Advances in signaling pathways in the non-bone phase mechanism of f luorosis
- 1. Department of Public Health, Medical College, Qinghai University, Xi’ning 810016, China
- 2. Department of Endemic Disease Prevention and Control, Qinghai Institute for Endemic Disease Prevention and Control, Xi’ning 811602, China
Abstract
Fluorine is an essential trace element, and excessive or insufficient intake can lead to damage. Currently, fluorosis has been studied more in the bone phase, but less in the non-bone phase of fluorosis. The pathogenesis of fluorosis is complex and unclear. Signaling pathways have been a popular area of exploration in disease pathogenesis, Signaling pathways play an important and irreplaceable role in the pathogenesis of fluorosis in recent years as well. In this paper, we summarize the relevant signaling pathways to provide a basis for the pathogenesis of fluorosis-induced non-osteopathic systems and hope to obtain effective preventive and therapeutic measures.
Keywords
• Fluorosis
• Signaling pathway
• Research progress
• Non-bone phase
CITATION
Yang R, Shen H, Wang M, Zhao Y, Jiang H, et al. (2023) Advances in signaling pathways in the non-bone phase mechanism of fluorosis. Ann Public Health Res 10(2): 1122.
INTRODUCTION
Fluorosis is a chronic systemic disease, which is caused by the accumulation of fluorine in the human body through excessive intake of fluorine in drinking water, food and air, resulting in the damage of certain organs and systems. Fluorosis is found in more than 20 countries around the world, and hundreds of millions of people are at risk of fluorosis [1]. In China, endemic fluorosis is found in 29 provinces, which brings great problems to people [2]. Chronic fluorosis was found to cause neurological damage and some degree of memory impairment in a clinical trial [3]. Fluorosis is not only manifested at the macroscopic level of human organs, but also at the microscopic level with new findings. Some scholars have randomized fluorine-exposed workers of an aluminum company’s occupational health checkup in the south of the Yangtze River through the whole group random sampling method, and found that patients with fluorine bone damage have abnormal cardiac function and DNA damage of peripheral lymphocytes [4]. Fluorosis is not only manifested in bone-phase damages, such as fluorosis and dental fluorosis [5], but also in non-bone-phase systems, such as kidney, liver, and nervous system [6-8]. Excessive fluoride exposure in the human body will cause the corresponding factors in the body to react with external compounds, and the corresponding signaling pathways will be activated to participate in the pathogenesis of fluorosis. Signaling pathway is an important and complex mechanism in the pathogenesis of fluorosis, which usually consists of multiple signaling pathways forming a complex network of multi-layer cross-regulation to regulate cell differentiation and apoptosis as well as alteration of cellular activity and function, thus showing different degrees of non-osteopathic damage. Therefore, it is important to recognize the mechanism of non-osteopathic damage in fluorosis, which can help to provide a strategy and basis for the prevention and treatment of fluorosis.
Expression of signaling pathways in the non-bone phase of fluorosis
MAPK signaling pathway: MAPK is a group of serine threonine kinases that can be activated by stimulation of factors external to the cell. It is a series of phosphorylation steps from MAPKKK (MKK) to MAPKK (MAPK) and back to MAPK, phosphorylating the related transcription factors, and is mainly involved in signaling from the surface of the cell membrane to the nucleus [9]. There are three key members in MAPK, namely, the extracellular signal-regulated protein kinase (ERK1/2), c-jun amino-terminal kinase (JNKS) and p38, experiments have shown that the MAPK signaling pathway is involved in the mechanism of fluorosis-induced non-bone phase injury. In rats suffering from chronic fluorosis, researchers found learning and memory deficits as well as many neuropathological changes in the brain, including cytoplasmic condensation, eosinophilia, and increased expression of extracellular regulated protein kinases (ERK1/2), members of the MAPK signaling pathway [10,11]. A cellular assay showed that in the presence of fluoride and MAPK inhibitors, mineralization levels were reduced in LS8 cells, with a more pronounced decrease in p38, which may be related to the inhibition of JNK and p38 phosphorylation [12]. In addition, oxidative stress is considered to be an important mechanism in the pathogenesis of fluorosis [13]. p38 is present in most animal cells, and it serves as an important signaling pathway that can participate in the oxidative stress response, modulate inflammatory factors, and is present in a wide range of physiopathologies [14], and activation of p38 phosphorylates relevant transcription factors, which in turn leads to alterations in gene expression [15]. In an animal experiment exploring the molecular mechanisms of brain damage induced by chronic fluorosis, it was found that chronic exposure to high doses of fluoride induced neurotoxicity in the rat brain through alterations in oxidative stress [16]. Another experimental study demonstrated that excessive fluoride exposure resulted in a significant increase in oxidative stress indicators such as malondialdehyde (MDA) in rats [17]. From this, it can be inferred that p38 may be involved in the expression of oxidative stress through the MAPK pathway in fluoride intoxicated non-bone phase organs.
NF-κB signaling pathway: Nuclear factor kappa B (NF κB) is a group of proteins responsible for regulating cytokine production and survival and participating in the expression of target genes [18], as well as the expression of inflammatory factors in inflammatory responses [19].NF-κB, as a multisubunit transcription factor, consists of dimers of five members of the ReI family, including mainly p65 and p50 [20].NF-κB signaling pathway activation is generally categorized into classical and non-classical pathways. The classical pathway refers to the inactivation of subunit dimers in the NF-κB signaling pathway through the inactivation of the NF-κB signaling pathway inhibitory protein (IκB), which is phosphorylated by inhibitory enzymes and then releases these dimers in the nucleus, thereby regulating the release of inflammatory factors; the non-classical pathway refers to the release of inflammatory factors that have a certain role in facilitating the activation of the NF-κB signaling pathway [21]. The activation of the NF-κB signaling pathway is inextricably linked to the non-bone phase pathogenesis of fluorosis. In the process of exploring the mechanism of brain injury in chronic fluorosis, the experimental correlates found that the expression of receptor for advanced glycosylation end products (RAGE) and nuclear factor-kB (NF-κB) in the brain of rat hippocampus was significantly increased after the establishment of a rat model of chronic fluorosis and administration of a small dose of fluoride [22]. Another animal experiment exploring the neurological effects of fluoride found that fluoride induced S-phase cell cycle arrest and up-regulated NF-κB protein in rat primary hippocampal neurons [23,24]. Observation of the effects of chronic fluoride poisoning on the rat kidney revealed that NF-κB expression was upregulated in the fluoride-treated experimental animals compared with the control group, which in turn caused some degree of pathological damage to the kidney [25].
Wnt signaling pathway: Wnt signaling is a combination of two names for the same gene, the Drosophila segment polarity gene as well as the mouse proto-oncogene Int1 [26].The Wnt signaling pathway mainly consists of the classical pathway, which refers to the Wnt/β-catenin pathway, and the non-classical pathway, which refers to the β-catenin-dependent pathway, and can be further divided into the Wnt/ planar cell polarity (PCP) and calcium pathways [27]. The secreted proteins of the family include 19 cysteine-rich glycoproteins i.e. 19 family members.Wnt signaling is essential for developmental processes, including cell proliferation and differentiation, and the Wnt signaling pathway plays an important role in embryogenesis, tissue homeostasis and regeneration in many organs [28]. Secreted Wnt ligands, which are highly conserved signaling molecules are involved in signal reception with gene expression, adhesion and migration in cells [29]. It has been reported that fluoride can alter oxidative stress and inflammatory changes in microglia by inhibiting the Wnt signaling pathway and consequently [30], suggesting that the Wnt signaling pathway is also involved in fluorosis target organogenesis. Another in vitro experiment showed that Wnt protein expression was significantly up-regulated in mouse enamel cell lineage cells (ALC) infiltrated with sodium fluoride (NaF), which may promote the activation of Wnt signaling pathway and thus participate in cell apoptosis and injury [31]. Domestic scholars exploring the renal pathogenesis caused by chronic fluorosis found that fluorine can stimulate the activation of the Wnt signaling pathway and induce renal injury [32], the relationship between fluorine and the Wnt signaling pathway at home and abroad is less studied, and it is necessary for scholars to further deepen their understanding of this signaling pathway under the pathogenesis of fluorosis.
Hedgehog signaling pathway: Hedgehog signaling pathway (Hh), or hedgehog signaling pathway, which is a pathway of signaling from cell membrane to cell nucleus, is highly conserved from Drosophila to human and plays an irreplaceable role in embryonic development. In vertebrates, three members of the Hh gene family have been detected: the Sonic hedgehog (SHh), Indian hedgehog (IHh), and Desert hedgehog (DHh) genes [33,34], all three of which activate the Hh signaling pathway by binding to the corresponding receptors [35]. Hh signaling activation involves three proteins: hedgehog (Hh) ligand, patching (Ptch), and smoothing (Smo), and Hh is a glycan. Hh is a glycoprotein that binds to the patch (Ptch) receptor on the cell membrane and activates the effector Gli1 to alter the structure of the receptor [36]. A study has shown that upregulation of Hh signaling in osteoblasts accelerates bone resorption [37], which is very similar to the mechanism of bone phase damage in fluorosis, and some researchers have found that the Hh signaling pathway also exhibits importance in the pathogenesis of bone phase due to fluorosis [38]. However, the Hh signaling pathway has also been reported in the pathogenesis of fluorosis in the non-bone phase, and an animal study found that significant differences in the expression of Ihh, Smo, and Gli1 proteins and mRNAs under the Hh signaling pathway were measured in the liver tissues of rats after high doses of fluoride were ingested [39]. In addition, another study showed that in a rat model of fluorosis, disorganized and swollen hepatocytes were observed in the fluoride-infected group, and Ihh and Gli1 protein expression were significantly upregulated by PCR compared with the control group, which led to the conclusion that the Hh signaling pathway may be a therapeutic target in the pathogenesis of fluorosis [40].
Interaction of signaling pathways in the pathogenesis of fluorosis
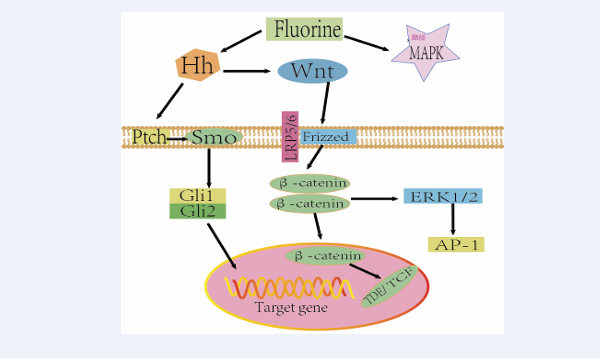
Not only one signaling pathway plays a role in the pathogenesis of fluorosis in the non-osteopathic phase, but often multiple signaling pathways work together to form a complex network of multi-layer cross-regulation, which transduces stimuli from signaling molecules in the extracellular environment to the cell and responds to extracellular signals in response to changes in the external environment [41]. The crosstalk between the signaling pathways of Hh and Wnt has been confirmed by studies [42,43], in addition, they are both involved in the non-bone phase mechanism of fluorosis, and studies have shown that the Wnt signaling pathway is downstream of the Hh signaling pathway [44]. Fluoride has been reported to stimulate the expression of IHH and Smo proteins as well as Gli2 transcription factors in the Hh signaling pathway [38]. Generally speaking, ptch receptors inhibit the expression of Smo proteins when the Hh signaling pathway is not activated, however, when this signaling pathway is activated Smo proteins will not be regulated by ptch receptors, and the accumulation of Smo receptors to a certain amount will promote the expression of Glis transcription factors, which in turn will promote the expression of target genes such as anti apoptotic genes Bcl-2 and pro-apoptotic genes such as Bax, Bad, etc. [45 ,46]. When cells are stimulated by external fluoride activates the Wnt signaling pathway, the extracellular Wnt factor binds to the membrane receptor frizzled protein (Frizzled), lipoprotein-related receptor 5/6 (LRP5/6) and further affects the degradation of β-catenin protein in the classical pathway [47], β-catenin protein continues to accumulate, and when it accumulates to a certain degree into the cell nucleus activate the transcription factor LEF1/TCF-1,and finally act on the expression of target genes [48,49], in addition, the ERK pathway in the MAPK signaling pathway activated by Wnt signaling can be developed on β-catenin protein [50], and the relationship between them is shown in Figure 1.
Figure 1: Interactive regulatory network of Wnt, Hh, and MAPK signaling pathways in fluorosis.
Summary and Outlook
China is a large country with endemic fluorosis. Over the years, under the strong support of the Party and the State, the incidence rate of fluorosis patients has decreased a lot in comparison with the past, and a certain degree of intervention measures have been carried out mainly from the sources of drinking water, coal burning and tea drinking. In recent years, the research on the non-bone phase damage of fluoride mainly focuses on the liver, kidney, reproductive system, nervous system and cardiovascular system, etc. Although many scholars have verified or explored the effects of fluoride on the body through in vivo and in vitro experiments, no matter from the molecular epidemiological level of the experiments or the human body field epidemiological to explore the pathogenesis or mechanism of its action, but from the point of view of the mechanism research is not very clear. In this paper, we reviewed the four signaling pathways of NF κB, MAPK, Wnt and Hh and their interaction mechanisms in the non-osteopathic phase of fluorosis, which provides theoretical guidance for exploring effective targets for the prevention and treatment of non-osteopathic phase of fluorosis.
Funding
2019QZKK0607
Authors’contributions
R.Y. made a substantial to literature review, H.S, G.P, Y.L, J.H, M.W, X.C, P.C, J.M, L.Q, Y.Z, S.Zand Q.Z conceptualized the study.
REFERENCES
1. SINGH G, KUMARI B, SINAM G, et al. Fluoride distribution and contamination in the water, soil and plants continuum and its remedial technologies, an Indian perspective– a review[J]. Environmental Pollution, 2018, 239(AUG.): 95-108.
2. ZHANG L, HUANG D, YANG J, et al. Probabilistic risk assessment of Chinese residents’ exposure to fluoride in improved drinking water in endemic fluorosis areas[J]. Environmental Pollution, 2017, 222(mar.): 118-125.
3. HUANG Yinsheng, MU Xujian, SHENG Gaoyang et al. Retrospective analysis of neurological impairment in patients with chronic fluorosis[J]. China Endemic Disease Control,2023,38(05):412-414.
4. LIU Chenxu, LI Ling, LI Lifen et al. Clinical significance of changes in cardiac function and peripheral lymphocyte DNA damage in patients with chronic fluorotic bone injury[J]. China Endemic Disease Control,2022,37(04):359-360.
5. DO L G, SPENCER A J. Risk-benefit balance in the use of fluoride among young children.[J]. Journal of Dental Research, 2007, 86(8): 723-728.
6. TIAN Wei, ZHAO Anjuku, YU Yanqin, et al. Relationship between IQ and urinary fluoride in children with coal-contaminated endemic fluorosis[J]. Chinese Journal of Endemic Diseases, 2022, 41(2): 3.
7. Mao Chunyan, Guan Zhizhong, Wu Changxue, et al. Analysis of DNA methylation in peripheral blood of patients with coal-contaminated endemic fluorosis[J]. Chinese Journal of Endemic Diseases, 2021, 40(2): 6.
8. JIANG Ruifeng, ZHANG Lifang, YAN Juzhen. Expression of transforming growth factor β1, aquaporin and osteoblastin in kidney tissues of rats with fluorosis[J]. Chinese Journal of Endemic Diseases, 2021, 40(7): 5.
9. HIRT C. Complexity, Cross Talk and Integration of Plant MAP Kinase Signalling[J]. Current Opinion in Plant Biology, 2002.
10. WANG Shengyuan[1], QIU Zhiwei[1], GUAN Zhizhong[1 2], et al. Study on the protective effect and mechanism of chondroitin sulfate on experimental chronic fluorosis brain damage in rats[J]. Chinese Journal of Endemic Diseases, 2018, 37(4): 7.
11. Yang Hongrui. The regulatory role of ERK-MAPK pathway in fluorine induced synaptic plasticity injury in hippocampal neurons[D]. North China University of Science and Technology.
12. B J J A, A F Y, A M Y, et al. P38/JNK signaling pathway mediates the fluoride-induced down-regulation of Fam83h[J]. Biochemical and Biophysical Research Communications, 2016, 471(3): 386-390.
13. FENG Jing, TIAN Xiaolin, DONG Nisha, et al. Progress of fluorine induced stress apoptosis in endoplasmic reticulum[J]. Environmental and Occupational Medicine, 2018, 35(6): 6.
14. ZHANG H, YUAN B, HUANG H, et al. Gastrodin induced HO-1 and Nrf2 up-regulation to alleviate H2O2-induced oxidative stress in mouse liver sinusoidal endothelial cells through p38 MAPK phosphorylation[J/OL]. Brazilian Journal of Medical and Biological Research = Revista Brasileira De Pesquisas Medicas E Biologicas, 2018, 51(10): e7439. https://doi.org/10.1590/1414-431X20187439.
15. HADJAL Y, HADADEH O, YAZIDI C E I, et al. A p38MAPK-p53 cascade regulates mesodermal differentiation and neurogenesis of embryonic stem cells[J/OL]. Cell Death & Disease, 2013, 4: e737. https://doi. org/10.1038/cddis.2013.246.
16. RAN L Y, XIANG J, ZENG X X, et al. Integrated transcriptomic and proteomic analysis indicated that neurotoxicity of rats with chronic fluorosis may be in mechanism involved in the changed cholinergic pathway and oxidative stress[J/OL]. Journal of trace elements in medicine and biology: organ of the Society for Minerals and Trace Elements (GMS), 2021, 64: 126688. https://doi.org/10.1016/j. jtemb.2020.126688.
17. ZHONG N, YAO Y, MA Y, et al. Effects of Fluoride on Oxidative Stress Markers of Lipid, Gene, and Protein in Rats[J/OL]. Biological Trace Element Research, 2021, 199(6): 2238-2246. https://doi. org/10.1007/s12011-020-02336-z.
18. TANIGUCHI K, KARIN M. NF-κB, inflammation, immunity and cancer: coming of age[J]. Nature reviews. Immunology, 2018.
19. LIAO X, LI Y. Nuclear Factor Kappa B in Autism Spectrum Disorder: A Systematic Review[J/OL]. Pharmacological Research, 2020, 159: 104918. https://doi.org/10.1016/j.phrs.2020.104918.
20. PULIYAPPADAMBA V T, HATANPAA K J, CHAKRABORTY S, et al. The role of NF-κB in the pathogenesis of glioma[J/OL]. Molecular & Cellular Oncology, 2014, 1(3): e963478. https://doi.org/10.4161/23 723548.2014.963478.
21. IANNETTI A, LEDOUX A C, TUDHOPE S J, et al. Regulation of p53 and Rb links the alternative NF-κB pathway to EZH2 expression and cell senescence[J/OL]. PLoS genetics, 2014, 10(9): e1004642. https:// doi.org/10.1371/journal.pgen.1004642.
22. Zhang KL. Expression of advanced glycation end-product receptor and nuclear factor κB in the cerebral hippocampus of rats with chronic fluorosis[J]. China Medical Abstracts: Internal Medicine (English Edition), 2014(1): 1.
23. ZHANG J, ZHU W J, XU X H, et al. Effect of fluoride on calcium ion concentration and expression of nuclear transcription factor kappa-B ρ65 in rat hippocampus[J/OL]. Experimental and Toxicologic Pathology: Official Journal of the Gesellschaft Fur Toxikologische Pathologie, 2011, 63(5): 407-411. https://doi.org/10.1016/j. etp.2010.02.017.
24. ZHANG M, WANG A, XIA T, et al. Effects of fluoride on DNA damage, S-phase cell-cycle arrest and the expression of NF-kappaB in primary cultured rat hippocampal neurons[J/OL]. Toxicology Letters, 2008, 179(1): 1-5. https://doi.org/10.1016/j.toxlet.2008.03.002.
25. ZHANG Kai-lin LOU Di-dong KUAN Zhi-zhong. Alterations of transmembrane transport proteoglycan-4 and cytokine-KB in the kidney of rats with chronic fluorosis[J]. Chinese Journal of Endemic Diseases, 2013, 032(002): P.133-135.
26. GAJOS-MICHNIEWICZ A, CZYZ M. WNT Signaling in Melanoma[J/OL]. International Journal of Molecular Sciences, 2020, 21(14): E4852. https://doi.org/10.3390/ijms21144852.
27. GRUMOLATO L, LIU G, MONG P, et al. Canonical and noncanonical Wnts use a common mechanism to activate completely unrelated coreceptors[J/OL]. Genes & Development, 2010, 24(22): 2517-2530. https://doi.org/10.1101/gad.1957710.
28. KAHN M. Wnt Signaling in Stem Cells and Cancer Stem Cells: A Tale of Two Coactivators[J/OL]. Progress in Molecular Biology and Translational Science, 2018, 153: 209-244. https://doi.org/10.1016/ bs.pmbts.2017.11.007.
29. STEINHART Z, ANGERS S. Wnt signaling in development and tissue homeostasis[J/OL]. Development (Cambridge, England), 2018, 145(11): dev146589. https://doi.org/10.1242/dev.146589.
30. CHEN R, ZHAO L D, LIU H, et al. Fluoride Induces Neuroinflammation and Alters Wnt Signaling Pathway in BV2 Microglial Cells[J/OL]. Inflammation, 2017, 40(4): 1123-1130. https://doi.org/10.1007/ s10753-017-0556-y.
31. SHUSTERMAN K, GIBSON C W, LI Y, et al. Wnt-RhoA Signaling Pathways in Fluoride-Treated Ameloblast-Lineage Cells[J/OL]. Cells Tissues Organs, 2014, 199(2-3): 159-168. https://doi. org/10.1159/000367840.
32. ZHANG Ying, YU Yanni, FAN Bin. Expression of Wnt signaling pathway in fluorosis kidney and its significance[J]. China Public Health, 2015, 31(5): 4.
33. KRAUSS S, CONCORDET J P, INGHAM P W. A functionally conserved homolog of the Drosophila segment polarity gene hh is expressed in tissues with polarizing activity in zebrafish embryos.[J]. Cell, 1993, 75(7): 1431-1444.
34. MARIGO V, TABIN C J. Regulation of patched by sonic hedgehog in the developing neural tube[J/OL]. Proceedings of the National Academy of Sciences of the United States of America, 1996, 93(18): 9346-9351. https://doi.org/10.1073/pnas.93.18.9346.
35. PATHI S, PAGAN-WESTPHAL S, BAKER D P, et al. Comparative biological responses to human Sonic, Indian, and Desert hedgehog[J/ OL]. Mechanisms of Development, 2001, 106(1-2): 107-117. https:// doi.org/10.1016/s0925-4773(01)00427-0.
36. VARJOSALO M, TAIPALE J. Hedgehog: functions and mechanisms[J/ OL]. Genes & Development, 2008, 22(18): 2454-2472. https://doi. org/10.1101/gad.1693608.
37. MAK K K, BI Y, WAN C, et al. Hedgehog signaling in mature osteoblasts regulates bone formation and resorption by controlling PTHrP and RANKL expression[J/OL]. Developmental Cell, 2008, 14(5): 674-688. https://doi.org/10.1016/j.devcel.2008.02.003.
38. DENG C, XU L, ZHANG Y, et al. The value of the hedgehog signal in osteoblasts in fluoride-induced bone-tissue injury[J/OL]. Journal of Orthopaedic Surgery and Research, 2021, 16(1): 160. https://doi. org/10.1186/s13018-021-02287-8.
39. ZHAO Li-Na, YU Yan-Ni, ZHU Zhi-Jian, et al. Expression and significance of hedgehog signaling pathway in the liver of rats with fluorosis[J]. China Public Health, 2015, 31(9): 5.
40. ZHAO L, YU Y, DENG C. [expression of sonic hedgehog signaling pathw ay and its inhibition by cyclopamine in rat liver with chronic fluorosis][J]. Zhonghua Bing Li Xue Za Zhi = Chinese Journal of Pathology, 2014, 43(12): 814-819.
41. PETROVA R, JOYNER A L. Roles for Hedgehog signaling in adult organ homeostasis and repair[J/OL]. Development (Cambridge, England), 2014, 141(18): 3445-3457. https://doi.org/10.1242/dev.083691.
42. MARCELLE C, STARK M R, BRONNER-FRASER M. Coordinate actions of BMPs, Wnts, Shh and noggin mediate patterning of the dorsal somite[J/OL]. Development (Cambridge, England), 1997, 124(20): 3955-3963. https://doi.org/10.1242/dev.124.20.3955.
43. MAEDA O, KONDO M, FUJITA T, et al. Enhancement of GLI1 transcriptional activity by beta-catenin in human cancer cells[J]. Oncology Reports, 2006, 16(1): 91-96.
44. HU H, HILTON M J, TU X, et al. Sequential roles of Hedgehog and Wnt signaling in osteoblast development[J/OL]. Development (Cambridge, England), 2005, 132(1): 49-60. https://doi.org/10.1242/dev.01564.
45. INGHAM P W, MCMAHON A P. Hedgehog signaling in animal development: paradigms and principles[J/OL]. Genes & Development, 2001, 15(23): 3059-3087. https://doi.org/10.1101/gad.938601.
46. ATHAR M, LI C, KIM A L, et al. Sonic hedgehog signaling in Basal cell nevus syndrome[J/OL]. Cancer Research, 2014, 74(18): 4967-4975. https://doi.org/10.1158/0008-5472.CAN-14-1666.
47. MACDONALD B T, HE X. Frizzled and LRP5/6 receptors for Wnt/β catenin signaling[J/OL]. Cold Spring Harbor Perspectives in Biology, 2012, 4(12): a007880. a007880. https://doi.org/10.1101/cshperspect.
48. WISNIEWSKA M B. Physiological role of β-catenin/TCF signaling in neurons of the adult brain[J/OL]. Neurochemical Research, 2013, 38(6): 1144-1155. https://doi.org/10.1007/s11064-013-0980-9.
49. TIMM A, GROSSCHEDL R. Wnt signaling in lymphopoiesis[J/OL]. Current Topics in Microbiology and Immunology, 2005, 290: 225 252. https://doi.org/10.1007/3-540-26363-2_10.
50. YUN M S, KIM S E, JEON S H, et al. Both ERK and Wnt/beta-catenin pathways are involved in Wnt3a-induced proliferation[J/OL]. Journal of Cell Science, 2005, 118(Pt 2): 313-322. https://doi.org/10.1242/ jcs.01601.