Rabies Outbreak in the Somali Region of Ethiopia: Public Health Significance and Potential of Canine Vaccination Using a one Health Approach
- 1. Swiss Tropical and Public Health, Switzerland
- 2. University of Basel, Switzerland
- 3. Jigjiga University, Ethiopia
- 4. Armauer Hansen Research Institute, Ethiopia
Abstract
Rabies is a neglected zoonotic disease that poses public health and economic challenges in developing countries. Rabies is endemic in Ethiopia causing 35 - 58 human deaths annually and resulting in 209 million USD in economic loss due to cattle death.
Method: From April to December 2023, an outbreak of rabies occurred in the Somali Regional State of Ethiopia (SRS). We collected rabies data from two districts of the Somali region, Awbare and Yocale districts, which represent agro-pastoral and pastoral areas of the region. We conducted a joint outbreak investigation, reviewing health facility data and collecting community data. To confirm the outbreak, brain samples were collected from animals (1 camel, 1 donkey, 1 cow and 1 dog) and tested at the Jigjiga Veterinary Regional Laboratory (JVRL) and the Ethiopian Public Health Institute (EPHI). In addition to the investigation, joint community awareness was conducted by the Health Bureau and Livestock Bureau regarding the transmission and public health consequences of the disease. To assess the cost-effectiveness of rabies control, dog vaccination costs, human hospitalization costs, and economic losses due to livestock death (ELLD) were calculated and compared.
Result: From May to December 2023, 34 people (Awbare 32, Yocale 2 people) were bitten by suspected rabid dogs, striped polecats, and camels. Thirty two victims were hospitalized and recovered, and two who did not seek health care or went to the hospital late died. The human attacking rate, or cumulative incidence, was
25.5 cases per 100,000 people (95% CI: 18.23_35.7/100,000), while the case fatality rate was 5.9% (95% CI: 0.015–0.22). Ninety one percent (91%) of human exposures were caused by dog bites, while cat, camel, and Ictonyx striatus caused 2.94% each. A majority of victims were male (70.59%) and children under 15 years old (57.14%). The most affected body parts were leg (50%), followed by hand or arm (23.53%), trunk (14.71%), and neck or head (11.76%). Twenty-eight animals, including camels, cattle, and donkeys, were killed or died following a reservoir host bite. Most of the animal suspected cases were caused by dogs (64.29%), followed by jackals (25%), striped polecat (3.57%) and unknown (7.14%). Our cost analysis estimated the PEP cost and ELLD at USD 6144 and USD 23601, respectively. In comparison, the cost required to vaccinate the entire dog population was significantly lower, estimated at USD 3’156. This outbreak also identified the first case of a camel bitten by a striped polecat testing positive for rabies in East Africa. Our economic analysis revealed that early response dog vaccination is economically advantageous and has the potential to reduce human and animal rabies exposure cases.
Conclusion and Recommendation: Our observation reveals that rabies is endemic in SRS, where it causes significant public health and economic costs. Rabies
vaccination is cost-effective, and we recommend dog vaccination campaign and promotion of health education on rabies using a one health approach.
Keywords
• Rabies
• Outbreak
• Cost-Effectiveness
• Camel
• Striped Polecat (Ictonyx striatus)
• Jackal
• SRS
• Ethiopia
Citation
Osman Y, Tschopp R, Hattendorf J, Crump L, Muhumed A, et al. (2025) Rabies Outbreak in the Somali Region of Ethiopia: Public Health Significance and Potential of Canine Vaccination Using a one Health Approach. Ann Public Health Res 12(1): 1139.
INTRODUCTION
Rabies is a zoonotic viral disease caused by the genus Lyssavirus within the family Rhabdoviridae, which infects all mammals [1,2], Dogs are the primary source of rabies transmission. The role of wildlife in the maintenance and transmission of rabies under Ethiopian circumstances is unclear and needs more research [3]. The route of transmission to humans and animals is through a bite, and the incubation period and clinical signs depend on the bite location and concentration of virus in the saliva [4]. The disease is preventable but if not treated in time causes 100% mortality [4]. Rabies causes 59’000 human death annually with 3.7 million disability adjusted life years (DALY) and 8.6 billion USD economic loss annually [5]. While industrial countries have eliminated dog-mediated rabies, it is endemic in developing countries [6]. The virus can be eliminated through sustained dog vaccination [7], and 70 percent of dog population vaccination interrupts transmission of rabies [8]. The World Health Organization [9] aims to eliminate dog-mediated rabies by the end of 2030, which is among the United Nation’s sustainable development goals to end neglected tropical diseases [10]. Rabies is recognized as an important zoonotic disease in Ethiopia. The first outbreak was reported in 1884 in Tigray, Wallo and Gojam [11]. Since then, rabies has been endemic in Ethiopia and ranked first in prioritized zoonotic diseases [12]. Deressa et al., reported 35-58 human deaths annually [13], while the annual economic loss in the cattle sector is estimated at 209 million USD [14]. Although the disease is among the list of notifiable diseases in both human and animal surveillance systems, its burden is underestimated due to poor surveillance systems, such as in other African countries [15,16]. Ethiopia developed a dog-mediated national rabies control and elimination strategy, which aims to eradicate the disease by the end of 2030 as part of this effort, dog vaccination programs were implemented in the main cities of the country by the Ministry of Agriculture and Health, in collaboration with global partners. International organizations such as the US Centers for Disease Prevention and Control, the Ohio State University Global One Health Initiative, the Global Alliance for Rabies Control, and the Health of Ethiopian Animals for Rural Development project support rabies vaccination programs [17]. Despite ongoing efforts, Ethiopia continues to experience frequent rabies outbreaks due to low national dog vaccination coverage. A recent rabies suspected outbreak in the SRS raised concern with the regional health and livestock bureau after several cases were reported from a number of districts in the region. This prompted the Jigjiga One Health Initiative (JOHI) research team to explore the epidemiological characteristics, public health, and cost effectiveness potential control measures within the SRS. The investigation aligns with JOHI’s objective of exploring research-based evidence to illuminate the potential impact and control measures for zoonotic diseases such as rabies.
MATERIALS AND METHODS
Description of Study Area
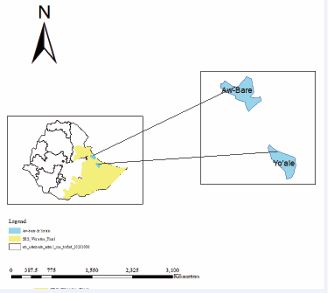
The study was conducted in Lafaissa town of Awbare district and Yocale districts where the outbreak occurred (Figure 1). Lafaissa’s total population is estimated at 53,300 while the total population of Yoale district is 80,000. The Awbare district lies in 9?18’N and 10?12’N Latitude and 42?37’E and 43?26’E Longitude. The district is agro-pastoralist, characterized by arid and semi-arid climate, with mean temperature 14?C and minimum and maximum temperatures of 20?C and 25?C, respectively.
Figure 1: Map of the study area.
Ethical Statement
The study protocol for integrated disease surveillance and response was reviewed and approved by the Ethics Committee of Northwestern and Central Switzerland (EKNZ Req-2019-01112) and the Ethics committee of Jigjiga University (RERC/022/ 2020). However, as rabies outbreak investigation is part of the public health response, specific ethical clearance for the investigation was not further submitted to the health bureau. Permission to conduct the investigation was obtained from the local authorities. To ensure informed participation, the purpose of the investigation was explained to all the participants. Verbal consent was obtained from the participants or their parents before the interviews.
METHODOLOGY/ APPROACH
Study Design: The rabies outbreak in 2023 in the SRS of Ethiopia emerged as a significant public health issue. Within the SRS One Health Task Force (SOHTF), the JOHI research team investigated the epidemiology and economic impact of the outbreak and evaluated the cost effectiveness of the implementation of a dog vaccination program. A combination of retrospective and prospective data collection was employed. We reviewed health facility documents, interviewed the victims, and held focus group discussions with community representatives and human and animal health staff to obtain epidemiological data on the outbreak. A structured questionnaire was used to collect information on the number of victims and associated risk factors from health facilities following an outbreak alarm. Similarly, from the livestock office, data on the number of livestock lost due to bites and associated risk factors and livestock prices were collected. To confirm the outbreak, brain samples were collected from 1 dog, 1 camel, 1 donkey and 1 cow. Anigen rapid rabies Ag test kit (www.cdc./rabies/pdf/rabiesdfaspv2.pdf) and florescent technique (https://www.fujirebio.com/en/products solutions/fitc-antirabies-monoclonal-globulin) were used to diagnose cases. Additionally, to estimate the direct economic loss and cost-effectiveness of the dog vaccination program, the cost of PEP and ELLD and a dog vaccination program were estimated.
Economic Loss Assessment in Human and Animals: Since dog bites in Yocale district were not involved and we did not have a dog population estimate for Yocale district, the economic analysis represents only Laffaissa town in Awbare district. Livestock prices were obtained from the owners of infected animals and crosschecked with the Laffaissa Town Livestock Market Center. To estimate PEP costs, we reviewed health facility data on hospitalization expenses and conducted interviews with the victims to determine additional expenses. We obtained bed and supportive treatment service costs from hospitals and health facilities. Victim interviews provided information on the cost of ambulance services (Table 1). We did not consider the time lost to work in our analysis. The details of the PEP and livestock loss costs are available in the supplementary document.
Table 1: Detailed cost break down of estimated rabies vaccination campaign.
|
Fuel |
Litter |
50 |
1.66 |
15 |
1245 |
|
Vaccine cost |
Dose |
1872 |
0.52 |
|
973 |
|
Vaccine transportation ( round trip flight from Jigjiga to Addis_Abeba) + round trip ticket to Bishoftu, vaccine production site) + 2 days accommodation in Addis Abeba |
lump sum |
|
|
|
240 |
|
Total cost |
|
|
|
|
3156 |
Estimation of Cost of Dog Vaccination Campaign: To estimate cost of dog vaccination in Laffaissa, a survey was conducted to estimate the canine population in the town. One hundred thirty-four households were randomly selected among the total households of the town, and based on the number of dogs per household surveyed, the total dog population of the town was projected to be 1872. This was used as the basis for cost of vaccination programs, assuming 100% vaccination in canine population. We used previous economic model assumptions to estimate vaccination program costs [18,19]. Accordingly, the costs of vaccine prices, vaccine transportation, and operational costs were assumed in the analysis. Operational costs included per diem costs for vaccinators, supervisors, and drivers, and fuel costs for vehicles. We did not consider the cost of consumables (detergents) and vaccination equipment (cold chain, vaccine syringe, needle, etc.), because these materials are available for other livestock vaccinations and would be shared for specific dog vaccination campaigns. A detailed breakdown of the estimated costs is provided in Table1 and equation 1.
Equation for vaccination cost
Total vcost: operational cost (( cosvac+cossu+ cosdr+ cosfue)+cosvacc+cosvacT))
Where vcost= total vaccination cost;
cosvac= cost of vaccinators;
cossu = cost of supervisors;
cosdr = cost of driver;
cosfue = cost of fuel;
cosvacc; cost of vaccine;
cosvacT = cost of vaccine transport
Statistical Analysis
Data obtained for the livestock and health facilities were compiled into an Excel spreadsheet and transferred to Stata 18 for analysis. Descriptive statistics were computed to characterize the frequency of rabies bites across various demographic factors, including sex, age, and species of animal. In addition, the Attacking Rate (AR) and case fatality rates were computed to assess the burden of rabies in the study areas.
Control Measures
Following the notification of the outbreak, regional livestock and health bureau through the SOHTF implemented a series of control measures including: 1) deployment of PEP to affected areas to ensure timely access of PEP for exposed individuals; 2) community awareness about disease transmission and health outcomes; 3) joint outbreak investigation; and 4) culling of stray dogs. Unfortunately, dog vaccination, which is the most effective control measure, was no implemented.
PRINCIPAL FINDINGS/ RESULT
Frequency and characteristics of victims (humans)
From April to December, 34 human rabies cases were detected in Lafaissa town and Yocale district of SRS in Ethiopia. Thirty-two (94.12%), were detected in Lafaissa town and two cases (5.88 %), were detected in Yocale district. Thirty-two victims were admitted to the hospital and recovered after PEP. There were two deaths: 60-year old women who did not take PEP after she was told it was not necessary (according to her family) and an 8-year old girl who went to the hospital late and received PEP but unfortunately died. According to family reports, both patients who died had excitation, excessive salivation, biting behavior, and loss of consciousness. The AR was 22.2 cases per 100,000 people ((95% CI: 15.9-31.1/100,000). The case fatality rate was 5.9%. Males (70.59%) and children under 15 years old (57.14%) were a majority of victims. Dogs caused a majority of bites (91%), while striped polecats, camels and cats caused one (2.94 %) bite each. The leg region (50%) was the most affected body part, followed by hand or arm (23.53%), trunk (14.71%), and neck or head (11.76%).
Frequency and Characteristics of Animal Bites
During the outbreak, twenty-eight animal bites were recorded. The highest number of cases were detected in Laffaissa town of Awbare district 23 (82.14%), followed by Yocale district 5 (17.86%). Cattle accounted for 19 (67.86%) cases followed by camels 5 (17.86%) goats 2 (7.14%), sheep 1 (3.17%) and donkey 1 (3.17%). In Laffaissa town of Awbare, the most bite exposures were caused by dogs 18 (78.26%), while jackals caused 3 (13%) bites. Conversely, in Yocale districts, jackals caused 4(80%) of the bite exposures, Ictonyx striatus caused 1 (20%), and dogs were not involved at all. According to the owners of the infected animals, the sampled animals showed aggressiveness, biting behaviors, self-infliction (donkeys), and excitement. Figure 2 shows a camel, which tested positive, with typical signs of rabies, and Figure 3 shows a goat with behavioral changes.
Laboratory Diagnosis
Dog (Figure 4), and camel (Figure 5), tested strongly positive, while cattle and donkey tested weakly positive with the rabies rapid kit test. Only the camel brain sample was shipped to the EPHI, where it tested positive for Florescence-Antibody Test (FAT). The remaining samples were stored at Jigjiga Regional veterinary laboratory (JRVL) for further molecular analysis. According to JRVL, jackals tested positive for rabies in the same geographic areas in 2020 (Figure 6). To our knowledge, this is the first report of camels bitten by striped polecat and testing positive for rabies in East Africa. A rabies outbreak was declared after the virus was confirmed in the animals.
Comparative Cumulative Cost Analysis
To estimate PEP cost and ELLD, we calculated the average cost of PEP and ELLD, and then total cost of PEP and ELLD for all human and animal cases. The average PEP and ELLD were estimated to be USD 180.20 and USD 622.97, respectively. The average PEP and ELLD were multiplied by the number of human and animal exposure cases to get total PEP and ELLD costs. According to our parameter estimate, the PEP cost of the exposed cases and ELLD were estimated to be USD 6144 and USD 23601, respectively (Table 2).
Table 2: Cost break down of PEP and ELLD.
|
# of cases |
Cumulative PEP cost1 (CPC) |
Cumulative LLED cost (CLC) |
Total economic loss (CPC+ CLC) |
Estimated dog vaccination cost |
|
1 |
1812 |
8433 |
1024 |
3156 |
|
2 |
362 |
1686 |
2048 |
3156 |
|
3 |
543 |
2529 |
3072 |
3156 |
|
4 |
724 |
3372 |
4096 |
3156 |
|
5 |
905 |
4215 |
5120 |
3156 |
|
6 |
1086 |
5058 |
6144 |
3156 |
|
7 |
1267 |
5901 |
7168 |
3156 |
|
8 |
1448 |
6744 |
8192 |
3156 |
|
9 |
1629 |
7587 |
9216 |
3156 |
|
10 |
1810 |
8430 |
10240 |
3156 |
|
11 |
1991 |
9273 |
11264 |
3156 |
|
12 |
2172 |
10116 |
12288 |
3156 |
|
13 |
2353 |
10959 |
13312 |
3156 |
|
14 |
2534 |
11802 |
14336 |
3156 |
|
15 |
2715 |
12645 |
15360 |
3156 |
|
16 |
2896 |
13488 |
16384 |
3156 |
|
17 |
3077 |
14331 |
17408 |
3156 |
|
18 |
3258 |
15174 |
18432 |
3156 |
|
19 |
3439 |
16017 |
19456 |
3156 |
|
20 |
3620 |
16860 |
20480 |
3156 |
|
21 |
3801 |
17703 |
21504 |
3156 |
|
22 |
3982 |
18546 |
22528 |
3156 |
|
23 |
4163 |
19389 |
23552 |
3156 |
|
24 |
4344 |
20232 |
24576 |
3156 |
|
25 |
4525 |
21075 |
25600 |
3156 |
|
26 |
4706 |
21918 |
26624 |
3156 |
|
27 |
4887 |
22761 |
27648 |
3156 |
|
28 |
5068 |
23604 |
28672 |
3156 |
|
29 |
5249 |
|
28853 |
3156 |
|
30 |
5430 |
|
29034 |
3156 |
|
31 |
5611 |
|
29215 |
3156 |
|
32 |
5792 |
|
29396 |
3156 |
|
33 |
5973 |
|
29577 |
3156 |
|
34 |
6144 |
|
29758 |
3156 |
Average number of PEP cost multiply by the number of exposure cases (eg,.1*180 , 2 * 180, 3*180n etc ) 2Average PEP cost 3Average LLED cost
Figures 4 Dog tested positive for rabies with rapid kit test.
Figures 5 Camel brain tested positive for rabies with rapid kit test.
Figures 6 Jackal tested positive for rabid rabies with rapid kit test.
Conversely, the cost of the dog vaccination campaign (DVC) was estimated at USD 3’156. As illustrated in Figure 7, at the beginning of the outbreaks, the cost of PEP is lower than that of DVC. However, as time progressed without any intervention in the dog reservoir, the number of cases in humans and animals progressively increased. When the number of human case exposures reaches approximately eighteen, the PEP cost surpassed the cost of the DVC and continued to increase. Similarly, the figure suggests that vaccinating the dog population in Laffaissa early in the outbreaks, potentially through the sale less than five animals, could have avoided the economic and public health impacts of the outbreak in Laffaissa town.
DISCUSSION
To our knowledge, this study represents the first comprehensive analysis of rabies epidemiology and cost effectiveness of rabies outbreak control measures in SRS. The first cases were detected in dogs and later jackals in Laffaissa town, while in Yocale district, a jackal caused the first notified bite, a striped polecat caused one bite,and dogs had no implication in the outbreak. Both wildlife (jackal and striped polecat) and dogs were involved in transmission dynamics in SRS. Jackal, Ictonyx striatus, and dog are the most common rabies reservoirs in Africa [20]. The involvement of jackals and striped polecat in the outbreak likely indicates that the disease is maintained in wildlife in pastoral areas of the Somali region. However, it is still unclear whether the disease is a spillover from wildlife to dogs or vice versa, so this requires further investigation. The recorded case fatality rate (5.8 %) was lower than that of the other regions of the country [21]. This could be because the communities in these areas have better knowledge of rabies and its health consequences if bitten by animals of unknown rabid status. This hypothesis was supported by our previous study on SRS [22], which revealed 87.9 % of the communities would seek medical attention if they were bitten by rabies reservoir animals, which shows better knowledge of the disease compare to other parts of the country [23,24]. The observed attacking rate of 25.5 cases per 100,000 people (95% CI: 18.23_35.7/100,000) is relatively higher. On the other hand, the reported incidence rate in different geographical areas varied considerably. For instance, in Gonder, north western Amhara and northern Tigria, incidence rates ranged from 1.27 to 4.6, 6.5 to 7.5, and 89.8 to 35.8, respectively [25-27]. This discrepancy in proportion of infected exposed people in different regions of the country could be due to variations in health data recording practices, methodological approaches, or dog management practices, including dog vaccination and the population of roaming/confined dogs. These factors can significantly influence the transmission dynamics of rabies in canine populations and increase the risk of human exposure to rabid dogs [28]. Additionally, missed diagnoses have been reported as one of the factors contributing to human death [29]. In line with this observation, the first patient died due to missed diagnosis, and the second patient went to the hospital at an advanced stage of disease. This indicates that health education, including of health professionals and communities, is essential to mitigate rabies deaths and risks [30]. The outbreak lasted approximately six months (May 20 until December 15, 2023). During this period, no intervention was made in the reservoir hosts, except for a dog culling operation conducted in July 2023, which resulted in elimination of 134 dogs. Dog culling is an inappropriate and insufficient strategy for controlling rabies [31]. The inability to implement appropriate control interventions in reservoirs contributes to the escalation of human and animal exposure cases. This lack of intervention, in addition to public and economic impacts, has resulted in stress among communities. Focus group discussion revealed how the community were anxious about the disease, exemplified by statements such as: “We are all at risk. We are unsure who will be the next victims. We understand the risk but we cannot do anything. We urge governments to find solutions to this problem.” Subsequent SOHTF meetings highlighted challenges associated with lack of vaccine, limited vaccination experience, and concerns related to community perception about vaccination, as dog vaccination had never been conducted in this area. In Ethiopia, dog vaccination coverage is very low compared to the dog population and is limited to the main cities despite the high risk of rabies in all regions of Ethiopia [20]. The regional government should consider immediate dog vaccination programs, as 70% coverage of dogs would interrupt with rabies transmission [8]. In addition, as most communities of the SRS are Muslim, community awareness should be raised to create good conditions for successful vaccination. To attain successful vaccination in SRS, we propose the model proposed by Mosimann et al. [32], a mixed model that assesses individual effectiveness parameters, which include accessibility, affordability, and adequacy. A triangulation methodology that combines both qualitative (participant observation and focus group discussion) and quantitative (household survey, empirical coverage estimation, and spatial analysis) methods would clarify the weight of the effectiveness of each determinant and underlining reasons embedded in local understandings of cultural practice, and social and political realities of the setting. Moreover, rabies control strategies for wildlife (jackal, striped polecat) should also be considered. A very important aspect of promoting rabies control and prevention strategies in developing countries lies in demonstrating cost effectiveness and presenting the economic and public health benefits of control to policy makers. Previous studies show the cost-effectiveness of rabies control [33,34]. As illustrated in Figure 7, absence of interventions in the reservoirs progressively increases the number of human and animal cases, cost of PEP and ELLD. Early intervention could significantly reduce the economic and public health impacts. However, the lack of a dog vaccination program in the region indicates a potential underestimation of rabies impact by the government and partners. This underestimation could stem from the limited understanding of comprehensive rabies epidemiology, public health, and economic loss posed by the disease, potentially due to poor surveillance systems in the country. Our findings highlight the importance of controlling zoonotic diseases early, before they spread throughout reservoirs. Early detection of zoonotic diseases in the environment or in animals would reduce the cost of public health measures [35]. Nevertheless, successful control and prevention of rabies in SRS depends on how the government prioritizes disease in strategic public health prevention and control measures. Given the endemic nature of rabies in African countries, regional collaboration to develop a joint control plan against rabies using game theory is indicated to eradicate the disease on the continent [36].
LIMITATIONS
The main limitations of this study are as follows.
1) Under reporting of animal cases: The number of reported cases is likely underestimated due to poor animal surveillance systems. Due to the absence of prophylaxis for livestock, communities may not report the exposed cases, meaning some cases go undetected.
2) Limited data for cost-effectiveness analysis: We estimated the cost-effectiveness for a small town. To draw more robust conclusions that can be generalized to a broader context, more comprehensive data are necessary.
REFERENCES
- Hemachudha T, Ugolini G, Wacharapluesadee S, Sungkarat W, Shuangshoti S, Laothamatas J. Human rabies: neuropathogenesis, diagnosis, and management. Lancet Neurol. 2013; 12: 498-513.
- Organization WH. WHO expert consultation on rabies: second report. World Health Organization. 2013.
- Ali JA. Prevention and control of rabies in animals and humans in Ethiopia. Health Sci J. 2021; 16: 1-10.
- Hemachudha T, Laothamatas J, Rupprecht CE. Human rabies: a disease of complex neuropathogenetic mechanisms and diagnostic challenges. Lancet Neurol. 2002; 1: 101-109.
- Hampson K, Coudeville L, Lembo T, Sambo M, Kieffer A, Attlan M, et al. Estimating the global burden of endemic canine rabies. PLoS neglected tropical diseases. 2015; 9: e0003709.
- Coleman PG, EM Fèvre, Cleaveland S. Estimating the public health impact of rabies. Emerging Infectious Dis. 2004; 10: 140-142.
- Hampson K, Dushoff J, Cleaveland S, Haydon DT, Kaare M, Packer C, et al. Transmission dynamics and prospects for the elimination of canine rabies. PLoS Biol. 2009; 7: e1000053.
- Léchenne M. Rabies control in N’Djamena, Chad, University of Basel.2015.
- WOAH WOfAH. Rabies in animals. 2009.
- Minghui R, Stone M, Semedo MH, Nel L. New global strategic plan to eliminate dog-mediated rabies by 2030. The Lancet Global Health. 2018; 6: e828-e829.
- Fekadu M. Rabies in Ethiopia. American J Epidemiol. 1982; 115: 266-273.
- Pieracci EG, Hall AJ, Gharpure R, Haile A, Walelign E, Deressa A, et al. Prioritizing zoonotic diseases in Ethiopia using a one health approach. One Health. 2016; 2: 131-135.
- Deressa A, Ali A, Bayene M, Selassie BN, Yimer E, Hussen K. The status of rabies in Ethiopia: A retrospective record review. Ethiopian J Health Development. 2010; 24.
- Jibat, T, Mourits MC, Hogeveen H. Incidence and economic impact of rabies in the cattle population of Ethiopia. Preventive Vet Med. 2016; 130: 67-76.
- Taame H. 4.2 Human Rabies Surveillance in Ethiopia. The NationalWorkshop on Rabies Prevention and Control in Ethiopia. 2012.
- Tschopp R, Bekele S, Aseffa A. Dog demography, animal bite management and rabies knowledge-attitude and practices in the Awash Basin, Eastern Ethiopia. PLoS neglected tropical diseases. 2016; 10: e0004471.
- Murphy SC, Negron ME, Pieracci EG, Deressa A, Bekele W, Regassa F, et al. One Health collaborations for zoonotic disease control in Ethiopia. Rev Sci Tech. 2019; 38: 51-60.
- Wera E, Velthuis AG, Geong M, Hogeveen H. Costs of rabies control: an economic calculation method applied to Flores Island. PLoS One. 2013; 8: e83654.
- Anyiam F, Lechenne M, Mindekem R, Oussigéré A, Naissengar S, Alfaroukh IO, et al. Cost-estimate and proposal for a development impact bond for canine rabies elimination by mass vaccination in Chad. Acta Trop. 2017; 175: 112-120.
- Rupprecht, CE, Mani RS, Mshelbwala PP, Recuenco SE, Ward MP. Rabies in the Tropics. Current Tropical Medicine Reports. 2022; 9: 28-39.
- Asfaw GB, Abagero A, Addissie A, Yalew AW, Watere SH, Desta GB, et al. Epidemiology of suspected rabies cases in Ethiopia: 2018–2022. One Health Adv. 2024; 2: 3.
- Yahya Osman, RT, Peter Odermatt,Jan Hattendorf,Lisa Crump,Abdifatah Muhumed,Jakob Zinsstag. Community based knowledge, attitude and practice regarding rabies risk in Shabele zone, Somali region, Ethiopia. 2024.
- Digafe RT, Kifelew LG, Mechesso AF. Knowledge, attitudes and practices towards rabies: questionnaire survey in rural household heads of Gondar Zuria District, Ethiopia. BMC Res Notes. 2015; 8: 1-7.
- Bahiru A, Molla W, Yizengaw L, Mekonnen SA, Jemberu WT. Knowledge, attitude and practice related to rabies among residents of Amhara region. Ethiopia. Heliyon. 2022; 8: e11366.
- Yibrah M, Damtie D. Incidence of human rabies exposure and associated factors at the Gondar Health Center, Ethiopia: a three-year retrospective study. Infect Dis Poverty. 2015; 4: 3.
- Gebreyohans GT, Teweldemedhn GH, Gebremedhin RE. High incidence of human rabies exposure in Northwestern Tigray, Ethiopia: a four-year retrospective study. 2017.
- Yizengaw E, Getahun T, Mulu W, Ashagrie M, Abdela I, Geta M. Incidence of human rabies virus exposure in northwestern Amhara, Ethiopia. BMC Infectious Diseases. 2018; 18: 1-7.
- Taylor LH, Wallace RM, Balaram D, Lindenmayer JM, Eckery DC, Mutonono-Watkiss B, et al. The role of dog population management in rabies elimination-a review of current approaches and future opportunities. Front Vet Sci. 2017; 4: 109.
- Salomão C, Nacima A, Cuamba L, Gujral L, Amiel O, Baltazar C, et al. Epidemiology, clinical features and risk factors for human rabies and animal bites during an outbreak of rabies in Maputo and Matola cities, Mozambique, 2014: Implications for public health interventions for rabies control. PLoS Negl Trop Dis. 2017; 11: e0005787.
- WHO. WHO Expert Consultation on Rabies. Geneva. WHO. 2005.
- Kaare M, Lembo T, Hampson K, Ernest E, Estes A, Mentzel C, et al. Rabies control in rural Africa: evaluating strategies for effective domestic dog vaccination. Vaccine. 2009; 27: 152-160.
- Mosimann L, Traoré A, Mauti S, Léchenne M, Obrist B, Véron R, et al. A mixed methods approach to assess animal vaccination programmes: The case of rabies control in Bamako, Mali. Acta Trop. 2017; 165: 203-215.
- Zinsstag JS, Dürr M, Penny R, Mindekem F, Roth SM, Gonzalez S. Transmission dynamics and economics of rabies control in dogs and humans in an African city. Proceedings of the National Academy of Sciences. 106: 14996-15001.
- Borse RH, Atkins CY, Gambhir M, Undurraga EA, Blanton JD, Kahn EB, et al. Cost-effectiveness of dog rabies vaccination programs in East Africa. PLoS neglected tropical diseases. 2018; 12: e0006490.
- People P, Planet O. The Economics of One Health. Washington, DC:World Bank. 2012.
- Bucher A, Dimov A, Fink G, Chitnis N, Bonfoh B, Zinsstag J. Benefit- cost analysis of coordinated strategies for control of rabies in Africa. Nature Commun. 2023; 14: 5370.