Mitigative Potentials of 5-Methoxyl-N-Acetyltryptamine (Melatonin) on Bisphenol a-Induced Epigenetic Changes in Wistar Rats
- 1. Department of Environmental Health Technology, Ekiti State College of Health Sciences and Technology, Ijero Ekiti, Nigeria
- 2. Department of Environmental Health Sciences, Faculty of Public Health, College of Medicine, University of Ibadan, Nigeria
Abstract
Introduction: Bisphenol A (BPA), as an endocrine disruptor, found in packaging materials for food and drugs is of public health concern. It accounts for the majority of daily human exposure. Health problems associated with BPA exposure in humans includes reproductive issues, changes in hormone levels and various cancers. Recognized therapeutic effects of melatonin have been established but limited study has been conducted on their protective potentials on BPA toxicity therefore this study was carried out to investigate the effect of 5-methoxy-N-acetyl-tryptamine on bisphenol a induced epigenetic changes in wistar rats.
Methods: Thirty five (35) adult albino rats was distributed into seven (7) groups of five (5) rats each: G1 (control), G2 (0.05 mg/kgbw/dy of BPA), G3 (10 mg/kgbw/dy daily of BPA) G4 (0.05 mg/kgbw/dy each of BPA and Melatonin), G5 (10 mg/kgbw/dy daily each of BPA and Melatonin), G6 (0.05 mg/kgbw/dy of Melatonin), G7(10 mg/kgbw/dy of Melatonin). Treatments were administered orally for 30 days. Haematological indices; haematocrit and hemoglobin were analysed. Histopathological examination of the testes, liver and brain were carried out. Epigenetic changes were measured using global DNA methylation kits. The results were analysed using descriptive statistics and ANOVA at α0.05.
Results: Haematocrit (26.9 ± 4.7%) and haemoglobin (8.98 ± 1.57 g/dl) were significantly reduced in G3 when compared with the control group (54.2 ± 3.8% and 18.06 ± 1.28 g/dl), respectively. These effects were considerably mitigated in G4 (44.70 ± 6.20% and 13.56 ± 2.06 g/dl) and G5 (42.3 ± 4.4% and 14.10 ± 1.47 g/dl), respectively. GSH was significantly reduced in G3 but this effect was considerably corrected in G4 and G5. The G3 (5.16 ± 0.12 nM) showed higher methyl group, while lower distributions of methyl group were observed in G4 (4.16 ± 0.31 nM), G5 (4.01 ± 0.11 nM) and G7 (3.03 ± 0.11 nM), respectively. There was severe portal and sinusoidal congestion, with degeneration of hepatic cells in the liver, mild congestion of the testicular vessel and a hemorrhage of the meninges with congestion of the capillaries in the brain of G3, while these effects were completely mitigated in G5.
Conclusion: The result of this study showed that melatonin demonstrated mitigative potentials on bisphenol a toxicity and prevented epigenetic changes in wistar rats.
KEYWORDS
- Bisphenol A
- 5-methoxy-N-acetyltryptamine
- Wistar rats
- Epigenetic changes
- Toxicity
CITATION
Dada A, Okareh O (2025) Mitigative Potentials of 5-Methoxyl-N-Acetyltryptamine (Melatonin) on Bisphenol a-Induced Epigenetic Changes in Wistar Rats. Ann Public Health Res 12(1): 1135.
BACKGROUND OF THE STUDY
One of the setbacks of living in modern and industrialized society is when we continuously exposed to synthetic compounds that can imitate body hormones and interfere with our endocrine glands, leading to a variety of health issues [1]. Everyday items like drinks, medicines, pesticides, cosmetics, plastics, detergents, electronics, flame retardants, personal hygiene products, and toys expose humans to hormone-disrupting chemicals like Bisphenol A (BPA). Bisphenol A was discovered by Aleksandr Dianin from Russia in the year 1891, when he combined acetone and phenol in addition to a known catalytic acid [2,3]. BPA is used in the production of Polycarbonate polymers with remarkable strength and stability and they are highly valuable when used as components of safety equipment such as face shields, guards, helmets, and impact-windows [4,5]. As a xenoestrogen, bisphenol A mimics the effects of estrogen and has hormone-like qualities, while the effects are not harmful, it is of concern how many things are made with bisphenol A. Since 2008, a number of nations and governments have verified its safety, leading some distributors to stop carrying polycarbonate items [6]. Bisphenol A is utilized in the manufacturing of internal coating resins and plastics, both of which have several applications. Some of these include baby feeding bottles, flash drives and food or beverage packaging materials. Certain dental composites and sealants may also increase a person’s exposure to bisphenol A [6,7].
The main ways at which humans are exposed to bisphenol A are through food, dust, and water consumption, other routes include skin penetration and inhalation of gasses or particulate matter in the air [8, 9]. Pregnant women and their unborn children are primarily affected by bisphenol A, when it is transferred from the mother through the placenta to the child, but the effects of exposure may be delayed). BPA has been found in fetal serum, human placental tissue, and to penetrate the placental barrier in rodents like rats and mice [10,11]. Numerous studies also support the idea that it may increase the risk of developing other non-transferable diseases [5,12,13]. The daily exposure of the human body to 10 μg of bisphenol A is known to occur in many bodily fluids, such as urine, breast milk, and semen [14]. It has been found that bisphenol A interferes with the activities and production of androgen hormones and has a poor tendency to connect the estrogen α and β receptor [1516]. Additionally, it has been documented to affect tight junction protein production and location, which compromises Sertoli cell function [17]. Moreover, it has been demonstrated that BPA has epigenetic consequences, such as DNA hypomethylation [18].
While maintaining the DNA sequence, epigenetic investigates the impact of the environment on a person’s genetic makeup. Cells can respond and adapt to environmental stresses through the regulation of suppressing gene expression by epigenetic mechanisms, which are activated by exposures to environmental stressors [19]. Epigenetic changes are strong indicators that there may be a connection between exposure to the environment and changes in gene expression that may lead to illness. In essence, an epigenetic effect is the result of chemical exposure that is passed down to subsequent generations. The gene sequence is unaffected by epigenetic influences. They have an impact on gene expression, therefore determining the expression profile of genes that are susceptible to epigenetic influences could be utilized as a biomarker for illness and exposure to the environment [20]. Animal studies have demonstrated the impact of environmental epigenetics in vulnerability to diseases [18- 21]. Melatonin (5-methoxy-N-acetyltryptamine) is produced originally from the pineal gland as a natural hormone [12]. Moreover, melatonin can be produced by various organs like the skin, brain, gut, eyes, and immune system cells [12,22]. The gastrointestinal system also produces melatonin. The body’s numerous metabolic activities are regulated by melatonin. Superoxide dismutase (SOD) and anti-oxidative enzymes can be stimulated by melatonin to minimize oxidative stress [23]. Furthermore, melatonin has the ability to shield lipids, proteins, and DNA [24, 25]. Despite the large number of studies demonstrating that environmental chemicals induce epigenetic changes, a clear link to adverse health outcome and mechanisms underlying such changes are rarely addressed. There are data showing that exposure to BPA may have effects on obesity and diabetes but significant knowledge gaps exist as to associations between exposures to BPA and their effect on DNA and other body indices, thus, this study investigates the mitigative effects of melatonin on bisphenol A-induced epigenetic changes in wistar rats.
METHODS
Animal Model
The study was experimental in design. Animal models (wistar strain albino rat) were obtained from a standard animal house at the Institute of Advanced Medical Research and Training (IAMRAT), College of Medicine, University College Hospital, Ibadan. The rats were authenticated by veterinarian to ascertain that the animals are in good conditions. They were obtained at three weeks old and placed on commercial rat pellet and water until a weight range between 120g-140g was obtained.
Chemical (Bisphenol A) and Melatonin (MEL) (5-methoxy-N-acetyltryptamine)
Bisphenol A (CAS No. 1478-61-1, 98% purity) was purchased from ADLAK chemical company in Onisha, Anambra State, Nigeria and raw melatonin (CAS No. 597- 10-1) was purchased from CELL BIOLAB INC, Ilorin, Kwara State, Nigeria.
Preparation of Stock and Working Concentration of BPA and Melatonin
Bisphenol A (5 mg) was dissolved in 100 ml of distilled water to give a concentration of 50 mg/l. Stock solution (1 ml) was dispensed in 9 ml of distilled water, thoroughly mixed. This was 1:10 dilution factor. It was kept in a refrigerator. Three serial dilutions were performed from the stock solution to obtain 0.05 mg. To obtain 10 mg working concentration, a serial dilution was performed by obtaining 2 ml from the stock concentration and dispensed in 8 ml of distilled water.
Procedure for Administration
The wistar strain albino rats (35) were distributed into 7 groups (G) of 5 rats each: G1 (control group), G2 (0.05 mg/kg.bw/dy of BPA), G3 (10 mg/kg.bw/dy daily of BPA), G4 (0.05 mg/kg.bw/dy of BPA and MEL), G5 (10 mg/kg.bw/dy of BPA and MEL), G6 (0.05 mg/kg.bw/dy of MEL), G7 (10 mg/kg.bw/dy of MEL). Treatments were administered orally for 30 days while maintaining the rat on pellets and distilled water. Daily weight and feed intake were recorded.
Collection of Blood Samples and Analysis
After 30 days of treatment administration, the rats were sacrificed through cervical dislocation and blood samples were collected through ocular puncture and preserved in both lithium heparin and ethylenediamine tetraacetic (EDTA) bottles. Heamatological indices (Table1) like Heamtocrit (HCT), heamoglobin count (HG) and procalcitonin (PLT) were analysed.
Table 1: Heamatological indices.
|
S. No |
Treatment Groups |
HCT (%) |
HGB (g/dl) |
PLT (×103 )MM |
|
1 |
Control |
54.20 ± 3.87 |
18.06 ± 1.29 |
800.00 ± 111.33 |
|
2 |
BPA (0.05mg) |
35.30 ± 8.69* |
11.76 ± 2.89* |
448.33 ± 122.86* |
|
3 |
BPA (10mg) |
26.90 ± 4.75* |
8.98 ± 1.58* |
307.00 ± 78.13* |
|
4 |
BPA + MEL (0.05mg) |
44.70 ± 6.20 |
13.56 ± 2.06 |
627.66 ± 138.74 |
|
5 |
BPA + MEL (10mg) |
42.30 ± 4.43 |
14.10 ± 1.47 |
581.66 ± 141.13 |
|
6 |
MEL (0.05mg) |
47.90 ± 1.51 |
15.96 ± 0.90 |
580.33 ± 126.30 |
|
7 |
MEL (10mg) |
48.36 ± 11.54 |
16.13 ± 3.91 |
692.66 ± 49.43 |
*P < 0.05, (n = 5)
HCT = Heamatocrit; HGB= Heamoglobin; PRT= Procalcitonin
(*) Indicate significant difference at P < 0.05 from the control group
Histopathological examination of the testes, liver and brain were carried out through standard analytical procedure. DNA methylation was measured using global DNA methylation kits.
Determination of Reduced Glutathione
Glutathione the levels of reduced glutathione (GSH) in the plasma of rat samples was determined by the method described by [26]. Plasma (0.5 ml) was deproteinised by the addition of an equal volume of 4% sulphosalicyclic acid (SSA) and centrifuged at 14000 g for 15 minutes. Thereafter, 0.5 ml of the supernatant was added to 4.5 ml of Ellman’s reagent. A blank was prepared with 0.5 ml of the diluted precipitating solution (diluted twice with phosphate buffer) and 4.5 ml of Ellman’s reagent. Reduced glutathione concentration in the sample was proportional to the absorbance at 412 nm.
Procedure for DNA Extraction from Blood Samples of Wistar Rats
Commercial DNA extraction kit (ZYMO-3700E, SA) was used to extract DNA from blood samples. Blood samples of 200 μL were transferred to zymo spin column and 400 μL of genomic lyses buffer was added which was cenfriguged at 100 rpm/min. The supernanant were discarded, followed by the addition of 200 μL of pre-wash buffer and centrifuged at 100 rpm/mins for 3-4 mins. The debris was discarded and 500 μL of DNA wash buffer was added. The extracted DNA samples was then stored in an eppendoff tube.
Procedure for DNA Methylation
The extracted DNA samples were diluted with 0.1–1 mg/ mL of water. Ice was used to quickly cool the DNA sample after it has been incubated at 95°C in hot water bath for five minutes in order to convert it to single-stranded DNA. In order to break down the DNA sample into nucleosides, the denatured DNA was incubated for two hours at 37ºC in 20 mM of Nuclease P1, and followed by addition of 5–10 units of alkaline phosphatase to break the phosphatase bond. The digested samples were then transferred into the ELISA plates that had been coated with 50 µL of the diluted anti-5MedCyd DNA conjugate antibody overnight. The mixture was stirred thoroughly, and was incubated on an orbital shaker at room temperature for two hours. 250 µL of 1X Wash Buffer was added to each plates after each wash were thoroughly aspirated. Then the wells were tapped on an absorbent pad after the last wash to get rid of extra 1X Wash Buffer. Secondary Antibody-HRP of 100 µL was added to every well, followed by addition of 100 µL of stop solution to halt the enzyme process. Spectrophotometer (SMP500-18658, USA) was used to measure the DNA methylation of each microwell.
Collection of Tissues for Histopathology
The rat models were slaughtered once blood samples were obtained. The cervical vertebrae are quickly disarticulated and the head was immobilized as part of the procedure. The base of the cranium was compressed firmly, and both of the forefinger and thumb were pinched and twisted. In order to give the animal a swift and painless death, the tail is simultaneously pulled back swiftly (Figure 1,2).
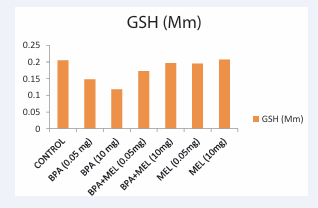
Figure 1: Level of oxidative stress across the groups. GSH: Glutathionec
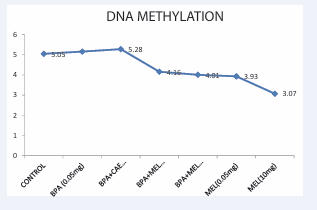
Figure 2: DNA Methylation of the Wistar rats across the groups.
The corpses were symmetrically sliced open, the brain, and testicles were removed, and they were then preserved in 10% formalin before being sent for histological examination.
RESULTS
Testes histopathology
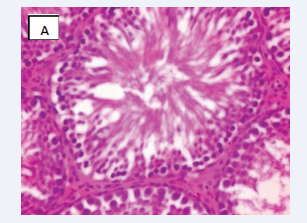
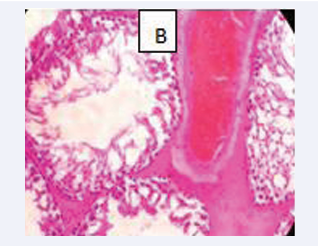
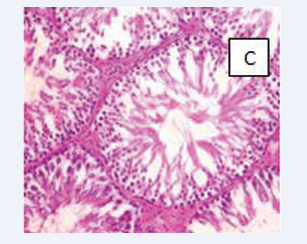
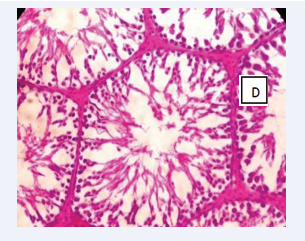
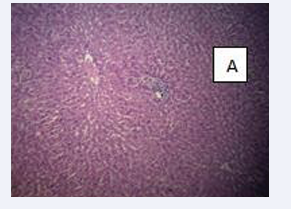
Photomicrograph of testicular section stained by Haematoxylin and Eosin (×400) (Figure 3-6).
Figure 3: CONTROL A-Normal testicular architecture with normal seminiferous tubules and insterstitial spaces contains normal leydig cells
Figure 4: BPA(10mg) B-Interstitial spaces show mild vascular congested vessel and Few normal seminiferous tubules showing normal maturation stage.
Figure 5: BPA (0.05 mg) C-The spermatogonia cells and the sertoli cells are normal.
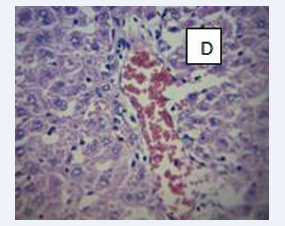
Figure 6: BPA+ MEL (10mg) D-The interstitial spaces show normal leydig cells andNormal maturation stages with presence of spermatozoa within their lumen
Brain histopathology
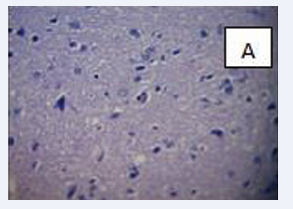
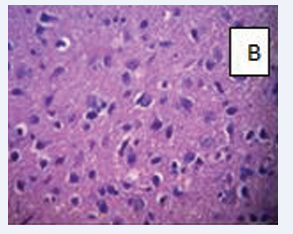
Photomicrograph of the brain section stained by Haematoxylin and Eosin (×400) (Figure 7-9.)
Figure 7: Control A- Normal brain parenchyma and no hemorrhage.
Figure 8: BPA+MEL (10mg) B- Normal brain parenchyma and no observable lesion.
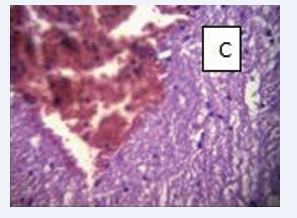
Figure 9: BPA (10mg) C-There is a severe hemorrhage of the meninges, with congestion of the cortical capillaries.
Liver Histopathology
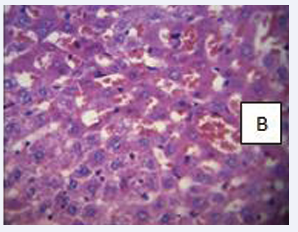
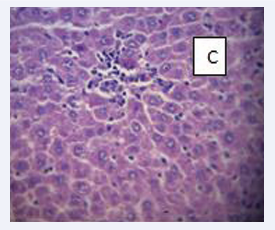
Photomicrograph of the liver section stained with haematoxylin and eosin (×400) (Figure 10-13).
Figure 10: Control A-No observable lesions.
Figure 11: BPA (0.05 mg) B- Mild hydropic degeneration and necrosis of hepatic cells.
Figure 12: BPA+MEL (10 mg) C- No observable lesions.
Figure 13: BPA (10 mg) D- Severe portal and sinusoidal congestion, with degeneration of hepatic cells.
DISCUSSION
The outcome of this study has provided data about the potential impact of bisphenol A administered at different doses on the haematological and biochemical indices in wistar rats. The reduction in the Haematocrit (HCT) and Haemoglobin (HGB) observed when 10 mg/kg of bisphenol A was administered suggested the occurrence of anemia in the wistar rats. This is confirmed in a study conducted by [27] when C. punctatus exposed to bisphenol A for 30 days had a significant decrease in its hemoglobin percentage, haematocrit, and red blood cell count. Bisphenol A was also reported to interfere with the synthesis of haemoglobin [28]. Also, the group concomitantly administered with melatonin and bisphenol A showed a substantial increase in most of the haematological indices assessed, hence melatonin played a significant role in mitigating the toxic effect exerted by the bisphenol A. This finding which aligns with a study conducted by [29] on protective effect of exogenous melatonin against neuroinflammation in high fat diet induced diabetic rats. This study also revealed that bisphenol A at moderately high doses increased Glutathione (GSH), suggesting that the cells’ levels of oxidative stress were higher in comparison with other exposed groups. The outcome of this study aligns with the findings of [30], who documented that exposure to bisphenol A causes oxidative damage. Another study showed that bisphenol A raised lipid peroxidation and lowered the activity of rat liver-produced antioxidant defense enzymes [31]. The increase in glutathione levels caused by bisphenol A could perhaps be attributed to its conjugation with hazardous derivatives of bisphenol A and subsequent oxidation to oxidized glutathione [32]. Also documented that administration of 20 mg/kg Bisphenol A resulted into oxidative stress, inflammatory gene expression, and histopathological changes in male wistar rats. However, in this study, administration of both bisphenol A and melatonin reduced the level of glutathione, an indication for the positive impact of the treatment on the bisphenol A toxicity. This is consistent with the study by [23] analyzing the ameliorative activities of melatonin on serobiochemical alterations and cardio- nephron diabetic vascular and cellular alterations.
This distribution of methyl group as a result of exposure to the chemicals was higher in the group exposed to high doses of bisphenol A while considerably lower distribution of methylation was documented in the groups co-administered with bisphenol A and melatonin This identifies the protective strength of melatonin against the DNA hypermethylation exerted by the toxic chemicals. The effect of bisphenol A is also confirmed in another study conducted by [33] in which wistar rats given a “secure” amount of bisphenol A for an extended period of time showed the a decrease in H3K9, and H4K12 histone acetylation, an increase in deacetylase Sirt1 expression accompanied by lower binding and, an increase in the binding of estrogen receptor β (ERβ) to caveolin-1 (Cav-1) [34]. However, according to a few scientists, early intake of bisphenol A may result in long-term damage to the prostatic epigenome, which could increase a person’s risk of developing cancer of the prostate [2, 35].
Testicular architecture was averagely normal in the group exposed to 10 mg/kg of bisphenol A, but interstitial spaces plus leydig vessels were clogged and few normal seminiferous tubular structure, indicating fairly normal testicular structure. All other groups displayed normal testicular architecture, and achieved healthy developmental stages. Both the sertoli and sperm cells are healthy having spermatozoa present in their lumen. This is consistent with a mouse study that found bisphenol A injection to damage testis shape, particularly the size of seminiferous tubules and the epithelium, which were both greatly reduced and showed various phases of spermatogenesis impairment [18].
In this study’s bisphenol A-exposed liver samples demonstrated detectable levels of vacuolar deterioration, sinusoidal dilation, and vascular congestion but no changes in the hepatic lobular structure which strongly suggests that bisphenol A can cause hepatocyte damage even with brief exposure at the current approved dosages, hence it indicated that wistar rats exposed to modest doses of bisphenol A orally develop liver damage. However, Treatment with 10 mg/kg ofboth bisphenol Aand melatonin in this study reduced the histopathology alterations caused by the toxic effect bisphenol A which is consistent with earlier research by [25] where, rats exposed to bisphenol A at doses of 50, 500, and 5000 mg/kg for 30 days had significantly higher levels of Triglycerides (TG) and total cholesterol levels while melatonin regulates antioxidant defense and inflammatory response by activating Nrf2- dependent mechanisms [22]. In another study, exogenous melatonin inhibits neuroinflammation in diabetic rats fed with high-fat diet, and promote hepatic functions [29], enhances anti-oxidative activity and moderately reduces the activities of the liver enzymes [24]. The liver may be more susceptible to low doses of bisphenol A compared to other organs [33]. This study also confirms that BPA at moderately high doses of 10mg/kg.bw causes severe hemorrhage of the meninges, with congestion of the cortical capillaries. This effect was completely mitigated by melatonin. Many studies on animals have shown that bisphenol A exposure has impacts on the development of the brain and behavior [36,37] and the ameliorative potentials of melatonin against brain related injuries caused by bisphenol A [38,39].
CONCLUSION
This study demonstrates that BPA at the doses used had a significant impact on the organs studied, heamopoiesis, and epigenetic modifications. Also the results of this study confirms that 5-methoxyl-N-acetyltryptamine (Melatonin) might have a promising role as detoxifier of bisphenol A toxicity, also reduces oxidative stress and protect DNA. The study also provides evidence for the potential use of melatonin as therapeutic interventions for bisphenol A toxicity. These natural substances may offer a complementary approach to conventional treatment or preventive strategies for other related health conditions and controlling oxidative stress. Therefore, knowing how bisphenol A functions and modifies bodily organs can help avoid and diagnose the ensuing health issues as well as develop more efficient methods and technologies for treating pathological conditions related to bisphenol A. It is therefore recommended that further researchneeds to be carried out to validate the ideal safe dose of exposure to bisphenol A and review the existing safe dose as provided by the European Food Safety Authority and Long-term studies are needed to assess the sustained mitigative effects and potential side effects of melatonin supplementation on bisphenol A toxicity. This will provide a more comprehensive understanding of their long term benefits and potential risks.
ACKNOWLEDGEMENT
The authors acknowledge all the laboratory assistant and technicians that assisted with analysis of blood samples and imaging of the tissues/organs.
Compliance with Ethical Guidelines
The study protocol was approved by UI Animal Care and Use Research Ethics (ACUREC) Committee with approval number: UI-ACUREC/071-0721/26.
Authors Contribution
The entire laboratory analysis was carried out by DADA A.O, but study design and project supervision was done by O. T. Okareh (Professor of Environmental Health Sciences).
REFERENCES
- EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids. Scientific opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs: Opinion on BPA. EFSA J. 2018; 13: 3978.
- Srivastava S, Dhagga N. Effect of bisphenol A on morphology and apoptosis in the mammary gland of adult Wistar rats. JOBAZ. 2019; 80: 20.
- Barali? K, Djordjevic A, Živan?evi? K, Antonijevi? E, An?elkovi? M, Javorac D, ?ur?i? M, Bulat Z, Antonijevi? B, ?uki?-?osi? D. Toxic effects of the Mixture of Phthalates and Bisphenol A-Sub acute Oral Toxicity Study in Wistar Rats. Int. J. Environ. Res. Public Health. 2020; 21: 121-136
- Molina-Molina J, Jiménez-Díaz I, Fernández M, Rodriguez-Carrillo A, Peinado F, Mustieles V, et al. Determination of bisphenol A and bisphenol S concentrations and assessment of estrogen- and anti- androgen-like activities in thermal paper receipts from Brazil, France, and Spain. Environ Res. 2019; 170: 406-415.
- Freire C, Molina-Molina M, Iribarne-Durán M, Jiménez-Díaz I, Vela- Soria F, Mustieles V, et al, Concentrations of bisphenol A and parabens in socks for infants and young children in Spain and their hormone- like activities. Environ Int. 2019; 127: 592-600.
- Ambreen S, Akhtar T, Hameed N, Ashfaq I, Sheikh N, In vivo evaluation of histopathological alterations and trace metals estimation of the small intestine in bisphenol A-intoxicated rats. Can. J. Gastroenterol. Hepatol. 2019; 17: 31-39.
- Dutta M, Paul G. Gallic Acid Protects Rat Liver Mitochondria Ex Vivo from Bisphenol A Induced Oxidative Stress Mediated Damages. Toxicol. Rep. 2019; 6: 578-589.
- Kazemi S, Mousavi S, Aghapour F, Rezaee B, Sadeghi F, Moghadamnia A. Induction Effect of Bisphenol A on Gene Expression Involving Hepatic Oxidative Stress in Rat. Oxid. Med. Cell. Longev. 2016; 16: 12-14
- Gao T, Yin Z, Wang M, Fang Z, Li J. The effects of pubertal exposure to bisphenol A on social behaviour in male mice. Chemosphere. 2020; 244:125-134
- Lombó M, Getino-Álvarez L, Depincé A, Labbé C, Herráez M, Embryonic exposure to bisphenol a impairs primordial germ cell migration without jeopardizing male breeding capacity. Biomolecules. 2019; 9: 307
- Hanan M A, Marwa A I, Samy A. Oxidative Stress and DNA methylation in male rat pups provoked by the transplacental and translactational exposure to bisphenol. AEVPR. 2020; 27: 4513-4519
- Guo R, The relationship between anesthesia and melatonin: A review. Front. Pharmacol. 2023; 14: 1255752.
- Chen H, Zhang Y, Zou M. et al, Bisphenol A aggravates renal apoptosis and necroptosis in selenium-deficient chickens via oxidative stress and PI3K/AKT pathway. J. Cell Physiol. 2022; 237: 3292-3304.
- Moreman J, Acute toxicity, teratogenic and estrogenic effects of Bisphenol A and its alternative replacements Bisphenol S, Bisphenol F and Bisphenol AF in zebrafish embryo-larvae. Environ. Sci. Technol. 2017; 51: b03283
- Santangeli S, Consales C, Pacchierotti F, Habibi H. and Carnevali O. Transgenerational effects of BPA on female reproduction. Sci. Total Environ. 2019; 685:1294-1305.
- Ikhlas S, Usman A, Ahmad M. In vitro study to evaluate the cytotoxicity of BPA analogues based on their oxidative and genotoxic potential using human peripheral blood cells. Toxicol. Vitr. 2019; 60: 229-236
- Batista-Silva K, Rodrigues K, de Moura N, Elie G, Van der Kraak C, Delalande F, Silva S. In vivo and in vitro short-term bisphenol A exposures disrupt testicular energy metabolism and negatively impact spermatogenesis in zebrafish. Reprod. Toxicol, 2022; 107: 10-21
- Lombó M, Fernández-Díez C, González-Rojo S, Herráez M, Genetic and epigenetic alterations induced by bisphenol A exposure during different periods of spermatogenesis: from spermatozoa to the progeny. Sci Rep. 2019; 9: 18029.
- Akhter A, Rahaman M, Suzuki T, Murono Y, Tokumoto T, Next- generation and further transgenerational effects of bisphenol A on zebrafish reproductive tissues. Heliyon. 2018; 4: e00788
- Ribas-Maynou J. and Benet J. Single and Double Strand Sperm DNA Damage: Different Reproductive Effects on Male Fertility. Genes (Basel). 2019; 10: 105
- González-Rojo S, Lombó M, Fernández-Díez C, Herráez M. Male exposure to bisphenol A impairs spermatogenesis and triggers histone hyperacetylation in zebrafish testes. Environ. Pollut. 2019; 248: 368-379.
- De-Melo I, Ferreira M, Da Silva Souza E, Almeida L, de Sá F, Neto C, de Castro P, Teixeira V, Melatonin regulates the expression of inflammatory cytokines, VEGF and apoptosis in diabetic retinopathy in rats. Chemico-Biological Interactions, 2020; 327: 109183.
- Alshariff K, Elmahallawy E, Alblihd M, Hamad A, Ali F. Melatonin ameliorates serobiochemical alterations and restores the cardio- nephro diabetic vascular and cellular alterations in streptozotocin- induced diabetic rats. Front Vet. Sci. 2023; 10: 1089733.
- Chang H.M, Lin HC, Cheng HL, Liao CK, Tseng TJ, Renn TY, Lan CT, and Chen Y. Melatonin successfully rescues the hippocampal molecular machinery and enhances anti-oxidative activity following early-life sleep deprivation injury. Antioxidants, 2021; 10: 774.
- Brazão V, Colato RP, Santello FH, Duarte A, Goulart A, Sampaio PA, Silva CB, Tirapelli CR, Costa RM, et al. Melatonin regulates antioxidant defense and inflammatory response by activating Nrf2-dependent mechanisms and inhibiting NFkappaB expression in middle-aged T. cruzi infected rats. Experimental Gerontology, 2022; 167: 111895
- Nuhu F, Seymour A, Bhandari S. Impact of intravenous iron on oxidative stressand mitochondrial function in experimental chronic kidney disease. Antioxidants 2019; 8: 498
- Rezaee-Tazangi F, Zeidooni L, Rafiee Z, Fakhredini F, Kalantari H, Alidadi H, et al. Taurine effects on bisphenol A-induced oxidative stress in the mouse testicular mitochondria and sperm motility. JBRA Assist Reprod. 2020; 24: 102-109
- Krishnapriya K, Shobana G, Narmadha S, Ramesh M. and Maruthappan V, Sublethal concentration of bisphenol A induces hematological and biochemical responses in an Indian major carp Labeo rohita. Ecohydrology and Hydrobiology. 2017; 17: 306-313.
- Maher AM, Salehm SR, Elguindy NM, Hashem HM, and Yacout GA. Exogenous melatonin restrains neuroinflammation in high fat diet induced diabetic rats through attenuating indoleamine 2, 3 dioxygenase 1 expression. Life Sciences, 2020; 247: 117427
- Li R, Luo X, Li L, Peng Q, Yang Y, Zhao L, Ma M, Hou Z. The protective effects of melatonin against oxidative stress and inflammation induced by acute cadmium exposure in mice testis. Biol. Trace Elem. Res. 2016; 170:152-164.
- Lin R, Jia Y, Wu F, Meng Y, Sun Q, Jia L. Combined Exposure to Fructose and Bisphenol A Exacerbates Abnormal Lipid Metabolism in Liver of Developmental Male Rats. Int. J. Environ. Res. Public Health. 2019; 16: 4152.
- Acaroz U, Ince S, Arslan-Acaroz D, Gurler Z, Demirel H, Kucukkurt I, Eryavuz A, Kara R. Bisphenol-A Induced Oxidative Stress, Inflammatory Gene Expression, and Metabolic and Histopathological Changes in Male Wistar Albino Rats: Protective Role of Boron. Toxicol. Res. 2019; 8: 262–269.
- Chen Z, Zuo X, He D, Ding S, Xu F, Yang H, et al, Long-term exposure to a “safe” dose of bisphenol A reduced protein acetylation in adult rat testes. Sci Rep. 2017; 7: 40337.
- D’Cruz C, Hao C, Labussiere M. Genome-wide distribution of histone trimethylation reveals a global impact of bisphenol A on telomeric binding proteins and histone acetyltransferase factors: a pilot study with human and in vitro data. Clin Epigenet 2022; 14: 186
- Burliba?a L, Ionescu AC, Dragusanu DM. Histone hyperacetylation and DNA methylation interplay during murine spermatogenesis. Zygote. 2019; 27: 305-14.
- Sinha B. Protection of melatonin in experimental models of newborn hypoxic-ischemic brain injury through MT1 receptor. J. Pineal Res. 2018; 64: e12443.
- Rybka V. Transmission electron microscopy study of mitochondria in aging brain synapses. Antioxidants 2019; 8: 171.
- Shen Q. Amelioratory effect of melatonin on cognition dysfunction induced by sevoflurane anesthesia in aged mice. Iran. J. Pharm. Res. 2022; 21: e133971
- Yang L. Melatonin improves neurological outcomes and preserves hippocampal mitochondrial function in a rat model of cardiac arrest. PLoS One 2018; 13: e0207098.