processes effecting heavy metal bioavailability, mobilization and the potential risk induced on biota.
Several sequential extraction schemes consisting of different steps, chemical reagents and operating conditions have been proposed and modified in the past years for the investigation of heavy metal distribution in sediments [14-19]. In this Study the modified SET of Badri and Aston [15],Tessier and Campbell, [18] which consists of four stages (exchangeable, aci-reducible, oxidizable-organics, and residual fractions) was applied to differentiate the heavy metals of anthropogenic origin from that of natural source. There are few limitations with SET such as non–specific phase extraction, elemental reabsorption and redistribution amongst phases during extraction [18,20,21]. In spite of the setback mentioned above, the sequential extraction procedure have been widely accepted and applied successfully in the investigation of heavy metal contamination in surface sediments [12,13],[22-26]. This study is aimed to (1) quantify the speciation of Cd, Ni, and Zn (2) identify the metals with higher percentage of anthropogenic origin and evaluate the possible bioavailability in the food web of Sungai Puloh intertidal mangrove ecosystem.
MATERIALS AND METHODS
Study Area
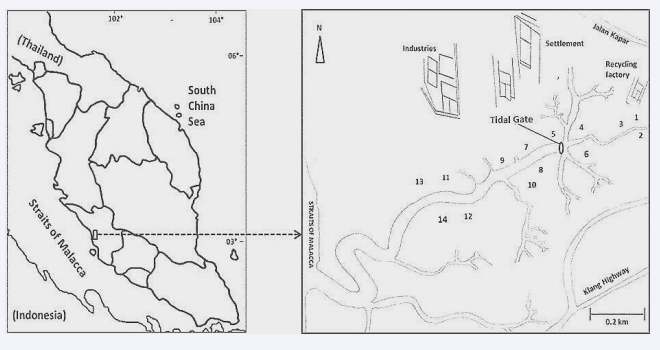
The mangrove area of Sungai Puloh (SGP N 03° 04.786 ?, E 101° 23.903 ?) is located in the state of Selangor, west coast of Peninsular Malaysia. Sungai Puloh forms a small linked estuary with the Straits of Malacca. The Straits of Malacca is a semidiurnal and meso-macrotidal system, with tidal ranges approximately 1-3m during neap tide and 3-5m during spring tides [27]. These conditions feed the mangrove during high tides. Sungai Puloh mangrove area sustains a great diversity of macro benthic organisms. It stretches about 6.87km in length and is situated in approximately less than 5km off the major intercity road of Jalan Kapar. There are several small and medium size industries (SMIs) operating in the vicinity. The industries involve various types such as scrap metal yards, recycling sectors (these factories handle a lot of scrap metals, polyethylene containers and various non- degradable materials), automobile workshops, oil palm mills, and aquaculture/agricultural activities. Most of their wastes are drained in this mangrove ecosystem. There is also a coal fired Power Generating Plant (Tenaga Nasional Berhad’s Sultan Salahuddin bin Abdul Aziz-SSA) at kapar near to Sg. Puloh mangrove area which is the country’s largest coal fired station generating a total of 1,420 MW of electricity into the national Grid network. Also a handful of residential houses were observed in this area, while, fishing and cattle rearing are the main domestic activity close to the Puloh River.
Sampling
Surface sediment samples were collected in March, 2012 from 14 sampling stations along the intertidal mangrove area of Puloh River (Figure I Sampling locations 1-14 for intertidal surface sediment of Sungai Puloh mangrove in the west coast of peninsular Malaysia: (N 03? 04.786?E 101 ? 23.903 ?).
Figure 1: Sampling locations 1-14 for intertidal surface sediment of Sungai Puloh mangrove in the west coast of peninsular Malaysia
Three replicate samples of surface sediment from each station were collected using a clean plastic scoop. The upper 2cm of each sample was placed in a polyethylene bag with a stainless steel spatula. All the samples were kept in an iced chest, taken to the laboratory and frozen until further analysis. Surface sediment samples were dried by using air-circulating oven to a constant dry weight at 80°C. Later the samples were ground using a clean dry pestle and ceramic mortar and sieved through a 63µm stainless steel sieve and stored in acid–washed container for metal speciation.
SAMPLE PREPARATION
Aqua Regia
The direct aqua regia method as described by Ismail [28] was employed for the pseudo-total heavy metal content analysis. About 1g of each dried sample is weighed and digested in a mixture of concentrated nitric acid (HNO? AnalaR grade, BDH 69%) and perchloric acid (AnalaR grade 60%) in a ratio 4:1. The samples were then placed in the digesting block, and the temperature was first set at a low temperature of 40°C for 1hr and later increased to 140°C for at least 3hrs [28-30]. After digestion, the samples were diluted to a 40ml volume with double distilled water and filtered using the Whatman No.1 filter paper into acid-washed polyethylene sample bottles and stored for metal determination.
Sequential Extraction
Metal speciation was analyzed using Sequential Extraction Technique (SET) (Table 1) as described by Badri and Aston [15] and Tessier and Campbell [18].
Table I: Extracts used in each extraction stages and various phases of sediment in the sequential extraction scheme
|
Extraction Stages |
Extrancts |
Sediment target phase |
|
Fraction 1 |
1.0 M NH?CH?COO, pH 7.0 |
Exchangeable |
|
Fraction 2 |
0.2 M NH?OH.HCl, pH 2.0 |
Acid- reducible |
|
Fraction 3 |
H?O? (35%), 1.0M NH?CH?COO, pH 2.0 |
Oxidizable - organics |
|
Fraction 4 |
HNO? (69%), HClO4 (60%) |
Residual |
Stage 1: Easily Freely Leachable and Exchangeable (EFLE) About 10g of sediment sample was placed into a clean Erlenmeyer flask then added 50ml of 1.0M ammonium acetate (NH?CH?COO) pH 7.0 and agitated continuously with orbital shaker (model GYROMAX 722) at constant speed of 2,500 rpm for 3hrs at room temperature. This first fraction liberates the metals that are loosely bound to carbonates.
Stage 2. Acid Reducible Fraction
The residue from fraction (1) was dried, weighed and placed into a clean Erlenmeyer flask, added 50ml of 0.2M hydroxyl ammonium chloride (NH?OH.HCl) acidified to pH 2.0 with hydrochloric acid at room temperature. After this it was agitated with orbital shaker as in the first fraction. This fraction releases the metals that are associated with Fe-Mn oxides and hydroxides. Iron and manganese-oxides bind to trace metals since the have high scavenging affinity for heavy metals [31].
Stage 3. Oxidisable Organic
The residue from (2) was dried, weighed and first oxidized with 15ml of H?O? (R&M Chemicals 35%) in water bath at 90- 95 ?C, until dried. After cooling, 50ml of 1.0M ammonium acetate (NH?CH?COO) acidified to pH 2.0 with HCl at room temperature was added and continuously shaken for 3hrs. The primary purpose is to liberate metals bound to organic complexes. The H?O? is used because it is a powerful and versatile oxidant. It hydrolyses formaldehyde, carbon disulfides, carbohydrates, organophosphorus and nitrogen compound, and various water soluble polymers. By its oxidant ability it liberates metals from these organic complexes.
Stage 4. Residual Fraction
The residue from fraction (3) was dried, weighed and digested in concentrated nitric acid (HNO? AnalaR grade, BDH 69%) and perchloric acid (HClO4 AnalaR grade 60%) in a ratio 4:1 as in direct aqua regia. This fraction can neither be is easily leached, acid-reduced nor oxidized, the metals are mainly embedded in the crystal lattice of the sediment fraction and should not be available for remobilization except under very harsh conditions [32] and are considered being of natural origin (Table I).
METAL DETERMINATION
The residue of each extraction stage was rinsed with to 20 ml double distilled water (DDW). It is then filtered through whatman No.1 filter paper into acid washed pill boxes and stored for metal determination (except for fraction 1 that was analyzed immediately because of its high sensitivity). Samples were determined for heavy metal concentrations by using an air- acetylene flame Atomic absorption Spectrophotometer (Analyst 800 model, by Perkin-Elmer), and the data are presented in μg/g dry weight basis.
Determination of pH in Sediments
About 10g of dried sediment sample was placed in glass beaker. Then 25ml distilled H?O was added and covered with plastic film. It is later shaken in orbital shaker for 4hrs at 175rm, the pH was read with a digital electrode pH meter model WTW pH 330 [33].
Total Organic Carbon (TOC) in Sediment
About 1g of dried sediment sample was thoroughly mixed with 1-2ml of I M HCl to remove inorganic carbon, and dried about 10hrs at 100-105°C to eliminate traces of HCl. The TOC% was analyzed using carbon analyzer (LECO CR-412) [34].
Quality Control
In order to avoid undue contamination, all glass wares were soaked in acid wash (10% HCl) for at least 24hrs and later rinsed with double distilled water, and air dried before use. To ensure precision and accuracy of the analytical method, quality control calibration curves were generated by analyzing Multiple-level Calibration standards, and standard solutions of each metal studied were prepared from 1,000mg/l (BDH SpectrosoL®) stock solution. Certified Reference Material (CRM) (International Atomic Energy Agency, Soil-5, Vienna, Austria) was used to check the quality of this method. The analytical results for reference material and its certified values showed satisfactory metal recovery percentages being about 109%, 96%, and 95% for Cd, Ni, and Zn respectively (Table II). Furthermore, an internal quality accuracy measure was performed by comparing the sum of metals extracted in different fractions during sequential extraction with the result of pseudo - total digestion (direct aqua regia), and the recovery percentages were satisfactory (Table III).
Table II: A comparison of measured values and certified values (µg/g) of CRM for soil, and the percentage recovery (n=3).
|
Metal |
Concentration (µg/g) |
Recovery (%) |
|
|
|
Certified values |
Measured values |
|
|
Cd |
1.50 ± 0.06 |
1.64 ± 0.07 |
109 |
|
Ni |
13.0 |
12.5 ± 1.97 |
96 |
|
Zn |
368 ± 3.20 |
351 ± 2.81 |
95 |
Table III: Mean comparison of the sum of sequential extraction with the direct aqua regia digestion based on 42 different sediment samples taken from Sungai Puloh intertidal mangrove ecosystem (n=3)
|
Metal |
Sum of extraction fractions (µg/g) |
Direct aqua regia (µg/g) |
Recovery (%) |
|
Zn |
1034.56± 410.79 |
1023.68 ±762.93 |
101.06 |
|
Ni |
37.76 ± 7.65 |
35.54 ± 10.74 |
106.25 |
|
Cd |
1.04 ±0.36 |
0.94 ±0.29 |
110.70 |
The recovery of the sequential extraction was calculated thus:
Recovery (%) = [(CFraction 1+ CFraction 2 + CFraction 3 + CFraction 4) / CDirectaqua regia] X 100
Statistical Analysis
The SPSS 20 software was used for all the statistical analyses and the Microsoft EXCEL for windows was employed for the graphs.
RESULTS AND DISCUSSION
Metal Speciation Patterns
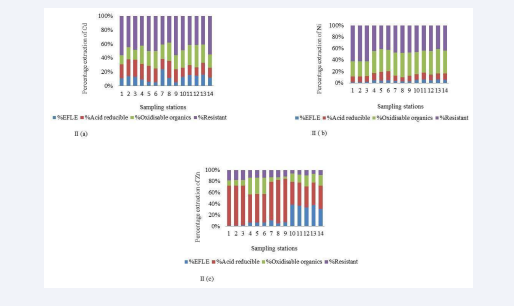
The spatial concentration distribution of all analyzed metals (Zn, Ni, and Cd) is summarized in [Tables IV a-c], while the percent fractions of these metals are shown in (Figure II a-c Percentage extractions of Cd, Ni, and Zn respectively in intertidal surface sediments of Sungai Puloh mangrove area).
Figure II: (a-c): Percentage extractions of Cd, Ni, and Zn respectively in intertidal surface sediments of Sungai Puloh mangrove area.
The comparison of the resistant and nonresistant percentage fractions are tabulated in [Table V]. Among these metals, the least residual fraction was observed in Zn (12.24%), followed by that of Cd (46.82%), and then Ni (48.32%) as shown in [Table VI]. The speciation profile also revealed that Zn is more dominant in exchangeable (15.90%), and acid reducible (56.23%) fractions compared to other metals, Ni (36.76%) is more dominant in oxidizable organics fraction compared to other metals (Table VI). This suggests that the rate of bioavailability and remobilization is much higher for Zn, and Cd, than for Ni. Therefore, the rate of chances of remobilization for these heavy metals in this intertidal mangrove ecosystem is Zn>Cd>Ni.
Table IV a: Zn speciation (µg/g) in Sungai Puloh intertidal mangrove
|
Stations |
Zn |
Total |
|||
|
No |
Exchangeable |
Acid reducible |
Oxidisable - organics |
Residual |
|
|
1 |
14.55 |
741.76 |
102.23 |
190.68 |
1049.22 |
|
2 |
13.79 |
756.69 |
108.87 |
191.11 |
1070.46 |
|
3 |
15.94 |
749.97 |
107.31 |
189.36 |
1062.58 |
|
4 |
114.56 |
869.52 |
517.48 |
237.37 |
1738.93 |
|
5 |
112.04 |
894.90 |
515.73 |
235.57 |
1758.24 |
|
6 |
113.39 |
871.36 |
505.52 |
236.36 |
1726.63 |
|
7 |
87.68 |
576.49 |
68.06 |
108.81 |
841.04 |
|
8 |
39.25 |
550.00 |
35.22 |
94.06 |
718.53 |
|
9 |
56.84 |
627.42 |
36.61 |
91.99 |
812.86 |
|
10 |
349.71 |
378.43 |
132.91 |
58.64 |
919.69 |
|
11 |
228.86 |
262.36 |
90.04 |
49.77 |
631.03 |
|
12 |
194.78 |
216.92 |
111.77 |
56.25 |
579.72 |
|
13 |
303.47 |
326.11 |
127.48 |
54.56 |
811.62 |
|
14 |
235.11 |
315.55 |
145.68 |
66.89 |
763.23 |
|
Max |
349.71 |
894.90 |
517.48 |
237.37 |
1758.24 |
|
Min |
13.79 |
216.92 |
35.22 |
49.77 |
579.72 |
|
Mean |
134.28 |
581.24 |
186.07 |
132.96 |
1034.56 |
Table IV b: Ni speciation (µg/g) in Sungai Puloh intertidal mangrove
|
Stations |
Ni |
|
|
|
Total |
|
No |
Exchangeable |
Acid reducible |
Oxidisable -organics |
Residual |
|
|
1 |
0.55 |
3.09 |
8.18 |
19.39 |
31.21 |
|
2 |
0.47 |
3.12 |
8.12 |
19.52 |
31.23 |
|
3 |
0.56 |
3.1 |
7.81 |
19.11 |
30.58 |
|
4 |
2.26 |
6.21 |
18.35 |
21.26 |
48.08 |
|
5 |
2.21 |
7.68 |
19.9 |
20.79 |
50.58 |
|
6 |
2.23 |
7.22 |
17.05 |
19.8 |
46.3 |
|
7 |
1.1 |
2.96 |
12.74 |
14.96 |
31.76 |
|
8 |
1.08 |
2.4 |
14.98 |
16.99 |
35.45 |
|
9 |
0.77 |
2.63 |
10.97 |
12.61 |
26.98 |
|
10 |
1.92 |
3.24 |
12.96 |
15.66 |
33.78 |
|
11 |
2.37 |
4.38 |
14.39 |
16.32 |
37.46 |
|
12 |
1.91 |
4.06 |
16.66 |
18.16 |
40.79 |
|
13 |
2.22 |
3.81 |
15.55 |
15.27 |
36.85 |
|
14 |
2.7 |
5.23 |
18.9 |
20.8 |
47.63 |
|
Max |
2.7 |
7.68 |
18.9 |
21.26 |
50.58 |
|
Min |
0.47 |
2.4 |
7.81 |
12.61 |
31.21 |
|
Mean |
1.6 |
4.22 |
14.04 |
17.90 |
37.76 |
Table IV c: Cd speciation (µg/g) in Sungai Puloh intertidal mangrove
|
Stations |
Cd |
Total |
|||
|
No |
Exchangeable |
Acid reducible |
Oxidisable -organics |
Residual |
|
|
1 |
0.08 |
0.15 |
0.1 |
0.42 |
0.75 |
|
2 |
0.08 |
0.14 |
0.1 |
0.26 |
0.58 |
|
3 |
0.08 |
0.15 |
0.09 |
0.3 |
0.62 |
|
4 |
0.1 |
0.25 |
0.29 |
0.47 |
1.11 |
|
5 |
0.1 |
0.39 |
0.36 |
0.86 |
1.71 |
|
6 |
0.09 |
0.34 |
0.44 |
0.88 |
1.75 |
|
7 |
0.19 |
0.12 |
0.17 |
0.33 |
0.81 |
|
8 |
0.08 |
0.18 |
0.19 |
0.28 |
0.73 |
|
9 |
0.05 |
0.17 |
0.19 |
0.52 |
0.93 |
|
10 |
0.14 |
0.15 |
0.28 |
0.55 |
1.12 |
|
11 |
0.17 |
0.15 |
0.31 |
0.45 |
1.08 |
|
12 |
0.18 |
0.15 |
0.4 |
0.52 |
1.25 |
|
13 |
0.17 |
0.18 |
0.28 |
0.44 |
1.07 |
|
14 |
0.13 |
0.16 |
0.22 |
0.62 |
1.13 |
|
Max |
0.19 |
0.39 |
0.44 |
0.88 |
1.75 |
|
Min |
0.05 |
0.12 |
0.09 |
0.26 |
0.58 |
|
Mean |
0.12 |
0.19 |
0.24 |
0.49 |
1.05 |
Table V: Comparison of non-resistant (anthropogenic) and residual (natural) percentage (%) of Cd, Ni, and Zn in intertidal surface sediments of Sungai Puloh mangrove ecosystem.
|
Station no. |
Non-resistant Percentage |
Resistant Percentage |
||||
|
|
Cd |
Ni |
Zn |
Cd |
Ni |
Zn |
|
1 |
44.00 |
37.87 |
81.83 |
56.00 |
62.13 |
18.17 |
|
2 |
55.17 |
37.50 |
82.15 |
44.83 |
62.50 |
17.85 |
|
3 |
51.61 |
37.51 |
82.18 |
48.39 |
62.49 |
17.82 |
|
4 |
57.66 |
55.78 |
86.35 |
42.34 |
44.22 |
13.65 |
|
5 |
49.71 |
58.90 |
86.60 |
50.29 |
41.10 |
13.40 |
|
6 |
49.71 |
57.24 |
86.31 |
50.29 |
42.76 |
13.69 |
|
7 |
59.26 |
52.90 |
87.06 |
40.74 |
47.10 |
12.94 |
|
8 |
61.64 |
52.07 |
86.91 |
38.36 |
47.93 |
13.09 |
|
9 |
44.09 |
53.26 |
88.68 |
55.91 |
46.74 |
11.32 |
|
10 |
50.89 |
53.64 |
93.62 |
49.11 |
46.36 |
6.38 |
|
11 |
58.33 |
56.43 |
92.11 |
41.67 |
43.57 |
7.89 |
|
12 |
58.40 |
55.48 |
90.30 |
41.60 |
44.52 |
9.70 |
|
13 |
58.88 |
58.56 |
93.28 |
41.12 |
41.44 |
6.72 |
|
14 |
45.13 |
56.33 |
91.24 |
54.87 |
43.67 |
8.76 |
|
Max |
61.64 |
58.90 |
93.62 |
56.00 |
62.50 |
18.17 |
|
Min |
44.00 |
37.50 |
81.83 |
41.12 |
41.10 |
6.38 |
|
Mean |
53.18 |
51.68 |
87.76 |
46.82 |
48.32 |
12.24 |
Zinc Speciation
The speciation profile of Zn (µg/g) for each sampling station is shown in [Table IV a]. The total Zn concentrations ranged from 579.72 to 1758.24µg/g. The exchangeable fraction for Zn ranged from 13.99 to 349.71µg/g with mean percentage of 15.9%. The acid-reducible fraction ranged from 216.92 to 894.90µg/g with mean percentage of 56.23%. The oxidizable organics range from 35.22 to 517.48 µg/g with mean percentage of 15.62%. The residual fraction ranged from 49.77 to 237.37µg/g with mean percentage of 12.24%. This result indicates that the speciation of Zn in the surface sediment of Sg. Puloh is dominated by the non-residual fraction 87.76% (Table V) with respect to acid- reducible fraction 56.23% (Table VI).
Table VI: Mean percentages of four chemical speciation fractions for heavy metal in Sg. Puloh intertidal sediment.
|
Metals |
%EFLE |
%Acid reducible |
%Oxidizable organics |
%Resistant |
|
Zn |
15.9 |
56.23 |
15.62 |
12.24 |
|
Ni |
4.05 |
10.87 |
36.76 |
48.32 |
|
Cd |
11.94 |
18.65 |
22.59 |
46.82 |
This is consistent with the findings of Perez-Cid et al [31], that Zn is the most liable metal because of its greater affinity to non-residual fractions. Similarly, many authors [22,35,36] reported higher concentrations of Zn in the non-residual fraction. The higher concentration of Zn in the acid-reducible fraction is in agreement with the reports of Qiao et al. [36], that Fe/Mn oxides and hydroxides which are mainly associated with heavy metal in the acid-reducible fraction are important scavengers of Zn. This indicates therefore, that appreciable portion of Zn could be remobilized and become bioavailable to the aquatic biota following a slight change in oxy- redox potential or changes in ionic strength [13]. Anthropogenic Zn in Sg. Puloh is considered to originate from city run offs, untreated discharges from recycling factories near the study area, and shipping activities in the straits of Malacca. The comparison of different percentage fractions of Zn in each sampling stations of surface sediments of Sg. Puloh reveals that the profile of Zn follows the pattern of acid-reducible>exchangeable>oxidizable organics>residual fraction.
Nickel Speciation
The speciation of Ni (µg/g) for each sampling station is presented in [Table IV b]. The total concentration of Ni ranged from 31.21 to 50.58µg/g. The exchangeable fraction for Ni ranged 0.47 to 2.7µg/g with mean percentage of 4.05%. The acid- reducible fraction for Ni ranged from 2.4 to 7.68µg/g with mean percentage of 10.87%. The oxidizable-organics fraction of Ni ranged from 7.81 to 18.9µg/g with mean percentage of 36.76%. The residual fraction for Ni ranged from 12.61 to 21.26µg/g with mean percentage of 48.32%. The speciation profile of Ni reveals that it is dominated by the residual fraction followed by the oxidizable-organics fraction, acid-reducible fraction, and lastly the exchangeable fraction (Table VI). This implies that if environmental conditions that favour the change in pH or the oxy- redox potential and ionic strength prevail, Ni may probably at a lesser extent be remobilized. Nickel is a plant micronutrient. It is referred as micronutrient because like other micronutrients it is less abundant (by 1-4 orders of magnitude) compared to the macronutrients like sulfur and phosphorus. Nickel has five valences (0, 1+ 2+ 3+ 4+), and the Ni2+ ion is the only known bioavailable form to plants [37]. It is one of the elements that serve as a broker for redox transformation. Various heavy metal sources such as engine oil, wash water, and metallic surfaces along Kapar road, as well as occasional oil spill along Straits of Malacca, and heavy metal-bearing fertilizer application by farmers contribute to the anthropogenic sources of Ni to this study area.
Cadmium Speciation
The speciation pattern for Cd (µg/g) for each of the sampling stations is in shown in [Table IV c]. The total Cd concentration ranged from 0.58 to 0.19µg/g. The exchangeable fraction ranged from 0.05 to 1.75µg/g with mean percentage of 11.94%. The acid-reducible fraction ranged from 0.12 to 0.39µg/g with mean percentage of 18.65%. The oxidizable-organics fraction ranged from 0.09 to 0.44µg/g with mean percentage of 22.59%. The residual fraction ranged from 0.12 to 0.88µg/g with mean percentage of 46%. Cadmium fractionation in this study follows the speciation pattern of Ni: Residual>oxidizable organics>acid reducible>exchangeable fraction. However, Cd showed higher percentage values of the exchangeable and acid- reducible fractions (11.94% and 18.65%) respectively than the percentage exchangeable and acid-reducible fractions observed for Ni (4.05% and 10.85%) respectively (Table VI).
This result suggests that probably Cd is associated with carbonates and Mn/Fe fractions in sediment more than Ni. Carbonates are sensitive to pH [38]; therefore if any environmental conditions that alter the sediment pH, water ionic strength and oxy redox potential prevail, Cd which is more liable compared to Ni in these fractions may be more bioavailable to aquatic biota than Ni. The mean nonresistant percentage fractions for Cd (53.1%) and Ni (51.68%) (Table V) respectively indicate that more than 50% of the total Cd and Ni in the surface sediment of Sg. Puloh mangrove may probably be remobilized and made bioavailable to aquatic organisms. The anthropogenic inputs of Cd in this mangrove surface sediment may probably result from discharges from recycling factory, refused dumps, auto shops and boating activities in the vicinity of this mangrove or other non- point sources.
The role of TOC and pH on Metal Partitioning and Bioavailability
The concentrations of total organic carbon (TOC %) and pH levels of all the surface sediment samples in different stations are shown in [Table VII].
Table VII: TOC concentration, pH levels in intertidal surface sediment at different stations
|
Station no. |
TOC% |
pH |
|
1 |
2.67 |
6.22 |
|
2 |
2.54 |
6.10 |
|
3 |
2.31 |
5.94 |
|
4 |
5.63 |
5.22 |
|
5 |
5.91 |
5.02 |
|
6 |
5.97 |
5.13 |
|
7 |
3.00 |
3.49 |
|
8 |
3.85 |
3.55 |
|
9 |
2.79 |
4.35 |
|
10 |
4.15 |
5.41 |
|
11 |
4.20 |
4.01 |
|
12 |
4.18 |
4.42 |
|
13 |
4.56 |
4.38 |
|
14 |
5.96 |
4.36 |
|
Max |
5.97 |
6.22 |
|
Min |
2.31 |
3.49 |
|
Mean |
4.12 |
4.83 |
The TOC and pH values in surface sediment of Sungai Puloh ranged between 2.31 to 5.97% and 3.49 to 6.22 respectively. The partitioning of metals between solid and liquid phases in sediment is strongly affected by some physiochemical parameters such as: pH, organic matter content, sediment solution ionic strength, Mn and Fe oxides, redox potential, and nature of sorbing soil surfaces [39]. The most prominent factors influencing the partitioning and bioavailability of heavy metals are pH, CEC (cation exchange capacity), clay content, and organic matter.
At low pH the metal availability increases since the hydrogen ion has a higher affinity for negative charges on the colloids, thus competing with the metal ions of these sites, therefore releasing metals. Most metal concentrations increase in aquatic environment with decreasing pH with highest at 4.0 [40]. High organic matter, mainly TOC favours the immobilization of metals that bind to example fulvic or humic acids. Some elements such as Pb do not form chloride complexes due to their strong binding to organic matter [41]. Organic matter in sediments is composed of carbon and nutrients in the form of carbohydrates, proteins, fats, and nucleic acids. Most sediment organic matter is derived from plant and animal detritus, bacteria or plankton formed insitu, or natural and anthropogenic sources. Sewage and effluents from processing plants, pulp mills and fish farms are example of organic waste human origin. The spill or release of contaminants into the environment increases the total carbon content present in soil or sediment. Notable characteristics of organic matter include their ability to form water- soluble and water non soluble complexes with metal ions and hydrous oxides; interact with clay minerals and bind particles together; sorb and desorb natural- occurring and anthropogenically induced organic compounds [42]. Total organic carbon (TOC) (often referred to the amount of organic matter preserved within sediment) is an essential part of any site characterization since its presence or absence can obviously influence how chemicals react in sediment [42].
The relationship between phase distributions of metals, TOC, and pH are determined and shown in [Table VIII].
Table VIII: Correlation of non-resistant fractions of metal speciation with TOC and pH in Sg. Puloh surface sediment
|
|
|
Cd |
Ni |
Zn |
TOC% |
pH |
|
Exchangeable fraction |
Cd |
1 |
|
|
|
|
|
|
Ni |
0.552b |
1 |
|
|
|
|
|
Zn |
0.706a |
0.811a |
1 |
|
|
|
|
TOC |
0.421 |
0.899a |
0.684a |
1 |
|
|
|
pH |
-0.390 |
-0.358 |
-0.279 |
-253 |
1 |
|
Acid- reducible fraction |
Cd |
1 |
|
|
|
|
|
|
Ni |
0.504 |
1 |
|
|
|
|
|
Zn |
0.389 |
0.200 |
1 |
|
|
|
|
TOC |
0.682a |
0.820a |
-0.15 |
1 |
|
|
|
pH |
-0.704 |
0.182 |
0.477 |
-0.253 |
1 |
|
Oxidizable- organics |
Cd |
1 |
|
|
|
|
|
|
Ni |
0.792a |
1 |
|
|
|
|
|
Zn |
0.562b |
0.670a |
1 |
|
|
|
|
TOC |
0.836a |
0.938a |
0.692a |
1 |
|
|
|
pH |
-0.201 |
-0.279 |
0.429 |
-0.253 |
1 |
a: correlation is significant at the 0.01 level; b: correlation is significant at the 0.05 level
For the exchangeable fraction, the phase distribution of Ni (r=0.899, p<0.01, and Zn (r=0.684, p<0.01) were strongly correlated with TOC, while Cd (r=0.421, p>0.05) showed weak positive correlation. Similarly, for acid-reducible fraction, all the examined metals showed strong positive correlation with TOC except for Zn (r= -0.15, p >0.05) in this distribution phase. This result indicates that in the acid-reducible fraction, the distribution of Zn may not be affected by TOC. However, for the oxidizable-organics fraction, the distribution of all the studied metals revealed significant strong positive correlations Cd (r=0.836, p<0.01), Ni (r=0.938, p<0.01), and Zn (r=0.069, p<0.01) with TOC.
The result of the investigation suggests that in the oxidizable- organics fraction, the distribution of Cd, Ni, and Zn may significantly be affected by the amount of TOC present in the sample. This is in agreement with the findings of Yuan et al. [13] who reported strong positive correlation of Pb, Cd, and Cu with TOC in fraction C. The relationship between pH and the phase distributions of all the studied metals revealed that there is no significant correlation between the pH and the metals in all the fractions. This is suggestive that TOC has a more significant effect than pH on the distribution of elements among phases. It is also in agreement with that of Yuan et al. [13], who concluded that the effect of pH on the distribution of elements in real sample analysis was not obvious.
Risk Assessment
The risk assessment code (RAC) as shown in [Table IX] indicate that surface sediments which can release in exchangeable phase (i.e. fraction 1) less than 1% of the total metal will be considered safe for the environment.
Table IX: Risk Assessment
|
Risk assessment Code (RAC) |
Criteria (%) |
|
No risk |
<1 |
|
Low risk |
1-10 |
|
Medium risk |
11-30 |
|
High risk |
31- 50 |
|
Very high risk |
>50 |
|
Metal |
Exchangeable fraction (%) |
|
Cd |
5.14 - 23.46 |
|
Ni |
1.50 – 6.33 |
|
Zn |
1.29 – 38.02 |
However, surface sediment releasing more than 50% of the total metal in the same fraction is considered to be of extreme risk to the environment and can easily be transferred from one trophic chain to another [43,44]. The metal speciation revealed that the percentage content of metals in the exchangeable phase ranged from 5.14-23.46% for Cd, 1.50-6.33% for Ni, and 1.29-38.02% for Zn. The RAC was applied to this study to determine the element/s that may pose a threat to food chain in this mangrove ecosystem. An average percentage content of 4.05% for Ni exists in the exchangeable fraction. Therefore, nickel falls into the low risk category which means that there is a low possibility that Ni may enter the food chain. Finally, an average percentage metal content of 11.94% for Cd and 15.91% for Zn exist in the exchangeable fraction. Therefore, cadmium and zinc come under the medium risk category, based on the RAC, it suggests that these metals may have a high possibility to be remobilized and thus becoming available to aquatic biota. Cadmium is a non-essential element which can posed serious problem to any ecosystem [44], therefore any trace of it in the exchangeable fraction should be of ecological concern.
In comparison of this data with other studies (Table X) Ni, Cd, and Zn were higher than earlier studies reported for Peninsular Malaysia, Hong Kong and Singapore, as reported by Zulkifli et al. [45], Tam and Wong [46] and Bayen et al. [3], respectively. However, when this study is compared to other studies in other parts of the world, Ni is found to be lower than those of Ayatan Lagoon Turkey, and Guanabara Bay, Brazil as reported by Davutluoglu et al. [44], and Neto et al. [47], respectively. The comparison of Cd, and Ni with those of Dumai coast Indonesia as reported by Amin et al. [48], revealed that the Cd concentration range are similar while Ni was higher in the present study. All the studied metals are comparable with the concentrations reported for Shantou Bay, China [36], except for Zn that shows higher concentration in the present study. The Zn concentrations in this study is comparable with those reported by Ismail et al. [49], and Chen et al. [50], but higher than other reported studies (Table X).
Table X: Comparison of Ni, Cd, and Zn (µg/g) in Sungai Puloh surface sediments with those from mangrove and coastal areas around the world.
|
Location |
Ni |
Cd |
Zn |
References |
|
West coast of Peninsular Malaysia |
- |
- |
50-1,400 |
Ismail et al. (1993) |
|
Bintulu coastal waters |
- |
- |
39-91 |
Ismail (1993) |
|
Sebarang Perai, Malaysia |
|
0.27- 4.68 |
30.03-513.20 |
Ismail and Asmah (1999) |
|
Hong Kong |
2.90 |
0.32 |
- |
Tam and Wong (2000) |
|
Port Jackson Australia |
- |
- |
10-287 |
MacFarlene et al. (2002) |
|
Intertidal west coast of Peninsular Malaysia |
- |
0.03-1.98 |
3.12-306.20 |
Yap et al. (2002; 2003) |
|
East China Sea |
27.24±1.32 |
0.99±0.05 |
59.82±2.74 |
Yuan et al. (2004) |
|
Mangrove area of Singapore |
7.44-11.65 |
0.18-0.27 |
51.24 -120.23 |
Bayen et al. (2005) |
|
Mandovy estuary India |
- |
- |
19.5-85.5 |
Alagarsamy (2006) |
|
Guanabara Bay, Brazil |
1-3,515.50 |
- |
5-755.1 |
Neto et al. (2006) |
|
Tg. Piai, Malaysia |
10.10-11.00 |
0.72-1.19 |
40.30-43.10 |
Yap et al. (2006) |
|
Kaoshiung Habour, Taiwan |
- |
0.1-6.8 |
52-1,369 |
Chen et al. (2007) |
|
Dumai coast Indonesia |
7.26-19.97 |
0.46-1.89 |
31.49-87.11 |
Amin et al. (2008) |
|
Akyatan Lagoon- Turkey |
80-219 |
- |
54-102 |
Davutluoglu et al. (2010) |
|
Peninsular Malaysia Northern part of Peninsular Malaysia |
2.41-36.29 |
< 1.06 |
23.70-607.20 |
Zulkifli et al. (2010) |
|
- |
- |
33.6-317.4 |
Yap and Pang (2011) |
|
|
Red Sea, Egypt |
5.5-52.4 |
0.65-5.75 |
- |
El-Said et al. (2012) |
|
Shantou Bay , China |
22.95 |
0.67 |
153.28 |
Qiao et al. (2013) |
|
Sungai Puloh, Malaysia |
31.21-50.58 |
0.58-1.75 |
579.72-1,758.24 |
Present study |
CONCLUSION
Based on the speciation profile, the probability of bioavailability and remobilization of these metals in Sg. Puloh mangrove surface sediments follow the following pattern: Zn>Cd>Ni. The high percentage range of TOC indicated that there is a considerable amount of organic matter accumulated within the surface sediment. This study further revealed that the effect of TOC on the distribution and partitioning of heavy metal is more pronounced than that of sediment pH. Risk assessment revealed that there is low possibility for Ni to enter into the food chain. However, there is a medium to high risk that Cd and Zn may enter the food chain in Sg. Puloh mangrove ecosystem. Therefore, for the fact that Cd (which has no known beneficial value in biota) is implicated, the effluents from recycling industries, power plant, and auto parts workshop, non-point sources from agricultural and aquacultures activities within the vicinity of the mangrove should be intensely and periodically monitored [51-55].
REFERENCES
- Vane C, Harrison I, Kim A, Moss-Hayes V, Vickers B, Hong K. Organic and metal contamination in surface mangrove sediments of South China. Mar Pollut Bull. 2009; 58: 134-144.
- Bosire JO, Dahdouh-Guebas F, Walton M, Crona B, Lewis III RR, Field C, et al. Functionality of restored mangroves: a review. Aqua Bot. 2008; 89: 251-259.
- Bayen S, Wurl O, Subramanian K, KAE SHING WONG K, Sivasothi N, et al. Heavy metal contamination in mangrove habitats of Singapore. Mar Pollut Bull. 2005; 50: 1732-1738.
- Hashim R, Kamali B, Tamin NM, Zakaria R. An integrated approach to coastal rehabilitation: Mangrove restoration in Sungai Haji Dorani, Malaysia. Estuarine, Coast Shelf Sci. 2010; 86: 118-124.
- Udechukwu BE, Ismail A. Copper and Zinc in commonly consumed bivalves from Pantai Remis Jeram, Selangor, Malaysia. J Trop Mar Ecosystem. 2013; 3: 1-8.
- Kamaruzzaman BY, Shuhada NT, Akbar B, Shahbudin S, Jalal KCA, Ong MC, et al. Spatial concentrations of Lead and Copper in bottom sediments of Langkawi coastal area, Malaysia. Res J Environ Sci. 2011; 5: 179-186.
- Udechukwu BE, Ismail A, Zulkifli SZ, Omar H. Distribution, mobility, and pollution assessment of Cd, Cu, Ni, Pb, Zn, and Fe in intertidal surface sediments of Sg. Puloh mangrove estuary, Malaysia. Environ Sci Pollut Res. 2014; 22: 1-14.
- MacFarlane G. Leaf biochemical parameters in Avicennia marina (Forsk.) Vierh as potential biomarkers of heavy metal stress in estuarine ecosystems. Mar Pollut Bull. 2002; 44: 244-256.
- Sanders JG, Riedel GF. Metal accumulation and impacts in phytoplankton. Metal Metabolism in Aquatic Environments. Chapman and Hall. New York. 1998; 59-76.
- Wang WX, Guo L. Bioavailability of colloid-bound Cd, Cr, and Zn to marine plankton. Mar Ecol Prog Series. 2000; 202: 41-49.
- Davidson CM, Thomas RP, McVey SE, Perala R, Littlejohn D, Ure AM. Evaluation of sequential extraction procedure for the speciation of heavy metals in sediments. Analytica Chimica Acta. 1994; 291: 277- 286.
- Naji A, Ismail A, Ismail AR. Chemical speciation and contamination assessment of Zn and Cd by sequential extraction in surface sediment of Klang River, Malaysia. Microchem J. 2010; 95: 285-292.
- Yuan CG, Shi, JB, He B, Liu JF, Liang LN, Jiang GB. Speciation of heavy metals in marine sediments from the East China Sea by ICP-MS with sequential extraction. Environment Int. 2004; 30: 769-783.
- Tessier A, Campbell PG, Bisson M. Sequential extraction procedure for the speciation of particulate trace metals. Analyt Chem. 1979; 51: 844-851.
- Badri M, Aston S. Observations on heavy metal geochemical associations in polluted and non-polluted estuarine sediments. Environ Pollut Series B, Chem Phy. 1983; 6: 181-193.
- Salomons W, Forstner U. Metals in the hydrocycle: Springer, Berlin Heidelberg New York. 1984.
- Kersten M, Förstner U. Chemical fractionation of heavy metals in anoxic estuarine and coastal sediments. Water Sci Technol. 1986; 18: 121-130.
- Tessier A, Campbell P. Partitioning of trace metals in sediments: relationships with bioavailability. Hydrobiol. 1987; 149: 43-52.
- Campanella L, D’Orazio D, Petronio B, Pietrantonio E. Proposal for a metal speciation study in sediments. Analytica Chimica Acta. 1995; 309: 387-393.
- Rendell PS, Batley GE, Cameron AJ. Adsorption as a control of metal concentrations in sediment extracts. Environ Sci Technol. 1980; 14: 314-318.
- Nirel P, Morel F. Pitfalls of sequential extractions. Water Res. 1990; 24: 1055-1056.
- Ngiam LS, Lim PE. Speciation patterns of heavy metals in tropical estuarine anoxic and oxidized sediments by different sequential extraction schemes. Sci Total Environ. 2001; 275: 53-61.
- Ismail A, Safahieh A. Copper and Zinc in intertidal surface sediment and Telescopium telescopium from Lukut River. Coastal Marine Science. 2004; 29: 111-115.
- Yap C, Rahim-Ismail A, Ismail A, Tan S. Analysis of heavy metal concentration data (Cd, Cu, Pb and Zn) in different geochemical fractions of the surface sediments in the Straits of Malacca by the use of correlation and multiple linear stepwise regression analyses. Malay Applied Biol. 2005; 34: 51-59.
- Obbard JP, Dang TC. Metal speciation in coastal marine sediments from Singapore using a modified BCR-sequential extraction procedure. Applied Geochem. 2006; 21: 1335-1346.
- Amin B, Ismail A, Yap C. Distribution and speciation of Zn and Pb in coastal sediments of Dumai Sumatera, Indonesia. Toxicol Environ Chem. 2008; 90: 609-623.
- Polgar G, Crosa G. Multivariate characterisation of the habitats of seven species of Malayan mudskippers (Gobiidae: Oxudercinae). Mar Biol. 2009; 156: 1475-1486.
- Ismail A. Heavy metal concentrations in sediments off Bintulu, Malaysia. Mar Pollut Bull. 1993; 26: 706-707.
- Ismail A, Ramli R. Trace metals in sediments and molluscs from an estuary receiving pig farms effluent. Environmental Technology. 1997; 18: 509-515.
- Yap C, Ismail A, Tan S, Omar H. Concentrations of Cu and Pb in the offshore and intertidal sediments of the west coast of Peninsular Malaysia. Environ Int. 2002; 28: 467-479.
- Pérez-Cid B, Lavilla I, Bendicho C. Analytical assessment of two sequential extraction schemes for metal partitioning in sewage sludges. Analyst. 1996; 121: 1479-1484.
- Ikem A, Egiebor N, Nyavor K. Trace elements in water, fish and sediment from Tuskegee Lake, Southeastern USA. Water, Air, and Soil Pollution. 2003; 149: 51-75.
- McLean E. Soil pH and lime requirement. Methods of soil analysis. 1982; 199-224.
- Nelson DW, Sommers LE. Total carbon, organic carbon, and organic matter. Methods of Soil Analysis Part 3—Chemical Methods (methodsofsoilan3). 1996; 961-1010.
- Usero J, Gamero M, Morillo J, Gracia I. Comparative study of three sequential extraction procedures for metals in marine sediments. Environ Int. 1998; 24: 487-496.
- Qiao Y, Yang Y, Gu J, Zhao J. Distribution and geochemical speciation of heavy metals in sediments from coastal area suffered rapid urbanization, a case study of Shantou Bay, China. Marine Pollution Bulletin. 2013; 68: 140-146.
- Klucas RV, Hanus FJ, Russell SA, Evans HJ. Nickel: a micronutrient element for hydrogen-dependent growth of Rhizobium japonicum and for expression of urease activity in soybean leaves. Proc Nat Acad Sci. 1983; 80: 2253-2257.
- Thomas R, Ure A, Davidson C, Littlejohn D, Rauret G, Rubio R, et al. Three-stage sequential extraction procedure for the determination of metals in river sediments. Analytica Chimica Acta. 1994; 286: 423- 429.
- McGrath S, Chang A, Page A, Witter E. Land application of sewage sludge: scientific perspectives of heavy metal loading limits in Europe and the United States. Environ Rev. 1994; 2: 108-118.
- Veselý J, Majer V. The effect of pH and atmospheric deposition on concentrations of trace elements in acidified freshwaters: A statistical approach. Water, Air, and Soil Pollution. 1996; 88: 227-246.
- Förstner U, Wittmann GT, Prosi F, Van Lierde J. Metal pollution in the aquatic environment. Springer-Verlag Berlin. 1979.
- Schumacher BA. Methods for the determination of total organic carbon (TOC) in soils and sediments. Environ Protect Agency. 2002.
- Perin G, Craboledda L, Lucchese M, Cirillo R, Dotta L, Zanette M, et al. Heavy metal speciation in the sediments of northern Adriatic Sea. A new approach for environmental toxicity determination. Heavy metals in the environment. 1985; 2: 454-456.
- Davutluoglu OI, Seck?n G, Kalat DG, Y?lmaz T, Ersu CB. Speciation and ?mplications of heavy metal content in surface sediments of Akyatan Lagoon-Turkey. Desalination. 2010; 260: 199-210.
- Zulkifli SZ, Mohamat-Yusuff F, Arai T, Ismail A, Miyazaki N. An assessment of selected trace elements in intertidal surface sediments collected from the Peninsular Malaysia. Environ Monit Assess. 2010; 169: 457-472.
- Tam N, Wong Y. Spatial variation of heavy metals in surface sediments of Hong Kong mangrove swamps. Environ Pollut. 2000; 110: 195-205.
- Alagarsamy R. Distribution and seasonal variation of trace metals in surface sediments of the Mandovi estuary, west coast of India. Estuar, Coast Shelf Sci. 2006; 67: 333-339.
- Amin B, Ismail A, Arshad A, Yap CK, Kamarudin MS. Anthropogenic impacts on heavy metal concentrations in the coastal sediments of Dumai, Indonesia. Environ Monit Assess. 2009; 148: 291-305.
- Ismail A, Badri M, Noor Ramlan M. The background levels of heavy metal concentration in sediments of the west coast of Peninsular Malaysia. Sci Total Environ. 1993; 134: 315-323.
- Chen CW, Kao CM, Chen CF, Dong CD. Distribution and accumulation of heavy metals in the sediments of Kaohsiung Harbor, Taiwan. Chemosphere. 2007; 66: 1431-1440.
- El-Said GF, Youssef DH. Ecotoxicological impact assessment of some heavy metals and their distribution in some fractions of mangrove sediments from Red Sea, Egypt. Environ Monit Assess. 2012; 1-12.
- Ismail A, Asmah M. Copper, zinc, lead and cadmium in intertidal molluscs and sediment off Seberang Perai coastline, Malaysia. Paper presented at the fourth Princess Chulabhorn International Science Congress, Bangkok, Thailand. 1999.
- Yap C, Pang B. Assessment of Cu, Pb, and Zn contamination in sediment of north western Peninsular Malaysia by using sediment quality values and different geochemical indices. Environmental Monitoring and Assessment. 2011; 183: 23-39.
- Yap CK, Choh MS, Berandah FE, Ismail A, Tan SG. Comparison of heavy metal concentrations in surface sediment of Tanjung Piai wetland with other sites receiving anthropogenic inputs along the southwestern coast of Peninsular Malaysia. Wetland Sci. 2006; 4: 48-57.
- Yap CK, Ismail A, Tan SG. Cd and Zn concentrations in the straits of Malacca and intertidal sediments of the west coast of Peninsular Malaysia. Mar Pollut Bull. 2003; 46: 1349-1353.