Seasonal Variation in the Diagnosis Onset of Type 1 Diabetes Mellitus in Children and Adolescents in Cameroon
- 1. Department of Internal Medicine and Specialties, University of Yaoundé I, Cameroon
- 2. University Hospital of Lausanne (CHUV), Lausanne, Switzerland
- 3. Université des Montagnes, Cameroon
- 4. Department of Anthropology, University of Yaoundé I, Cameroon
Abstract
Background: Although the seasonality of type 1 diabetes has been described in most regions of the world, there are few data from sub-Saharan Africa. The aim of this study was to determine if there is a seasonality in the occurrence of type 1 diabetes, based on the “Changing Diabetes in Children” (CDiC) registry where all cases of type 1 diabetes in children and adolescents in Cameroon are registered since 2010.
Methods: We identified the files of all children and adolescents enrolled in the CDiC project between October 2010 and December 2015. For each participant, we gathered information concerning demographic data and history of diabetes. In addition, we collected for each case the region of residence at the time of diagnosis, with the precipitation and temperature data corresponding to the month and year of diagnosis. A month was dry if the average monthly rainfall was <100mm and wet if the average monthly rainfall was ≥ 100mm. The data were analyzed using SPSS (Statistical Package for Social Sciences) software version 20.0.
Results: Fourth hundred forty-six patients were included in the study. We found a repeating trends over the years with a low number of cases diagnosed during dry months (March to May) and a higher number of cases during wet months (August to October). Overall, most of the diabetes new cases occurred between August and November with a peak in September (69/446), while the lowest number of cases were diagnosed in April and May (23/446). There was a positive correlation between the number of new T1DM cases and the rainfall and a negative correlation with the mean temperature.
Conclusion: Our data support the existence of a clear seasonal pattern in the onset and diagnosis of type 1 diabetes mellitus in Cameroon. Most of the new cases appear between August and October, which represent the cold and wet months.
Keywords
• Type 1 diabetes
• Onset
• Children and adolescents
• Seasonality
CITATION
Yefou MD, Tankeu A, Takam CYM, Awah PK, Etoga MCE, et al. (2023) Seasonal Variation in the Diagnosis Onset of Type 1 Diabetes Mellitus in Children and Adolescents in Cameroon. Ann Pediatr Child Health 2023; 11(5): 1326.
INTRODUCTION
Seasonality of Type 1 diabetes mellitus (T1DM) was first reported about one century ago in 1926 [1]. Following this report by Adams, several studies have confirmed the link between seasonality and T1DM in almost every region of the world [2-11]. For instance, there is compelling evidence for an association between seasonality and T1DM in Scandinavia [12- 15] which is most affected region by this condition across the world [16]. Most of these findings are consistent with a peak incidence during cold months as opposed to the warm months [4-6]. Based on the excessive onset in colder/darker months than in warmer/lighter months, it is suggested that environmental factors have an etiological role in the onset of T1DM in children [3]. While some authors claimed that cold could trigger some physiological seasonal changes that modulate carbohydrate and lipid metabolism leading to higher insulin resistance [17], others pointed the viral infections with seasonal variations such as Influenza viruses [18]. Other mechanisms such as raised winter levels of pituitary [19], adrenal [20] and thyroid hormones [21]. Although less important, a role for seasonal variation in exercise and nutrition has also been considered [22]. However, among the numerous studies reporting a seasonality of T1DM all over the world, there have been very few studies carried out in sub- Saharan Africa as witnessed by the review of Moltchanova et al. [2]. The authors analyzed the seasonality in the diagnosis of type 1 diabetes, based on the incidence data in 0- to 14-year-old children, collected over one decade period (1990–1999) in one hundred and five centers from 53 countries worldwide [2]. As surprising as it may seem, of these 105 centers reporting data on the seasonality of T1DM worldwide, only one center was located in sub-Saharan Africa. This shows the scarcity or even the inexistence of data on the subject in our context. Moreover, since then, there is not a single study reporting exclusively the seasonality of T1DM in the region. Given that, there is a need to increase research output on the topic in Sub Saharan Africa region which is facing a rapid increase in T1DM cases and present a different climate profile from the Nordic countries where the majority of studies have been conducted [2]. Lontchi-Ymagou reported data on the relationship between diabetes admission rates and climate variations in Yaoundé, Cameroon [23]. However, this previous study reported seasonality of both types of diabetes and included a mixed population of children and adults and different hospital settings. This paper focuses on the seasonality of T1DM in children. The data were generated from the “Changing Diabetes in Children (CDiC)” project conducted in Cameroon from 2010. CDiC aims at providing free diabetes care to children and adolescents living with diabetes in Cameroon. Investigations inform us that there may be seasonal variation in the diagnosis of new cases, requiring the adaptation of approaches for this modelled care for T1DM.
METHODS
The research was retrospective. It was conducted from October 2010 to December 2015.
Study population, settings, sampling, and procedures
The research was conducted on a population of children and adolescents living with T1DM and monitored as part of the “Changing Diabetes in Children” project in Cameroon.
The research site was the Children Diabetic Clinic of the Yaoundé Central Hospital, where all data of diabetic children and adolescents monitored in the CDiC project are centralized. For each participant, we obtained monthly data of precipitation and temperature corresponding to the month and year of diagnosis according to the region of residence from the national meteorological database of the Weather Station of IRAD Nkolbisson, Yaoundé in Cameroon.
We identified the files of all children and adolescents enrolled in the “Changing Diabetes in Children (CDiC)” project between October 2010 and December 2015. For each participant, we gathered information concerning sex, age at diagnosis of diabetes, date of diagnosis of diabetes (month and year), region of residence at the time of diagnosis, circumstances of discovery of diabetes, blood sugar, ketonuria and HbA1c at diagnosis. In addition, we collected for each case the region of residence at the time of diagnosis, with the precipitation and temperature data corresponding to the month and year of diagnosis. A month was dry if the average monthly rainfall was < 100 mm and wet if the average monthly rainfall was ≥ 100 mm.
Statistical analysis and calculations
The data were analyzed using SPSS (Statistical Package for Social Sciences) software version 20.0. The variables are presented as mean ± standard deviation. We assessed the correlation between the number of cases diagnosed per month and climatic parameters with the Pearson’s coefficient. The one-sample chi-square test was used to assess the uniformity of monthly totals and the two-sample Kolmogorov-Smirnov goodness-of fit test (K-S) was used to assess the similarity of monthly distribution between the years (24). A P-value ≤ 0.05 was statistically significant.
We processed the meteorological data using the following operations.
Average precipitation calculation
Pm = Σ Px / n
Where Pm = average precipitation in (mm). n = number of years, months, seasons.
Px = amount of precipitation for a year x, a month x, a season x
Average deviation index.
Δx = (Px - Pm)
If Δx = 0 year / month / constant season. Δx < 0 year / month / deficit or arid season. Δx> 0 year / month / wet season.
Average temperature calculation
Tm = Σ (Tmax + Tmin) / 2 or Tm = Σ Tx / n Where Tm = average temperature (°C) Tmax = maximum temperature
Tmin = minimum temperature
Tx = temperature of a year x, a month x, a season x n = number of years, months, seasons.
Ethical considerations
The Institutional Ethical Committee of the Université des Montagnes (ethical clearance number 2016/031/UdM/PR/CAB/ CIE) approved this study. As the study is retrospective and based on already collected data, informed consent is not mandatory.
RESULTS
Participants characteristics
Overall, 549 patients were enrolled in the CDiC project during the study period (2010 to 2015). We excluded one hundred and three files that did not meet our selection criteria. Therefore, 446 files were included in the final study. Among the 446 participants, 239 (53.60%) were male and 207 (46.40%) females. The mean age at diagnosis for T1DM was 14.2±3.6 years. The youngest child diagnosed was one year old. Participants came from ten regions of Cameroon. Clinical presentation at diagnosis was polyuria and polydipsia for 269 patients (60.31%), followed by coma for 48 patients (10.76%), ketoacidosis without coma for 39 patients (8.74%) and weight loss for 38 patients (8.52%). The mean blood sugar at diagnosis was 411.2±120.2 mg/dl with a minimum of 200 mg/dl and a maximum of 842 mg/dl. The average HbA1c was at diagnosis was 11.6 ± 3%.
Seasonal patterns of Type 1 diabetes mellitus cases
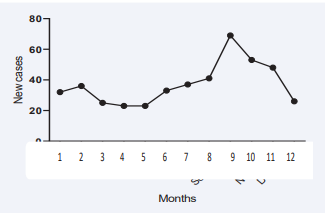
We first studied the variation in the onset of T1DM cases over the five years of our study period. We found a repeating trends over the years with a low number of cases diagnosed from March to May and a higher number of cases from August to October (Figure 1).
Figure 1: Overall variation in diagnosis of type 1 diabetes during the year (five years period). Caption: 1 = January, 2 = February, 3 = March, 4 = April, 5 = May, 6 = June, 7 = July, 8 = August, 9 = September, 10 = October, 11 = November and 12 = December.
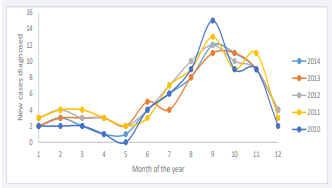
Overall, most of the diabetes new cases occurred between August and November with a peak in September (69/446), while the lowest number of cases were diagnosed in April and May (23/446) as illustrated in Figure 2.
Figure 2: Yearly variation of type 1 diabetes onset from 2010 to 2014. Caption: 1 = January, 2 = February, 3 = March, 4 = April, 5 = May, 6 = June, 7 = July, 8 = August, 9 = September, 10 = October, 11 = November and 12 = December
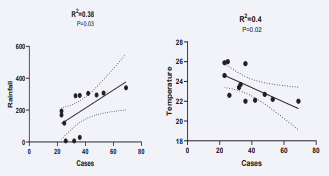
Finally, we study the correlation between temperature, rainfall, and diabetes cases. There was a positive correlation between the number of new T1DM cases and the rainfall and a negative correlation with the mean temperature (Figures 3a &3b).
Figure 3: correlation between new T1DM cases vs rainfall (3a) and vs temperature (3b).
Then, we grouped the new cases according to the season of diagnosis (dry season or wet season). The number of new cases diagnosed during the wet season (302) was more than twice that of the dry season (144), showing a clear pattern with a trend toward an increase in T1DM onset during the wet season (p<0.001).
DISCUSSION
This study investigated the seasonal variation in the diagnosis onset of type 1 T1DM in children and adolescents in Cameroon.
We found a clear pattern of variation in new cases with a low number of cases diagnosed from March to May and a higher number of cases from August to October (peak in September) in each of the five years period of our study. In order to confirm a relation between this variation in onset and rainfall and/or temperature, we study the correlation between these parameters. There was a positive correlation between the number of new T1DM cases and the rainfall and a negative correlation with the mean temperature.
In the previously cited review by Moltchanova et al. [2], published in 2009, the authors were already highlighting the need of more data on the population living below the 30th parallel (north of South America and sub-Saharan Africa) in order to complete the picture as suggested by the final sentence of their abstract. Surprisingly, since then, there have been no reports on the topic in sub–Saharan Africa. The findings of the present study represent the first reports in sub–Saharan Africa on the seasonality of T1DM since the paper by McLarty in 1989 three decades ago. Our findings are consistent with those of McLarty et al., who reported a seasonal pattern in the presentation of patients with both insulin-requiring and non-insulin-requiring diabetic patients over a six-year period. Their peak months of presentation were also August through to November, with the greatest number of patients presenting in September and the smallest number in June, similar to our results [24]. The authors of this review suggested that there was a northern/ southern hemisphere dichotomy in the seasonality pattern of T1DM since their seasonal pattern appears to be dependent on the geographical position of the country. However, our findings are similar to those of the majority of studies from northern countries with a seasonal clear pattern and peak of T1DM cases during cold months and lower cases during warm months despite the fact that the maximal occurrence of cases might not be found at the same months [4,5,25]. The significant correlation between the number of new cases and the rainfall and temperature support the fact that seasons have a real influence on the onset of T1DM in our context. The onset of T1DM tends to increase during the cold season. Many studies in the literature pointed out viruses as the cause of these variations since they also present a seasonal pattern and have the ability to trigger an immune response leading to beta cell dysfunction and destruction [26]. A starting point to answer to this question can be obtained from an epidemiological perspective by putting a side by side a map of seasonal variations of those incriminated viruses and T1DM. This could support and pave the way for more in depth studies on the viral infections as a potential trigger for T1DM onset as it has to be done for type 2 diabetes and ketosis-prone atypical diabetes [27,28].
Our study has limitations. We did not stratify children or adolescents by age group as most of the other study, and this does not allow us to describe a clear pattern in each group of age since the seasonal variation can change according to age as shown by previous study [25,29]. However, our findings already revealed a global seasonal pattern during each of the five years of the study.
CONCLUSION
Our data support the existence of a clear seasonal pattern in the onset and diagnosis of Type 1 diabetes mellitus in Cameroon. Most of the new cases appear between August and October, which represent the cold and wet months. The confirmation of the seasonality of T1DM in sub-Saharan Africa is important data concerning the early diagnosis of the disease. Indeed, many cases remain undiagnosed due to the ignorance of signs and symptoms by health care professionals and the general population. Sensitization campaigns are useful to prevent misdiagnosis. In the context of seasonality, those campaigns would be more cost effective in months with high incidence.
Data Availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
AUTHORS’ CONTRIBUTIONS
MDY: study conception and design, draft, reviewing, and editing. AT: draft, data analysis, reviewing, and editing. CYMT: data collection, data analysis, and reviewing. PA: reviewing and editing. MCEE: reviewing and editing. JCM: study conception and design, reviewing, and editing. ES: study conception and design, reviewing, and editing. All authors read and approved the final version for publication.
REFERENCES
- Adams F. The seasonal variation in the onset of acute diabetes. The age and sex factors in 1000 diabetic patients. Arch Intern Med. 1926; 37: 861-864.
- Moltchanova EV, Schreier N, Lammi N, Karvonen M. Seasonal variation of diagnosis of Type 1 diabetes mellitus in children worldwide. Diabet Med. 2009; 26: 673-678.
- Douglas S, McSporran B, Smail P. Seasonality of presentation of type I diabetes mellitus in children. Scottish Study Group for the Care of Young Diabetics. Scott Med J. 1999; 44: 41-46.
- Kalliora MI, Vazeou A, Delis D, Bozas E, Thymelli I, Bartsocas CS. Seasonal variation of type 1 diabetes mellitus diagnosis in Greek children. Hormones (Athens, Greece). 2011; 10: 67-71.
- Spaans EA, van Dijk PR, Groenier KH, Brand PL, Reeser MH, Bilo HJ, et al. Seasonality of diagnosis of type 1 diabetes mellitus in the Netherlands (Young Dudes-2). J Pediatr Endocrinol Metab. 2016; 29: 657-661.
- Waldhoer T, Schober E, Tuomilehto J. Long-term patterns in seasonality of insulin-dependent diabetes mellitus diagnosis in Austrian children. J Clin Epidemiol. 1997; 50: 159-165.
- McKinney PA, Europe ESOBG, Diabetes. Seasonality of birth in patients with childhood Type I diabetes in 19 European regions. Diabetologia. 2001; 44: B67-74.
- Scott RS, Brown LJ, Darlow BA, Forbes LV, Moore MP. Temporal variation in incidence of IDDM in Canterbury, New Zealand. Diabetes Care. 1992; 15: 895-899.
- Levy-Marchal C, Patterson C, Green A. Variation by age group and seasonality at diagnosis of childhood IDDM in Europe. The EURODIAB ACE Study Group. Diabetologia. 1995; 38: 823-830.
- Kida K, Mimura G, Ito T, Murakami K, Ashkenazi I, Laron Z. Incidence of Type 1 diabetes mellitus in children aged 0-14 in Japan, 1986- 1990, including an analysis for seasonality of onset and month of birth: JDS study. The Data Committee for Childhood Diabetes of the Japan Diabetes Society (JDS). Diabet Med. 2000; 17: 59-63.
- Higgins T, Saw S, Sikaris K, Wiley CL, Cembrowski GC, Lyon AW, et al. Seasonal variation in hemoglobin A1c: is it the same in both hemispheres? J Diabetes Sci Technol. 2009; 3: 668-671.
- Ludvigsson J, Afoke AO. Seasonality of type 1 (insulin-dependent) diabetes mellitus: values of C-peptide, insulin antibodies and haemoglobin A1c show evidence of a more rapid loss of insulin secretion in epidemic patients. Diabetologia. 1989; 32: 84-91.
- Padaiga Z, Tuomilehto J, Karvonen M, Dahlquist G, Podar T, Adojaan B, et al. Seasonal variation in the incidence of Type 1 diabetes mellitus during 1983 to 1992 in the countries around the Baltic Sea. Diabet Med. 1999; 16: 736-743.
- Karvonen M, Jantti V, Muntoni S, Stabilini M, Stabilini L, Muntoni S, et al. Comparison of the seasonal pattern in the clinical onset of IDDM in Finland and Sardinia. Diabetes Care. 1998; 21: 1101-1109.
- Karvonen M, Tuomilehto J, Virtala E, Pitkaniemi J, Reunanen A, Tuomilehto-Wolf E, et al. Seasonality in the clinical onset of insulin- dependent diabetes mellitus in Finnish children. Childhood Diabetes in Finland (DiMe) Study Group. Am J Epidemiol. 1996; 143: 167-176.
- Maahs DM, West NA, Lawrence JM, Mayer-Davis EJ. Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am. 2010; 39: 481-497.
- Fahlen M, Oden A, Bjorntorp P, Tibblin G. Seasonal influence on insulin secretion in man. Clinical science. 1971; 41: 453-458.
- Szopa TM, Titchener PA, Portwood ND, Taylor KW. Diabetes mellitus due to viruses--some recent developments. Diabetologia. 1993; 36: 687-695.
- Weitzman ED, deGraaf AS, Sassin JF, Hansen T, Godtlibsen OB, Perlow M, et al. Seasonal patterns of sleep stages and secretion of cortisol and growth hormone during 24-hour periods in northern Norway. Acta Endocrinol (Copenh). 1975; 78: 65-76.
- Johansson G, Frankenhaeuser M, Lambert WW. Note on seasonal variations in catecholamine output. Percept Mot Skills. 1969; 28: 677-678.
- Touitou Y, Sulon J, Bogdan A, Reinberg A, Sodoyez JC, Demey-Ponsart E. Adrenocortical hormones, ageing and mental condition: seasonal and circadian rhythms of plasma 18-hydroxy-11-deoxycorticosterone, total and free cortisol and urinary corticosteroids. J Endocrinol. 1983; 96: 53-64.
- Ma Y, Olendzki BC, Li W, Hafner AR, Chiriboga D, Hebert JR, et al. Seasonal variation in food intake, physical activity, and body weight in a predominantly overweight population. Eur J Clin Nutr. 2006; 60: 519-528.
- Lontchi-Yimagou E, Tsalefac M, Tapinmene LM, Noubiap JJ, Balti EV, Nguewa JL, et al. Seasonality in diabetes in Yaounde, Cameroon: a relation with precipitation and temperature. BMC Public Health. 2016; 16: 470.
- McLarty DG, Yusafali A, Swai AB. Seasonal incidence of diabetes mellitus in tropical Africa. Diabet Med. 1989; 6: 762-765.
- Gerasimidi Vazeou A, Kordonouri O, Witsch M, Hermann JM, Forsander G, de Beaufort C, et al. Seasonality at the clinical onset of type 1 diabetes-Lessons from the SWEET database. Pediatr Diabetes. 2016; 17: 32-37.
- Filippi CM, von Herrath MG. Viral trigger for type 1 diabetes: pros and cons. Diabetes. 2008; 57: 2863-2871.
- Karim S, Mirza Z, Kamal MA, Abuzenadah AM, Azhar EI, Al-Qahtani MH, et al. An association of virus infection with type 2 diabetes and Alzheimer’s disease. CNS Neurol Disord Drug Targets. 2014; 13: 429- 439.
- Sobngwi E, Choukem SP, Agbalika F, Blondeau B, Fetita LS, Lebbe C, et al. Ketosis-prone type 2 diabetes mellitus and human herpesvirus 8 infection in sub-saharan africans. JAMA. 2008; 299: 2770-2776.
- Mooney JA, Helms PJ, Jolliffe IT, Smail P, Scottish Study Group for the Care of Diabetes in the Y. Seasonality of type 1 diabetes mellitus in children and its modification by weekends and holidays: retrospective observational study. Arch Dis Child. 2004; 89: 970-973.