The Neuroprotective Potential of Omega-3 in Terms of Preventive and Therapeutic Aspects in Ischemic CVA
- 1. School of Medicine, Federal University of Cariri – UFCA, Barbalha, Ceará, Brazil
Abstract
Introduction: Cerebrovascular Accident (CVA), is a neurological disorder whose pathophysiology of ischemic side occurs along with the development of a central inflammatory process. Omega-3 fatty acids appear as a potential agent in the prevention and treatment of the repercussions of this ischemic event, presenting the potential for neuroprotection in successive studies.
Objective: We aim to carry out a systematic review of the literature about the neuroprotective potential of omega-3 in terms of preventive and therapeutic aspects in ischemic CVA.
Method: From a search in the electronic databases PubMed and Scopus following the items of Guidelines for Systematic Reviews and Meta-analyzes (PRISMA), 12 published articles were included in the final sample and applied for discussion according to the literature.
Results: The studies pointed out the decrease in omega-3 as prejudicial to the regeneration of nervous tissues, as well as the neuroprotective role of omega-3 in cerebral ischemia after ischemia-reperfusion injury in animal models.
Conclusion: Reduction of cerebral edema, promotion of angiogenesis, preservation of the integrity of the blood-brain barrier and tissue protection were observed. Therefore, a greater understanding of the molecular role of omega-3 fatty acids and their consequences for supplementation in humans is needed.
Keywords
• Cerebral ischemia
• Omega-3 Polyunsaturated Fatty Acids
• Neuroprotection
CITATION
de Alencar Viana Melo L, Costa Silva AM, Ribeiro Grangeiro SE, Neto WM, Neto LL, et al. (2021) The Neuroprotective Potential of Omega-3 in Terms of Preventive and Therapeutic Aspects in Ischemic CVA. Ann Psychiatry Ment Health 9(2): 1166.
INTRODUCTION
Cerebrovascular Accident (CVA), is one of the neurological disorders with the highest mortality and permanent sequelae rates [1]. Among 240 causes of mortality, CVA is the second globally, surpassed only by ischemic heart disease (IHD), and the continuity of the rates is projected until 2030. Survivors of this morbidity may be affected by organic and psychological functional complications, requiring temporary or permanent assistance that demands high human and economic costs. Globally, the gross numbers of new stroke events increased from 6.8 million in 1990 to 11.9 million in 2017 [2].
There are two types of CVA: ischemic and hemorrhagic. Briefly, the first includes atherothrombotic cerebral infarction, cardioembolic stroke and lacunar infact, while the second, intracerebral hemorrhage and subarachnoid hemorrhage. Both have common risk factors, such as smoking, alcoholism, chronic arterial disease and previous strokes. In addition, studies suggest the influence of some genetic component on Cerebrovascular Accident [3].
Analyzing the pathophysiological pathway of CVA, the important role of neuroinflammation in the pathogenesis of ischemic stroke and the installation of brain injury are understood. Experimentally, focal cerebral ischemia induces time-dependent recruitment and activation of inflammatory cells, including neutrophils, T cells and macrophages. The inhibition of the neuroinflammatory response decreases the extent of the infarction and provides less neurological deficit in the experimental lesion [4]. Polyunsaturated fatty acids (PUFAs), are able to partially inhibit many aspects of inflammation, including leukocyte chemotaxis, expression of adhesion molecules and leukocyte-endothelial adhesive interactions, eicosanoid production and production of inflammatory substances [5].
Regarding the biochemical characteristics, PUFAs of the omega-3 family include α-linolenic acid (ALA), stearidonic acid (SDA), eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA) and docosahexaenoic acid (DHA). The oils that contain these fatty acids (FAs), or some of them, originate mainly from plant sources or are modified in plants, as well as marine, algae and unicellular sources [6].
Under the neuroprotective perspective of omega-3 PUFAs, studies have linked the effects of EPA to the suppression of oxidative stress and endothelial activation which were induced after cerebral ischemia. In this context, there is an evaluation of the anti-inflammatory and antinociceptive actions of omega-3 polyunsaturated fatty acid (n-3 PUFA), whose suggested mechanisms may involve the inhibition of cyclooxygenases and microglial activation, leading to a reduced release of pro-inflammatory cytokines, such as TNF-α [7].
Later studies by Nobre et al [7], also demonstrated that the administration of low doses (5mg and 10mg/kg), of omega-3 caused a protective effect in rats against global ischemic injuries, a potential attributed to their anti-inflammatory properties [7]. This article, therefore, aims to conduct a systematic review of the literature on the neuroprotective potential of omega-3 in ischemic CVA, given the important findings that can contribute to mitigate the effects of this serious public health problem.
METHOD
A systematic revision was made, following the PRISMA protocol (Preferred Reporting Items for Systematic Reviews and Meta-Analysis).
Inclusion criteria
The inclusion criteria were studies published in English, Portuguese, french and german suitability for the purpose of this review, methodological rigor applied and full-text available for free. Review articles, as well as comments on literature, editorials, communications and letters to the editor were excluded. We had no restrictions related to study design, methodological quality, or language. The language problem was solved through a reasonable degree of comparability, which allowed us to systematically analyze the selected evidence, its critical evaluation process, and its success in including relevant studies in other languages.
We classify assessments according to their level of inclusion of studies in other languages. Reviews that excluded non-English studies with an explicit justification in the research question or research objectives were categorized as justified by R1 (that is, justified in English), while those that excluded non-English studies without justification were categorized as restricted to RR1 (that is, languages that are not restricted to English). Reviews that did not explicitly exclude studies that were not in English were categorized as RR1-open, unless they successively included studies that were not in English, in which case they were RR1-inclusive. Finally, revisions that did not declare language criteria were considered to be RR1-open.
Literature research and selection of articles
To search for studies, the following databases were used: The keywords “ cerebral ischemia ”; “ Stroke”; “Omega-3 fatty acids ”; “DHA”; “EPA”; “Neuroprotection” and “neurological function” were applied to identify articles published between January 2015 and March 2020.
Data extraction and methodological quality assessment
One researcher (MLRN), extracted data and another verified the extraction. Two researchers (MEPN and AOC) independently assessed the methodological quality of systematic reviews using the AMSTAR tool and qualitative studies using the CASP checklist (Critical Appraisal Skills Programme – CASP, 2020). A researcher (FT) assessed the quality of cross-sectional studies using the JBI Prevalence or the JBI Cross-sectional analytical checklist and longitudinal studies using the JBI Cohort checklist (Johanna Briggs Institute).
Data presentation and analysis
We summarized the results narratively. We described interventions and outcomes based on the information provided in the studies. When studies showed neuroprotective properties of omega-3 in ischemic cerebrovascular accident results in numbers without numbers, we extracted them using online software (https://apps.automeris.io/wpd/). We decided not to perform a quantitative analysis of summaries of the associations between the various correlates and health factors, due to a combination of heterogeneity in the measures and lack of control groups, and an embraced lack of descriptions necessary to confirm sufficient homogeneity. We rated the certainty of the evidence using the GRADE approach – (Grading of Recommendations Assessment, Development and Evaluations.
DISCUSSION
In the post-stroke period, the metabolic demand of tissues increases considerably, leading the patient to lack several essential nutrients, such as omega-3[8]. This malnutrition slows down and impairs the regeneration of brain tissue. To reverse this situation, studies have shown how long-chain omega-3 fatty acids (DHA and EPA) [9], and their alpha-linoleic acid precursor (ALA) [8], administered, respectively, by injections and by food supplementation have neuroprotective properties. As much as there are disagreements regarding these studies, such as the statement by Berressem et al. [9], that there is a reduction in the infarcted area in the action of omega-3 and the statement by Bourourou et al. [8], that the neuroprotective effect is due to the improvement in learning and memory and not to the reduction of the infarcted area, both confirm mechanisms by which omega-3 has the beneficial effects in the post-stroke period.
The administration of a single dose, in female rats, of a long-chain omega-3 lipid emulsion (Omegaven 10% ®, OGV) containing fish oil (DHA 18mg/ml; EPA21mg/ml) and alpha-tocopherol (0,2mg/ml) demonstrated great efficacy in the acute treatment of ischemic stroke. When this treatment is performed exclusively with EPA, it has been shown to be ineffective in the hypoxia-ischemia brain injury model, unlike DHA, which showed great efficacy in the same experiment; however, EPA demonstrated effects such as vasodilation and better fluidity of the membrane, which, in combination with DHA, it can enhance the neuroprotective effects [9]. Studies by Nobre et al. [7], demonstrate that small doses (5 and 10 mg/kg/ day, orally) of omega-3 fatty acids administered for seven days show neuroprotective action, showing reversal of biological changes resulting from cerebral ischemia, such as the presence of anxiety, loss of spatial memory, and decreased expression of pro- inflammatory cytokines in the studied nervous tissue.
Cerebral ischemia in neonates may have its consequences mitigated with the administration of omega-3 polyunsaturated fatty acids (n-3 PUFA) [10-12], with various pathophysiological mechanisms for this interaction being described in the literature. Zhang et al. [12], inferred that a n-3 PUFA enrichment diet, in the period from the second day of pregnancy to two weeks after delivery, acts in maintaining the integrity of the blood-brain barrier in Sprague-Dawley rats. This is due to the suppression of the activity and of the synthesis of matrix metalloproteinases, enzymes that play a role in the degradation of the basement membrane proteins and the stock junction, and that, when their production is suppressed, the levels of pro-inflammatory cytokines such as TNF-alpha and IL-1beta are also suppressed.
Zhang et al. [11], also presents another mechanism by which n-3 PUFA acts in a neuroprotective manner, in which the dietary supplementation of n-3 PUFA enriched in female rats on the second day of gestation will considerably increase the content of this fatty acid in the cerebral cortex, suppressing, by n-3 PUFA, the inflammatory response, in addition to promoting phosphatidylserine biosynthesis in neuronal cell membranes. Phosphatidylserine acts as a protective factor for cell survival, as it preserves and increases a survival path called PI3K/Akt, which has been increasingly linked to neuronal survival in post-ischemic damage. Another study that reports the protective action of omega-3 in cerebral ischemia, in Zhang et al. [10], states that n-3 PUFAs show a decrease in their amount after CVA due to the increased metabolic demand. However, when omega-3 supplementation occurs, the neuroprotective effect occurs by partially suppressing the inflammatory response mediated by microglia.
In addition, Mayurasakorn et al. [13], describes that mice that, at 10 days of age, experienced brain damage due to ischemia showed short (24 hours), and long term (8 to 9 weeks),improvement in the neurological progress after treatment with two administrations via intraperitoneal doses (0.375 g n-3 TG/ kg/dose), of tri-DHA, and not of tri-EPA. One administration was performed immediately after the ischemic damage and the other after 1 hour. Such improvement was associated with an increase in DHA in brain mitochondria and bioactive metabolites derived from DHA in brain tissue. This fact was associated with mitochondria that suffered hypoxia-ischemia becoming resistant to calcium-induced membrane permeability (Ca2+), reduced oxidative brain damage and permanent neuroprotection. This corroborates the studies by Zhang et al. [10], Zhang et al. [11], Zhang et al. [12].
The presence of a low proportion of n-3 polyunsaturated fatty acids (n-3 PUFAs), may be associated with ischemic and hemorrhagic damage, in cerebral small vessel diseases (CSVD), including the total score, which measures the severity of the disease. Thus, a diet rich in n-3 PUFA, could reduce the severity of CSVDs, regardless of their hemorrhagic or ischemic origin [14].
In a population-based study in South Korea with 220 patients, high levels of n-3 PUFAs in the blood were associated with better executive functions and beneficial effects on the white matter microstructure, while low levels of n-3 PUFAs were related to more serious changes in white matter. One of the protection mechanisms would be the production of anti-inflammatory molecules, resolvins and protectins, through the lipoxygenase or cyclooxygenase pathways. In addition, n-3 PUFA had an effect on the stabilization of atheromatous plaques and lesser macrophage infiltration in patients waiting for a carotid endarterectomy who were treated with fish oil [14]. In contrast, in 3 independent prospective cohorts, which included different samples of men aged 40 to 65 and women aged 30 to 55, performed in the United States, it was found that not all active forms of n-3 PUFA, acid eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA), and docosahexaenoic acid (DHA), have the same effectiveness in reducing risk of total ischemia, atherothrombotic and cardioembolic CVA [15].
Thus, among the types of ischemic infarction, circulating DHA is inversely associated with atherothrombotic stroke, whereas DPA, with cardioembolic stroke. However, EPA was not associated with a lower risk of ischemic CVA, total atherothrombotic or cardioembolic. It was found that higher levels of DHA represented 47% lower risk when assessing atherothrombotic stroke, without significant relevance of circulating DPA and EPA in these cases. When considering cardioembolic stroke, patients with the highest levels of DPA, compared to the lowest levels, had 42% lower risk, without significant relevance of the amounts of blood DHA and EPA in such cases [15].
DHA is also a precursor to neuroprotectin D1, a docosanoid anti-inflammatory derivative which reduces apoptosis, promoting cell survival in ischemic stroke models [16].
Furthermore, Lin et al. [17], suggests that DHA would also act in the hemorrhagic transformation (HT), a feared complication of ischemic stroke, frequent after the use of thrombolytic therapy. In this sense, DHA would act in order to reduce the risk of HT, which is increased due to hyperglycemia after focal ischemic injury. The studies by Lin et al. [17], used adult male rats that received 50% dextrose injection (6 ml/kg intraperitoneal) in order to induce hyperglycemia and, consequently, HT, 10 min before suffering a middle cerebral artery occlusion. Treatment with DHA (10mg/ kg), 5min before reperfusion reduced HT, decreased the volume of infarction, in addition to having demonstrated better neurological progress after seven days. It was also confirmed that DHA inhibited the inflammatory reaction mediated by ICAM- 1, a transmembrane protein in endothelial cells that facilitates leukocyte transmigration and adhesion. DHA also acted by inhibiting the collagen type IV degradation, in order to stabilize the blood-brain barrier, since collagen IV is the main component of capillary endothelial cells.
Still under this bias, Sumiyoshi et al. [18], evaluated that in ovariectomized female rats that were subjected, after the eleventh week of life, to cerebral ischemia due to middle cerebral artery occlusion, there is an increase in the expression of HMG- 1, a DNA-binding ubiquitous nuclear protein that is released by necrotic cells and secreted by activated leukocytes. Such protein has relevance in post-ischemia damage, acting as an activator of antigen-presenting cells through the receptor for advanced glycation end products (RAGE), and toll-like receptors (TLRs), triggering and exacerbating the inflammatory response. It has also been shown that a pretreatment with EPA supplementation (500 mg/kg/day, orally), for 4 weeks attenuates these changes regardless of estrogen levels, which may point to a future treatment in cases of human women affected by stroke and who are in the post-menopausal stage. EPA, in addition to having antiarrhythmic, anticoagulant, antioxidant, and anti- inflammatory actions, is also an agonist of the PPARγ receptor, which modulates the immune system of the central nervous system by inhibiting the activation of macrophages and by modulating the release of pro- inflammatory mediators [18].
In a study with an animal model of oxygen-glucose ischemia/ reperfusion injury or oxygen-glucose deprivation/reperfusion (OGD / R), fat-1 type rats, that is, those with a higher level of endogenous n-3 PUFA, were fed with 10% corn oil. Shi et al. [14], then, demonstrated that both endogenous and exogenous n-3 PUFA increased the survival of a culture of cortical neurons after OGD / R. In this model, the fat-1 gene increased the expression of neuronal n-3 PUFA, including the active forms DPA and DHA. Thus, when comparing fat-1 rats with wild-type rats (WT), it was found that WT neurons showed cellular damage and neuritis 24h after OGD/R considerably greater than fat-1 neurons. In addition, lower intracellular accumulation of reactive oxygen species (ROS) in fat-1 neurons was attested, which points to inhibition of ROS activation by endogenous n-3 PUFA. There was also greater expression of cleaved caspase-3 in WT neurons. In the exogenous administration of DHA and GSH, there was also a significant reduction in ROS activation. Such neuroprotection is directly related to the elimination of ROS and positive regulation of anti-apoptotic proteins. Recently, it was discovered that DHA modulates neuronal defenses by activating the expression of GSH in brain cells. The observations also showed a significant improvement in the integration of motor and sensory functions, 2 weeks after OGD / R, in fat-1 rats.
Shi et al. [19], added that, by combining n-3 PUFA treatment, especially DHA, with Lycium barbarum Polysaccharide (LBP),better results can be obtained in the rescue of cortical neurons after OGD/R, particularly by activating the TrkB receptor and alleviating intracellular Ca2+ overload. In this context, studies have pointed out the role of n-3 PUFA in inhibiting the release of Ca2+ by the endoplasmic reticulum. However, in this research, treatment with only LBP proved to have limited influence on the control of Ca2+ overload. Furthermore, the observations demonstrated that LBP and n-3 PUFA perform neuroprotection by activating the anti-apoptotic Bcl-2 cascade, a fact that also contributes to maintaining Ca2+ homeostasis in the endoplasmic reticulum. This study, for the first time, also reported that LBP can perform neuronal protection from apoptosis by modulating the neurotrophin pathway, which is initiated from the cell membrane. However, contrary to Shi et al. [14], it was stated that there are inconsistencies in the dietary supplementation of n-3 PUFA in animal studies due to the variance of the component in the diet, in addition to a certain neglect of the relevance in the proportion between n-3 PUFA and n-6 PUFA [19-27].
CONCLUSION
Omega-3 can possibly act as a neuroprotective agent in several ways, playing an important supporting role in the prevention and/or treatment of CVA. Studies have shown its vasodilator activity, providing improvement in spatial memory and learning and decreased depression and anxiety after global ischemia in animals.
Its anti-inflammatory potential has also been reported, decreasing the activation of microglia and release of pro- inflammatory mediators, restoring mitochondrial function and reestablishing glucose level in brain tissue. Studies also report that omega-3 can act to reduce cerebral edema, promote angiogenesis, preserve blood-brain barrier integrity and protect tissue by reducing reactive oxygen species. This study was limited due to the existence of few records in the literature that address the direct relationship between protective properties of omega-3 and ischemic stroke, in addition to sparse research that includes human models. Thus, there is a need for further research in the area, in order to provide greater understanding about the molecular action of omega-3 fatty acids and their role in supplementation in humans.
REFERENCES
- Azarpazhooh M, Mandzia J, Thrift A, Sposato L, Morovatdar N, Amiri A, et al. Age, sex, and setting in the etiology of stroke study (ASSESS): Study design and protocol. J Neurol Sci. 2019; 399: 209-213.
- Avan, A, Digaleh H, Di Napoli M, Stranges S, Behrouz R, Shojaeianbabaei G, et al. Socioeconomic status and stroke incidence, prevalence, mortality, and worldwide burden: an ecological analysis from the Global Burden of Disease Study 2017. BMC Med. 2019; 17.
- Yamada Y. Molecular basis of stroke. Clin Mol Med. 2019; 189-216.
- Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol. 2010; 87: 779-789.
- Calder P. Omega-3 fatty acids and inflammatory processes: from molecules to man. Biochem Soc Trans. 2017; 45: 1105-1115.
- Shahidi F, Ambigaipalan P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu Rev Food Sci Technol. 2018; 9: 345-381.
- Nobre M, Correia A, Mendonça F, Uchoa L, Vasconcelos J, de Araújo C, et al. Omega-3 Fatty Acids: Possible Neuroprotective Mechanisms in the Model of Global Ischemia in Rats. J Nutr Metabol. 2016; 1-13.
- Bourourou M, Heurteaux C, Blondeau N. Alpha-linolenic acid given as enteral or parenteral nutritional intervention against sensorimotor and cognitive deficits in a mouse model of ischemic stroke. Neuropharmacol. 2016; 108: 10-72.
- Berressem D, Koch K, Franke N, Klein J, Eckert G. Intravenous Treatment with a Long-Chain Omega-3 Lipid Emulsion Provides Neuroprotection in a Murine Model of Ischemic Stroke – A Pilot Study. PLOS ONE. 2016; 11: e0167329.
- Zhang W, Hu X, Yang W, Gao Y, Chen J. Omega-3 Polyunsaturated Fatty Acid Supplementation Confers Long-Term Neuroprotection Against Neonatal Hypoxic–Ischemic Brain Injury Through Anti-Inflammatory Actions. Stroke. 2010; 41: 2341-2347.
- Zhang W, Liu J, Hu X, Li P, Leak RK, Gao Y, et al. n-3 Polyunsaturated Fatty Acids Reduce Neonatal Hypoxic/Ischemic Brain Injury by Promoting Phosphatidylserine Formation and Akt Signaling. Stroke. 2015; 46: 2943-2950.
- Zhang W, Zhang H, Mu H, Zhu W, Jiang X, Hu X, et al. Omega-3 polyunsaturated fatty acids mitigate blood–brain barrier disruption after hypoxic–ischemic brain injury. Neurobiol Dis. 2016; 91: 37-46.
- Mayurasakorn K, Niatsetskaya Z, Sosunov S, Williams J, Zirpoli H, Vlasakov I, et al. DHA but Not EPA Emulsions Preserve Neurological and Mitochondrial Function after Brain Hypoxia-Ischemia in Neonatal Mice. PLOS ONE. 2016; 11: e0160870.
- Shi Z, Ren H, Luo C, Yao X, Li P, He C, et al. Enriched Endogenous Omega-3 Polyunsaturated Fatty Acids Protect Cortical Neurons from Experimental Ischemic Injury. Mol Neurobiol. 2015; 53: 6482-6488.
- Saber H, Yakoob M, Shi P, Longstreth W, Lemaitre R, Siscovick D, et al. Omega-3 Fatty Acids and Incident Ischemic Stroke and Its Atherothrombotic and Cardioembolic Subtypes in 3 US Cohorts. Stroke. 2017; 48: 2678-2685.
- Marcheselli V, Hong S, Lukiw W, Tian X, Gronert K, Musto A, et al. Novel Docosanoids Inhibit Brain Ischemia-Reperfusion-mediated Leukocyte Infiltration and Pro-inflammatory Gene Expression. J Biol Chem. 2003; 278: 43807-43817.
- Lin Y, Xu M, Wan J, Wen S, Sun J, Zhao H. Docosahexaenoic acid attenuates hyperglycemia-enhanced hemorrhagic transformation after transient focal cerebral ischemia in rats. Neurosc. 2015; 301: 471-479.
- Sumiyoshi M, Satomi J, Kitazato K, Yagi K, Shimada K, Kurashiki Y, et al. PPARγ-Dependent and -Independent Inhibition of the HMGB1/TLR9 Pathway by Eicosapentaenoic Acid Attenuates Ischemic Brain Damage in Ovariectomized Rats. J Stroke Cerebrovascular Dis. 2015; 24: 1187- 1195.
- Shi Z, Wu D, Yao J, Yao X, Huang Z, Li P, et al. Protection against Oxygen-Glucose Deprivation/Reperfusion Injury in Cortical Neurons by Combining Omega-3 Polyunsaturated Acid with Lyciumbarbarum Polysaccharide. Nutrients. 2016; 8: 41.
- Bazan N. Docosanoids and elovanoids from omega-3 fatty acids are pro-homeostatic modulators of inflammatory responses, cell damage and neuroprotection. Mol Aspects Med. 2016; 64: 18-33.
- Hossmann K, Heiss W. Neuropathology and pathophysiology of stroke. Textbook of Stroke Medicine. 2010; 1-27.
- Jackova J, Sedova P, Brown R, Zvolsky M, Volna M, Baluchova J, et al. Risk Factors in Ischemic Stroke Subtypes: A Community-Based Study in Brno, Czech Republic. J Stroke Cerebrovasc Dis. 2020; 29: 104503.
- Jauch E, Peacock W, Morgan J, June J, Ireland J. RNA Gene Expression to Identify the Etiology of Acute Ischemic Stroke: The Biomarkers of Acute Stroke Etiology (BASE) Study. Neuromethods. 2019; 157-169.
- Ministry of Health. Health Brazil 2018: An analysis of the health situation and of chronic diseases and conditions: challenges and perspectives (1st ed.). 2018.
- Nobili V, Bedogni G, Alisi A, Pietrobattista A, Rise P, Galli C, et al. Docosahexaenoic acid supplementation decreases liver fat content in children with non-alcoholic fatty liver disease: double-blind randomised controlled clinical trial. Arch Dis Child. 2011; 96: 350- 353.
- Nobre M, Correia A, Borges M, Sampaio T, Chakraborty S, Gonçalves D, et al. Eicosapentaenoic acid and docosahexaenoic acid exert anti-inflammatory and antinociceptive effects in rodents at low doses. Nutrition Res. 2013; 33: 422-433.
- Da Silva C, Schwartz J, Belli V, Ferreira L, Cabral N, França P. Ischemic Stroke and Genetic Variants: In Search of Association with Severity and Recurrence in a Brazilian Population. J Stroke Cerebrovasc Dis. 2020; 29: 104487.
- Ueno Y, Miyamoto N, Yamashiro K, Tanaka R, Hattori N. Omega-3 Polyunsaturated Fatty Acids and Stroke Burden. Int J Mol Sci. 2019; 20: 5549.
RESULTS
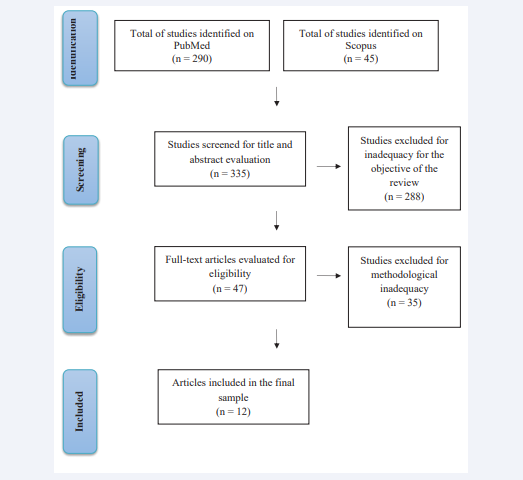
A total of 335 articles were designated for screening using the search strategy. After screening by title and abstract, 47 articles were selected for full-text evaluation. Out of these studies, 35 were excluded due to the unsuitability for the objective of this review. Thus, 12 articles were included for study and discussion in the literature (Figure 1).
Figure 1: Flowchart summarizing the search strategy for studies
The main characteristics of the included studies are presented in Table 1. This review included
12 studies published between 2015 and 2017, carried out between Brazil, China, the United States, Germany, South Korea, France and Japan (Table 1).
|
Table 1: Author and year of publication, country of study, objective, methodology, results and conclusion of the 12 studies included. |
|||||
|
Author and year |
Coun- try |
Objective |
Methodology |
Results |
Conclusion |
|
Zhang et al. [11]. |
China; United States |
To elucidate the mechanisms underly- ing the protection af- forded by n-3 polyun- saturated fatty acids (n-3 PUFAs) against neuronal ischemic injuries. |
N-3 PUFAs supplementation was started on the 2nd day of gestation in rats. Hypoxia- ischemia (HI) damage was induced in 7-day-old puppies by occlusion of the ip- silateral common carotid artery followed by hypoxia. Loss of brain tissue, cell death, and activation of signaling events were as- sessed after HI. The effects of n-3 PUFAs on cell death induced by oxygen-glucose deprivation and the underlying protection mechanism were also examined |
N-3 PUFAs reduced brain tissue loss at 7 days after HI and im- proved neurological outcomes, while inhibition of PI3K/Akt signaling partially canceled out this neuroprotective effect. DHA/EPA prevented ischemic neuronal death and increased phosphatidylserine production. |
N-3 PUFAs protect against brain damage induced by HI in neo- nates, activating the Akt pathway of survival in compromised neurons, promote the formation of phosphatidylserine and promote Akt activ- ity. |
|
Shi et al. [14] |
China; United States |
To verify the effect of omega-3 polyun- saturated fatty acids (n-3 PUFAs) on the increased survival of cortical neurons cul- tured in the oxygen- glucose deprivation/ reperfusion (OGD/R) injury model. |
Rats were fed a diet containing 10% corn oil, for a period of up to 10 weeks. Cell culture and OGD/R, fatty acid analysis, bio- chemical, immunocytochemical and histo- pathological tests, TUNEL model, intracel- lular ROS evaluation in vivo were used. The statistics were made using the ANOVA and Turkey tests. An ischemic model with re- producible cortical infarction and function deficits was also used. Gait analysis and aadd-- hesive removal test were also performed. |
Fat-1 neurons exhibited sig- nificantly attenuated reactive oxygen species (ROS) activa- tion induced by OGD / R injury, increased presence of anti- apoptotic proteins Bcl-2 and Bcl-xL, and reduced cleavage of caspase-3. Exogenous adminis- tration of docosahexaenoic acid (DHA), resulted in similar pro- tective effects on neurons in the cultured cortex. |
Therefore, we provide evidence that n-3 PUFA exert its protective ef- fects against ischemic injury both in vitro and in vivo, partially by in- hibiting ROS activation. |
|
Sumiyo- chi et al [18]. |
Japan |
To verify if HMG-1 plays a role in isch- emic brain damage in ovariectomized rats (OVX +) and if eicosapentaenoic acid (EPA) inhibits the activation of this pathway and attenu- ates brain damage in a PPARg-dependent manner. |
Seven-week-old Sprague-Dawley rats, divided into 3 groups were used; non- ovariectomized rats (OVX-)and OVX + rats treated with EPA and not treated with EPA before induction of cerebral ischemia. Another set of OVX + mice treated with EPA was injected with the inhibitor of Per- oxisome Proliferator-Activated Receptor gamma (PPARγ) antagonist, GW9662. |
The rats (OVX +) reduced the level of PPARg mRNA and increased that of HMG-1, re- ceptor for advanced glycation end products (RAGE), toll-like receptor 9 (TLR9) and tumor necrosis factor alpha (TNFa) simultaneously with brain dam- age. EPA restored PPARγ expres- sion, down-regulated molecules related to the HMG-1 signal and reduced brain damage. |
The volume of cortical infarction suffered by OVX + is associated with positive regulation of the HGM-1/TLR9 pathway. Suppression of this pathway can help limit this damage to postmenopausal women. |
|
Lin et al. [17] |
China; United States |
To examine whether the docosahexaenoic acid (DHA) can pro- tect the brain from injury and decrease the risk of hemor- rhagic transforma- tion (HT) increased by hyperglycemia after focal ischemia. |
A substance with 50% dextrose (6ml / kg intraperitoneally) was injected into male Sprague-Dawley rats to induce hypergly- cemia 10 min before 1.5 hours of occlusion of the middle cerebral artery (MCAO) of the filament combined with treatment with DHA (10mg / kg) 5min before reperfusion. |
Treatment with DHA 5 min before reperfusion reduced HT and improved the neurological outcome of 7 days. It reduced the volume of the infarction and improved the integrity of the blood-brain barrier (BBB) in rats treated with DHA. In addition, DHA reduced the expression of intercellular adhesion mol- ecule-1 (ICAM-1) in the brain. |
DHA attenuated hyper- glycemia and improved neurological function, preserving the integrity of the BBB and reduc- ing inflammation. |
|
Song et al. [14]. |
South Korea |
To verify the hypoth- esis that a low pro- portion of plasma fat- ty acids (FA) would be associated with cerebral small vessel diseases (CSVD). |
Study with 220 patients with first-episode cerebral infarction, up to 7 days after the onset of symptoms. The composition of plasma FAs was analyzed using gas chro- matography. The presence and load of cerebral micro-hemorrhages (CMH), high degree of white matter changes (WMC), enlarged perivascular spaces (EPVS) and asymptomatic lacunar infarctions (ALI) were investigated |
CMHs, WMCs and EPVSs were negatively correlated with the proportion of EPA, DHA and n-3 PUFA in the univariate analy- sis; as for multivariate, a lower proportion of EPA, DHA and n-3 PUFAs was associated with the presence of CMHs, WMCs and EPVS, but not ALIs. The total score of CSVD was inversely cor- related with the proportion of EPA, DHA and n-3 PUFA. |
An association was found between low proportions of plasmids and n-3 PUFA and cere- bral CSVD pathologies. |
|
Bourour- ou Heu- rteaux & Blon- deau [8]. |
France |
To evaluate the use of alpha-linolenic acid (ALA) as adjunctive therapy for stroke recovery, comparing whether oral or intra- venous AAL supple- mentation would best assist in the recovery from ischemia. |
Male rats, 4 weeks old, with a diet enriched with ALA were used, the lipids from rape- seed oil represent 10% and AAL, 0.75% by weight. Body weight and water and food consumption were monitored. Regarding parenteral supplementation, the animals were injected into the penile vein 2 h and 3, 7, 10, 14, 17 and 21 days after the middle cerebral artery occlusion (MCAO). |
ALA supplementation in the diet was better than intravenous treatment in improving motor coordination, but this improve- ment was not due to a neuropro- tective effect, since the size of the infarction was not reduced. Both types of supplementation improved spatial learning and memory after a stroke. This cog- nitive improvement correlated with the longer survival of neu- rons in the hippocampus. |
It was identified that the preservation of the hippocampus has an intrinsic effect in the treatment with ALA, leading to reduced cog- nitive deficits, which is not due to the biocon- version of DHA. |
|
Ber- ressem et al [9]. |
Ger- many |
To test the effective- ness of an acute treatment with a long-chain omega-3 lipid emulsion (OGV), containing fish oil and α-tocopherol in a transient middle ce- rebral artery occlu- sion (MCAO) model of ischemic stroke. |
Female mice were subjected to 90 minutes of MCAO. A single dose of OGV is applied af- ter stroke or reperfusion induction. Motor function, neurological outcome and stroke- related parameters were assessed 24 hours after MCAO. Samples were collected from the extracellular space of the striatum. Mitochondrial function was determined in isolated mitochondria or dissociated brain cells and inflammation markers were mea- sured in cerebral homogenate. |
Intravenous OGV injection re- duced stroke size and severity, restored mitochondrial function and prevented the release of ex- citotoxic glutamate. The increase in pro-inflammatory markers was attenuated, the neurological severity score and neurochemi- cal data demonstrated that the acute treatment with OGV im- mediately after stroke induction was more efficient and capable of improving the short-term neurological outcome. |
Acute treatment of CVA with a single in- travenous OGV dose provided strong neu- roprotective effects and was most effective when administered im- mediately after the on- set of ischemia. Thus, it proved to be a possible early treatment of isch- emic stroke to prevent further brain damage. |
|
Mayura- sakorn et al. [13]. |
United States |
To test the hy- pothesis that the administration of docosahexaenoic acid (tri-DHA) enriches the brains that have suffered hypoxia- ischemia (HI) lesions with DHA / DHA metabolites, reducing the permeabilization of the mitochondrial membrane induced by Ca2 +. |
10-day-old mice, after HI injury, received tri-DHA, tri-EPA or vehicle. The composi- tion of mitochondrial fatty acids and the buffering capacity of Ca2 + at 4-5 hours of reperfusion were analyzed; at 24 hours and at 8-9 weeks, oxidative damage, neu- rofunctional and neuropathological results were evaluated. In vitro, mitochondrial ROS generation induced by hyperoxia and Ca2 + buffering capacity were measured in the presence or absence of DHA or EPA. |
Post-treatment with tri-DHA reduced oxidative damage and improved short- and long-term neurological outcomes, associ- ated with increased DHA content in brain mitochondria and DHA- derived bioactive metabolites in brain tissue.
In vitro, hyperoxia increased the mitochondrial production of ROS and reduced the buffering capacity of Ca2 +; DHA has miti- gated these effects. |
The interaction of DHA with the mitochondria changes the release of ROS and improves the buffering capacity of Ca2 +. This may explain the neuroprotective ac- tion of post-HI adminis- tration of tri-DHA. |
|
hi et al. [19]. |
China |
To investigate the neuroprotective ef- fects of combining omega-3 polyunsatu- rated fatty acids (n-3 PUFAs) with Lycium barbarum polysac- charide (LBP) in cor- tical neurons using an in vitro ischemic model. |
Primary cultures of cortical neurons and oxygen-glucose deprivation/reperfusion (OGD/R), immunohistochemistry, genomic DNA extractions and PCR amplification, fat- ty acid analysis, cell viability assay, TUNEL staining, intracellular calcium measure- ments were performed (Ca2 +) and West- ern Blotting analysis were performed. |
Treatment with docosahexae- noic acid (DHA) inhibited the increase of intracellular Ca2 + in cultured WT cortical neurons submitted to OGD/R. Treat- ment with LBP activated Trk-B signaling in cortical neurons and attenuated cell apoptosis. Combining LBP with n-3 PUFAs in WT neurons showed effects on the protection of WT neurons against OGD/R injuries |
It was pointed out that omega-3 polyunsatu- rated fatty acids and LBP are promising can- didates for combined pharmacotherapy for ischemic stroke. |
|
Nobre et al [7] |
Brazil |
To study the neuro- protective effects of omega-3 (????-3) in a model of global isch- emia. |
Male Wistar rats were submitted to carotid occlusion (30 min), followed by reperfu- sion. The groups were the false operated (FO), ischemic untreated (NT) with ????-3 and ischemic treated (ST) with ????-3 (5 and 10mg / kg for 7 days). The animals in the FO and NT groups were treated by oral with 1% cremophore and, 1 hour after the last admin- istration, they were treated and tested be- haviorally and sacrificed for neurochemical, histological analysis and immunohistochemi- cal evaluations. The data were analyzed by ANOVA and Newman-Keuls. |
Ischemia increased locomotor activity and breeding behavior which were partially reversed by ± 3. Ischemia decreased the striated content of dopamine (DA) and DOPAC and increased the content of NE, effects re- versed by ????-3. This medication protected the hippocampus from neuron degeneration and increased immunostaining for TNF-alpha, COX-2 and NOSs were partially or totally blocked by ????-3. |
This study showed a neuroprotective effect of ????-3, largely due to its anti-inflammatory ef- fect, stimulating trans- lational studies focus- ing on its use in stroke management clinics. |
|
Zhang et al [12]. |
China; United States |
To examine the effects of n-3 poly- unsaturated fatty acids (n-3 PUFAs) on the integrity of the blood-brain barrier (BBB) after neonatal hypoxic-ischemic (HI) injury. |
The rats were fed a diet with or without enrichment of AGPI3 from the second day of pregnancy until 14 days after delivery. H / I was introduced into the 7-day-old offspring. |
AGPI3 reduced H / I-induced BBB damage, as shown by reduc- tions in tracer efflux and IgG ex- travasation, preservation of BBB ultrastructure and increased ex- pression of the narrow-junction protein. In addition, AGPI3 pre- vented increased matrix metal- loproteinases (MMPS) activity in the brain and blood after H / I. |
Thus, n-3 PUFAs can protect newborns against BBB damage by reducing the activation of matrix metallopepti- dases after HI. |
|
Saber et al. [15]. |
United States |
To investigate the relationship between circulating eicosap- entaenoic acid (EPA), docosapentaenoic acid (DPA) and doco- sahexaenoic acid (DHA) with the risk of ischemic stroke, atherothrombotic and cardioembolic. |
The levels of circulating phospholipid fatty acids (PLFAs) were verified at the begin- ning of the study in 3 cohorts. Ischemic strokes were classified as atherothrom- botic or cardioembolic. The risk according to PLFA levels was assessed using Cox proportional hazards or conditional logistic regression according to the study design. Cohort findings were grouped using fixed- effect meta-analysis. |
After multivariate adjustment, a lower risk of total ischemic CVA was observed with the highest levels of DPA and DHA, but not with those of EPA. DHA was associated with a lower risk of atherothrombotic stroke and DPA with a lower risk of cardio- embolic stroke. |
In 3 large US cohorts, the highest circulating levels of DHA were inversely associated with incident athero- thrombotic stroke and APD with |
Evaluation of the methodological quality of the included studies
The most common methodological weaknesses in all the studies arose from insufficient reporting: samples, scenarios, and recruitment procedures were often not fully described.
Interventions in mental health
Eight studies reported the urgent need to implement interventions in order to prevent or reduce neuroprotective properties of omega-3 in ischemic cerebrovascular accident. Four studies demonstrate the need for interventions to organizational adjustments in the reduce neuroprotective properties of omega-3 in ischemic cerebrovascular accident.