Evaluation of Testis Biometry, Sperm and Hormone Profiles of Naturally Unilateral Cryptorchid West African Dwarf Goats
- 1. Department of Veterinary Anatomy, University of Nigeria, Nigeria
- 2. Department of Veterinary Obstetrics and Reproductive Diseases, University of Nigeria, Nigeria
Abstract
The present study was aimed at evaluating the parameters that sustain reproductive capacity of unilateral cryptorchid West African dwarf goats. Forty adult bucks were used for the study. Twenty of the bucks were unilateral cryptorchids while the remaining 20 bucks had fully descended testes. Semen were collected by electroejaculation and analyzed quantitatively and qualitatively. Hormone profiles were analyzed using Enzyme- linked immune-sorbent assay. The results demonstrated that the serum levels of testosterone were significantly higher (p < 0.05) in the unilateral cryptorchid bucks compared to the normal bucks. Similarly, serum luteinizing hormone levels were significantly higher (p < 0.05) in the unilateral cryptorchids (3.28 ± 0.05ng/ml) when compared to the normal bucks (2.31 ± 0.06ng/ml). There was no significant difference (p > 0.05) between the serum follicle stimulating hormone levels of the unilateral cryptorchids (31.47 ± 0.54ng/ml) and the normal bucks (31.98 ± 0.08ng/ml). Semen ejaculate volume, sperm concentration, sperm motility, percentage live spermatozoa, were not significantly different (p > 0.05) between the normal and the unilaterally cryptorchid bucks. The differential and total percentage morphological abnormalities were also not significantly different (p > 0.05) between the two groups. The contralateral scrotal testes of the cryptorchid bucks were hypertrophied. These results suggest that the severely impaired undescended testes probably induced compensatory morphological changes, which enhanced the endocrine and exocrine functions of the contralateral scrotal testes of the unilateral cryptorchids. These findings tend to elucidate the positive fertility potentials of naturally hemicryptorchid West African dwarf bucks.
Keywords
Semen parameters; Endocrine profiles; Hemicryptorchidism
Citation
Okpe GC, Anya KO (2017) Evaluation of Testis Biometry, Sperm and Hormone Profiles of Naturally Unilateral Cryptorchid West African Dwarf Goats. Ann Reprod Med Treat 2(1): 1007.
INTRODUCTION
The West African Dwarf (WAD) goat is a short-legged goat indigenous to the humid rain forest ecological zone of West Africa. Among the WAD goats of Eastern Nigeria, unilateral cryptorchidism has been described as a common condition [1]. Local goat owners claim that unilateral cryptorchid animals possess superior reproductive efficiency than normal bucks with fully descended testes [2]. The above claims obviously appear tenuous as the imperfection of an undscended testis has been recognized since John Hunter reported about retention of testes. The degenerative nature of the retained testis in unilateral cryptorchidism is well documented [3]. The histological appearance and function of the contralateral descended testes; however, is the subject of controversy. Development of bilateral testicular impairments has been suggested as an explanation for decreased sperm density in unilateral cryptorchid males [4]. Semen quality was also reported to be affected in human, porcine and canine unilateral cryptorchids [5]. In contrast to the above reports [6], observed that unilateral cryptorhidism even with altered histology of testis and spermatic tract did not affect fertility [7] also found no significant differences in semen quality and quantity between the normal and unilateral cryptorchid bucks.
Data on gonadotrophin levels in cryptorchid animals are also inconsistent. In the ram, cryptorchidsm has been found to cause elevations in blood concentrations of both follicle stimulating hormone (FSH) and luteinizing hormone (LH) [8]. However, [9] did not detect any change in the gonadotrophin levels of cryptorchid rams. Bilateral cryptorchidism in bulls was reported to be associated with elevated plasma luteinizing hormone and follicle stimulating hormone levels [10]. Reports on the influence of unilateral cryptorchidism on hormone profiles have been inconsistent in human patients [11]. Testosterone regulates multiple aspects of sexual development. It is essential for the continual production of sperm by supporting multiple aspects of spermatogenesis [12]. There is a local assumption in south eastern Nigeria that unilateral cryptorchid bucks are more salacious than their counterparts with fully descended testes, this assumption implies an enhanced testosterone secretion. The present study was therefore aimed at evaluating the quantity and quality of sperm, and hormone profiles of adult unilateral cryptorchid West African dwarf bucks. The findings may provide an explanation for improved salaciousness of hemicryptorchid West African Dwarf bucks.
MATERIALS AND METHODS
Forty adult male West African Dwarf (WAD) goats weighing between 6-10kg were used for the study. The bucks were divided into two groups as follows:
Group A: 20 bucks with fully descended testes (control) and Group B: 20 bucks with unilaterally descended testis.
Animal management: All the animals were managed intensively throughout the study. The animals were dewormed using ivomectin (IvomecR) and fed with giant star grass and spent maize grain. Water was provided ad libitum.
Semen Collection
The bucks were influenced to ejaculate repeatedly by introducing does (female goat) into the pen. This was done to deplete the spermatozoa reserve in the tail of the epididymis. The bucks were then rested for one week for stabilization. Semen was collected from each buck once every week; for a period of 12 weeks, using the electro-ejaculation method as described by [13].
Semen Analysis
Semen volume was measured using a calibrated collection bottle. Colour and consistency of the ejaculate were determined by visual observation. Sperm mass activity was determined by placing a small drop of semen on a warm glass slide, and observed under a low power light microscope objective. The mass activity was observed as recurrent swirling waves. The score depended on the presence or absence of distinct dark waves and ranged from 0-5. Sperm motility was determined by examining the semen at x400 magnification. Progressive forward motility, with a characteristic swinging of the head and tail was estimated as the ideal. Sperm concentration was determined using an improved Number haemocytometer, after dilution of semen with normal saline. To determine the morphology, of the spermatozoa, a drop of semen was placed on a clean warm glass slide with two drops of Well and Awa stain. These were gently mixed and a smear was made on another clean warm slide (to avoid cold shock) and air dried. The slide was observed under light microscope (x1000 magnification.) for the presence of abnormal sperm cells, and percentage of abnormal sperm cells were noted and recorded. The abnormal forms were then classified using the method of [14] as follows: Primary abnormalities and Secondary abnormalities Morphological abnormalities among 400 counted sperm cells were noted and percentage of abnormal cells calculated and recorded by 3 independent observers. The abnormal forms were then classified using the method of [14]. Vital differential staining technique (Eosin-Nigrosin stain) was used to determine the live / dead spermatozoa in semen smears.
Hormone Analysis
Sample collection: Blood was collected from the jugular vein of each animal once a week, between 0900 and 1200h at every visit, for a period of twelve weeks. The blood samples were allowed to clot in a slanting test tube and the sera were harvested and stored under -200 C. Enzyme- Linked Immunosorbent Assays were used for the quantitative determination of testosterone, luteinizing hormone and follicle stimulating hormone. Assay Kits were obtained from Diagnostic Automation Int. 23961 Cratsman Road, Suite EF Calabas, CA91302. Intra- and interassay coefficients of variation were both below 8% in follicle stimulating hormone and luteinizing hormone assays, while for testosterone they were below 10%. Goat antibodies were utilized for the analysis.
Biometric data measurement: The animals were castrated under local anesthesia (lindocane). Testis weight determined using digital analytical balance (U.S. Solid), while the length and thickness were measured using digital Vernier caliper (Shanghai Huxu Trade Co. Ltd, China). Gonado-somatic index was determined by calculation of the gonadal weight as a proportion of the total body weight.
Statistical Analysis: Quantitative data generated were analyzed by repeated measure ANOVA using IBM 2015 SPSS statistics. Significance was accepted at P < 0.05.
RESULTS
The semen characteristics of the natural unilateral cryptorchid bucks and the normal (Control) bucks are summarized in (Table 1). The semen volume produced by the cryptorchid bucks and the normal bucks ranged from 0.41 to 0.51ml. The semen volumes of the control and unilateral cryptorchid bucks were not significantly different (P > 0.05).
Table 1: The sperm physiological parameters in normal and unilateral cryptorchid goats (Means ± Standard error of the Mean (SEM)).
|
Sperm characteristics |
Normal bucks |
Cryptorchid bucks |
|
Semen concentration (109//ml) |
2.67 ± 0.04a |
2.66 ± 0.03 a |
|
Sperm motility |
84.36 ± 0.85 a |
83.98 ± 0.80 a |
|
Ejaculate volume Progressive motility (%) Semen colour Wave motion (0-4) Percentage live spermatozoa |
0.47 ± 0.02a 84.36 ±0.85a Milky or creamy 4 97.10±0.21a |
0.45 ± 0.02a 83.98±0.08 a Milky or creamy 4 96.39±0.44 a |
|
Means with different superscripts on the same row are significantly different (P< 0.05). |
||
The semen from the two groups of bucks appeared milky or creamy in colour.
Semen concentration
The sperm concentrations in both the cryptorchid and normal bucks were within the reference range. The unilateral cryptorchid bucks and the control bucks had similar sperm concentration (p > 0.05).
Sperm Motility
The mean percentage sperm motility was 84.36 ± 0.85% and 83.98 ± 0.08% for the normal bucks and natural unilateral cryptorchid bucks respectively. Significant difference in percentage sperm motility was not observed between the natural unilateral cryptorchid bucks and normal bucks (p > 0.05) (Table 1). Sperm mass movement (Wave motion): Mass sperm movement manifested swirling waves and scored 4 (very Good) in all the groups. There was no significant difference between the groups in mass activity (Table 1). Percentage live spermatozoa: The mean percentage live spermatozoa were 97.10±0.21% and 96.39±0.44% for the normal and natural unilateral cryptorchid bucks respectively. There was no significant (p > 0.05) difference in the percentage live spermatozoa between the normal and the cryptorchid bucks (Table 1). Spermatozoa morphological abnormalities: The sperm morphological abnormalities are presented in (Table 2).
Table 2: Morphological sperm abnormalities of normal and natural unilateral cryptorchid goats (Means ± Standard error of the Mean (SEM)).
|
Parameter |
Normal buck |
Cryptorchid buck |
|
Detached heads Coiled tails Double tails proximal cytoplasmic droplet Distal cytoplasmic droplet Total abnormalities |
2.12±0.14b 1.64±0.05 b 0.00±0.00 b 3.24± 0.34 b 2.43± 0.27b 9.43±0.73b |
1.46±0.21 b 1.83±0.30b 0.06±0.44b 3.83±0.08b 2.81±0.33b 9.99±0.80b |
|
Means with same superscripts on the same row are not significantly different (P> 0.05). |
||
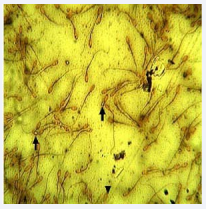
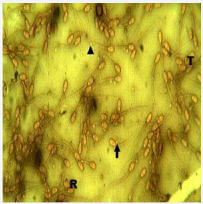
The sperm abnormalities observed in the normal and cryptorchid bucks were detached head, coiled tails, double tails, proximal cytoplasmic droplet and distal cytoplasmic droplets (Figures 1,2). The differential and total percentage abnormalities observed in the normal and cryptorchid bucks were not significantly different (p> 0.05).
The mean percentage live spermatozoa were 97.10±0.21% and 96.39±0.44% for the normal and natural unilateral cryptorchid bucks respectively. There was no significant (p > 0.05) difference in the percentage live spermatozoa between the normal and the cryptorchid bucks (Table 1). Spermatozoa morphological abnormalities: The sperm morphological abnormalities are presented in (Table 2). The sperm abnormalities observed in the normal and cryptorchid bucks were detached head, coiled tails, double tails, proximal cytoplasmic droplet and distal cytoplasmic droplets (Figures 1,2). The differential and total percentage abnormalities observed in the normal and cryptorchid bucks were not significantly different (p> 0.05).3.
Figure 1: Micrograph of semen smear from normal (non-cryptorchid) buck. Showing coiled tails (arrows) and detached head (arrow head). Well and Awa stain x400.
Figure 2: Micrograph of semen smear from the unilteral cryptorchid buck. Note proximal cytoplasmic droplet (arrow); distal cytoplasmic droplet (arrow head), detached head (R) and coiled tail (T). Well and Awa stain x400.
Hormone Analysis
Mean testosterone concentrations of 2.97 ± 0.03ng/ml and 3.60 ± 0.02ng/ml were recorded for the normal (control) and unilateral cryptorchid bucks respectively. The mean plasma testosterone concentration of the unilateral cryptorchid bucks was significantly higher (p < 0.05) than those of the normal bucks (control). The concentration of follicle stimulating hormone was higher in the cryptorchid bucks compared to the control. However the differences observed were not statistically significant (p > 0.05). The mean plasma luteinizing hormone concentrations were 2.31±0.06 ng/ml and 3.28±0.05 ng/ml for the normal (control) bucks and the unilateral cryptorchid bucks respectively. The plasma concentration of luteinizing hormone in the unilateral cryptorchid bucks was significantly (p < 0.05) higher than those of the normal bucks. Body weight, testicular weight, gonadosomatic index and other gross testicular biometric data of the normal and unilateral cryptorchid bucks are shown in Table (3).
Table 3: Body weight and gross testicular dimensions of normal and unilateral cryptorchid goats (Mean± Standard error of the Mean (SEM)).
|
Morphological Parameters Body weight (kg) |
Cryptrochid bucks |
Normal bucks |
||
|
7.52±1.45 |
7.16±1.78 |
|||
|
Retained testis |
Scrotal testis |
Left testis |
Right testis |
|
|
Testis Weight (g) |
2.68 ± 0.95a |
14.43 ± 0.74b |
11.51 ± 0.55c |
12.16 ± 0.84c |
|
Gonado Somatic Index (%) |
0.34 ± 0.08 a |
1.91 ± 0.03 b |
1.47 ± 0.10c |
1.70 ± 0.22b |
|
Length of Testis (cm) |
2.63 ± 0.93 a |
4.68 ± 0.72 b |
4.17 ± 0. 45 b |
4.01 ± 0.30b |
|
Mid Circumference (cm) |
2.58 ± 0.25 a |
4.32 ± 0.32 b |
3.15 ± 0.17 c |
3.19 ± 0.22c |
|
Thickness of testis (cm) |
0.88 ± 0.14 a |
3.67 ± 0.32 b |
2.48 ± 0.17c |
3.01 ± 0.50c |
|
Means with different superscripts on the same row are significantly different (P< 0.05) |
||||
The weight of the retained testes was significantly (p < 0.05) less than those of the contralateral scrotal testes and the normal testes, respectively. Compared with the normal, the testicular weight of the contralateral scrotal testes of the unilateral cryptorchid bucks was significantly higher (p < 0.05). The gonado-somatic index of the controlateral scrotal testes of unilaterally cryptorchid bucks was significantly higher (p < 0.05) than those of the normal bucks. The contralateral scrotal testes of the cryptorchid bucks were observed to be significantly (p < 0.05) longer than those of the normal testes and the retained testes. Similarly, both the circumference and thickness of the testes were greater (p < 0.05) in the contralateral scrotal testes compared to the normal testes.
DISCUSSION
The ejaculate colour obtained in this study was common to both the normal and the unilateral cryptorchid bucks. The colour similarity is an indication that unilateral cryptorchidism did not affect the colour of the ejaculate as the colour agrees with the standard [15]. The normal semen colour suggests absence of overt clinical condition. Semen colour is considered insignificant in assessing sperm fertilization potential; however, it may be agood sign of associated clinical condition [16]. Semen volume obtained in this study fell within the reference range as reported for goats [17]. This finding is an indication that unilateral cryptorchidism does not reduce ejaculate volume. Thus, the functions of accessory sex glands were not compromised in unilateral cryptorchidism as normal semen volume is an indication of the positive functional status of the accessory sex glands [15]. The spermatozoa from the unilaterally cryptorchid and normal bucks were morphologically similar. This similarity indicates that at testicular level, germ cell differentiation in the contralateral scrotal testis, the only source of spermatozoa, is not disturbed by unilateral cryptorchidism. The spermatozoa morphology reported in this study is similar to earlier reports on the characteristics of the semen of West African Dwarf bucks [17,18]. Sperm morphological abnormalities found in the present study were probably not associated with the cryptorchid condition. The presence of abnormal forms of spermatozoa in both the normal and cryptorchid bucks is consistent with the report of [19], that a number of abnormal forms of spermatozoa are normally encountered in all ejaculates. The abnormalities are of clinical importance only when the percentage is high. In contrast to the present finding, [20] reported a significantly higher frequency of primary abnormalities in unilateral cryptorchidism. The finding of [20] may have been influenced by the number of animals used, since statistical evaluation may be biased by the great differences between sample numbers. The cryptorchid buck’s and the normal buck’s sperm concentrations were within the reference range for goats [21]. The sperm concentration in the normal and cryptorchid bucks were also found to be comparable. This observation suggests that the descended scrotal testes of the cryptorchid bucks were able to supply similar sperm cell concentration as two scrotal testes of the normal bucks. Thus, unilateral cryptorchidsm in West African Dwarf goats did not impair the sperm production of the scrotal testis. Similar result was reported by [7] in unilaterally cryptorchid Sahel goats. Although [22] reported that 90% of unilateral cryptorchid human patients showed normal sperm concentration. [23] found a drastic impairment of semen parameters in men with history of cryptorchidism. The disparity between these studies in humans may be attributed to the experimental procedures employed as most of the patients used for the latter study were obtained from infertility clinics.
The high percentage sperm motility observed in the present study suggests that the semen were of high quality, an indication that at epididymal level, the morphological maturation of spermatozoa was not altered. However, [19] reported an alteration in sperm motility in spontaneous unilateral abdominal cryptorchid boars and [24] reported similar observation in rats. The alteration was attributed to dysfunction in the epididymal epithelium or the accessory sex glands. The reason for this disparity may be due to the age of the animal model and duration of the cryptorchidism. The increased serum testosterone level observed in the present study is most probably a result of hyperplasia of the Leydig cells and enhanced development of organelles associated with steroid synthesis in the contralateral scrotal testis [25]. Similar finding was reported in cryptorchid rats [26]. The elevated testosterone concentration is expected to induce secondary sexual characteristics and improved libido. Conflicting data exist concerning the effects of cryptorchidism on serum hormone levels. Serum testosterone has been reported to increase [27], decrease [28] or remain unchanged [29] following cryptorchidism. The discrepancies in these reports may be attributed to the heterogeneity in the various study populations, differences in the exact location of the retained testis and experimental procedures utilized. The serum level of luteinizing hormone was observed to be significantly higher in the unilateral cryptorchid bucks compared to the normal bucks. It is probable that the suprascrotal temperature interfered with testosterone synthesis in the retained testis, prompting an increase in luteinizing hormone secretion. This suggests a compensatory action on the scrotal testis. The elevated serum luteinizing hormone level associated with increased testosterone level also suggests the existence of compensated Leydig cell function in unilateral cryptorchidism. Elavated luteinizing hormone [30], normal levels [31] sub normal levels [32] have been reported in unilateral cryptorchidism. The differences observed in the follicle stimulating hormone concentrations were not significant between the groups. However, the absolute values of follicle stimulating hormone were slightly elevated in the unilateral cryptorchid bucks. The elevated follicle stimulating hormone suggests compromised function of Sertoli cell in the retained testis. Similar opinion was expressed by previous authors [33]. The weight of the contralateral scrotal testis of the unilateral cryptorchid bucks was significantly higher than those of the retained testis as well as the testes of the normal bucks. The observed increase in weight may be due to compensatory hypertrophy of the contralateral scrotal testes. The increase in weight of the contralateral scrotal testis could be physiological phenomenon to enable the contralateral scrotal testis perform the function of two normal testes as suggested by [34]. Several authors have reported hypertrophy of the contralateral scrotal
testes in different animal models [35,36]. Generally, these authors agreed that removal or damage to one of a pair of glandular organs frequently result in compensatory hypertrophy of the cells of the remaining intact gland. In the present study, the weight of the retained testes was significantly lower than that of the contralateral scrotal testis of the cryptorchid bucks and also lower than weight of the testis of the normal bucks. The observed atrophy most probably resulted from the impact of the abdominal temperature on the retained testis which may have caused degenerative changes in the testis. Similar opinion has been expressed by other authors [37].
CONCLUSION
In conclusion, the study demonstrated contralateral scrotal testes hypertrophy, elevated testosterone levels and normal semen profiles in unilateral cryptorchid West African Dwarf bucks. These findings have provided an explanation for the improved salaciousness of hemicryptorchid West African dwarf bucks as observed by local farmers in South east Nigeria.
REFERENCES
1. Emehelu CO, Ekwueme EC, Chah FK. Cryptorchidism in West African Dwarf goat in Nsukka Agricultural zone of Enugu State, Nigeria. Sahel J Vet Sci. 2005; 4: 59-61.
13.Blom E. Physiology and pathology of reproduction. Med Weter. 1981; 4: 239-242.
14.Bearden HJ, Fuquay JW. Applied Animal Reproduction. 4th edn. New Jersey, Upper Saddle River, USA, Prentice-Hall Inc. 1997.
16.Oyeyemi MO, Akusu MO. Response of West African Dwarf Goats to concentrate supplementation. Vet Arhiv. 2002; 72: 29-38.
20.Refsal KR. Collection and evaluation of caprine semen. In: D. A. Morrow (Ed.): Current Therapy in Theriogeneology. W. B. Saunders, Philadelphia, 1986; 619-621.
22.Hadziselimovic F. Cryptorchidism, its impact on male fertility. Eur Urol. 2002; 41: 121-123.
25.Risbringer GP, Kerr JB, de Krester DM. Evaluation of Leydig cell function and gnadotrophin binding in unilateral and bilateral cryptorchidism: evidence for local control of Leydig cell function by seminiferous tubule. Biol Reprod. 1981; 24: 534-540.
29.Hedinger E. Histopathology of undescended testes. Eur J Pediatr. 1982; 139: 266-71.
34.Gaudino R, Cavarzere P, Camilot M, Teofoli F, Zampieri N, Tato L. Prepubertal serum inhibin B in cryptorchid infants and in monorchid boys with compensatory testicular testicular hypertrophy. Fertil. Steril. 2008; 90: 2217-2221.