Primary Oral or Intramuscular Vaccination Followed by a Vaginal Pull Combine to Reduce Recurrent Genital Pathology in HSV-Infected Guinea Pigs
- 0. LKT and ERB contributed equally to the study/manuscript
- 1. Department of Centre for Immunology and Infection Control and School of Biomedical Sciences, Queensland University of Technology, Australia
- 2. Department of Otago Innovation Ltd, University of Otago, New Zealand
Abstract
HSV-2 infected over 491 million people in 2016. Reactivation of infection is frequent, causing ulceration of the genital mucosae and viral shedding. Recent studies have suggested that local tissue-resident memory CD8 cells (Trm) are important for protection against HSV infection. Using a guinea pig model, we asked if oral or intramuscular vaccination, followed by transient induction of local inflammation by poly I:C or R848 could protect against recurrent genital pathology in a therapeutic setting. HSV-2 infected female guinea pigs were immunised either orally with live-attenuated HSV-2 in (LiporaleTM) or intramuscularly (IM) with killed HSV-2 in Alum/MPL, either alone or followed by local vaginal application of the inflammatory TLR agonists poly I:C or R848 and monitored for recurrent pathology for 60 days. Both oral immunisation and IM immunisation reduced recurrent pathology in HSV-2-infected guinea pigs. Protection elicited by oral immunisation was further enhanced by vaginal application of both R848 and Poly I:C, while IM immunisation induced protection was only increased by application of R848. Activation of either mucosal or systemic immunity by oral or IM immunisation, combined with induction of transient local genital inflammation provides significant protection against severity of recurrent HSV-2 pathology in guinea pigs.
Keywords
• Herpes
• Guinea pig
• Tissue-resident memory
• Oral vaccination
• Intramuscular vaccination
• Prime and pull
• CD8
CITATION
Trim LK, Bryan EB, Mulvey P, Aaskov JG, Sweeney EL, et al.(2023) Primary Oral or Intramuscular Vaccination Followed by a Vaginal Pull Combine to Reduce Recurrent Genital Pathology in HSV-Infected Guinea Pigs. Ann Reprod Med Treat 6(1): 1027.
INTRODUCTION
Herpes simplex virus 2 (HSV-2) is the most common cause of genital herpes (WHO https://www.who.int/news-room/fact- sheets/detail/herpes-simplex-virus) with prevalence in people aged 15-49 exceeding 50% in some parts of Africa and South America (WHO). Infection is life-long and recurrent symptoms are common. Virus can be transmitted to new sexual partners during both symptomatic and asymptomatic infections. Over time recurrences become less frequent and severe, suggesting that local immunity may occur [1]. Recent studies have suggested that local tissue-resident memory CD8 cells (Trm) [2-5] are essential for protection against both primary and recurrent infection. These cells can be elicited by either natural infection or vaccination (prime) but must be actively recruited (pulled) into the epithelium, where they reside for months/years, providing immediate local protection upon challenge or reactivation.
To date, there are no prophylactic or therapeutic vaccines available to prevent human HSV-2 infections, despite large human trials of subunit-based vaccines produced by Chiron (gB/gD- MF59 Vaccine) and GSK (gD-ASO4 Vaccine, SimplirixTM). However, the recent development of two therapeutic vaccines for another alphaherpes virus, Varicella zoster (Chickenpox) suggests that a therapeutic vaccine for HSV may be achievable. Zostavax® is a live attenuated vaccine, based on the varicella Oka strain (VARIVAX®) [6]. ShingrixTM contains a single viral glycoprotein, gpE adjuvanted with the ASO1B adjuvant [7]. Both vaccines prevent/ reduce Shingles/postherpetic neuralgia caused by reactivation of Varicella. Perhaps surprisingly, the greatest efficacy (>90%) was obtained using the ShingrixTM vaccine even in patients over 80 years of age [8,9]. The success of these therapeutic vaccines maybe due to the less stringent requirements for reactivation and expansion of existing long-lived, infection-induced memory T cells [10] as opposed to prophylactic vaccines which must activate naïve T cells using dendritic cell co-stimulation. With almost half a billion people already suffering from genital HSV infections (WHO) the development of a therapeutic HSV vaccine would have a major public health benefit.
In mice, priming of protective HSV-specific CD8 T cells was achieved by subcutaneous [2] or oral [5] administration of attenuated thymidine kinase-negative HSV (TK-HSV) [11]. Recruitment into the genital tract was achieved by local vaginal application of chemokines CXCL9 and CXCL10 [2] or the non- specific inflammatory agent DNFB [5]. In the mouse model however, infection with wild-type HSV is lethal in control mice, and recurrent infections do not occur, so studies of therapeutic vaccines that prevent reactivation cannot be undertaken. In guinea pigs however, the initial vaginal infection resolves, but recurrent infections and associated genital pathology occur as in humans, making it ideal for evaluating a therapeutic vaccine [12]. Like humans, guinea pig HSV infection also elicits CD8 memory cells that could potentially be mobilised with a therapeutic vaccine. We have therefore used the guinea pig model to evaluate a ‘Prime and Pull’ vaccine approach in a therapeutic setting. Specifically, we investigated (i) if oral immunization of guinea pigs with attenuated HSV in LiporaleTM [13, 14], or intramuscular immunization (IM) with killed virus in Alum/MPL, initiated after challenge infection reduced the incidence and severity of recurrent HSV pathology in guinea pigs. (ii) if local vaginal application of R848 and poly I:C, initiated after challenge infection reduced the incidence and severity of recurrent HSV infection in guinea pigs and (iii) would an oral or IM immunization followed by vaginal pull synergize to further enhance protection.
MATERIALS AND METHODS
Animals, Cells and Viruses
Female outbred tri-colour guinea pigs (6-8 weeks) were obtained from the Flinders University (Adelaide, South Australia). All procedures were performed at the QIMR Berghofer Medical Research Institute under QIMRB, Ethics Approvals P2161 and P2314. TK- 11and WT HSV-2 virus strains were kindly provided by Professor Nicholas King at the Sydney Institute of Emerging Infectious Diseases and Biosecurity (SEIB).
Thymidine kinase negative (TK-) and HSV-2 strain 333 were grown in Vero cells and purified as described [5].
Guinea pig model
Guinea pigs were anaesthetised using isoflurane. The vaginal membrane was ruptured using a sterile swab moistened with sterile saline. Guinea pigs were inoculated intravaginally with 1x106 PFU WT HSV-2 strain 333 in 50uL PBS and monitored until normal breathing returned.
Vaccine Preparation
LiporaleTM [13] adjuvant was provided by Immune Solutions Ltd (Dunedin, New Zealand). The oral vaccine was prepared by combining live TK-HSV with LiporaleTM adjuvant heated to 37oC to a final concentration of 1x108 PFU in 200uL. LiporaleTM only controls were combined with sterile PBS. The intramuscular vaccine was formulated by combining 1x108 PFU of UV-inactivated TK-HSV with 275ug Alum (InivoGen, Jomar Life Research, VIC, Australia) plus 12.5ug MPL (InivoGen). Vaccines were mixed for 5 minutes before being administered to guinea pigs.
Oral Vaccination
Guinea pigs (n=6/group) were immunised on days 7, 14, 21 and 28 post-infection, using a sterile syringe fitted with a ball- end feeding needle (1x108 PFU in 200uL). Guinea pigs were restrained, the syringe was inserted into the back of the mouth and expelled. All animals were observed to swallow the vaccine.
Intramuscular Vaccination
On days 14 and 28 post-infection, guinea pigs (n=6/group) were anaesthetised using isoflurane and administered 100uL vaccine into the biceps femoris using a 26-gauge needle. Guinea pigs were monitored until ambulant then returned to the cage.
Vaginal Pulls
On day 35 post-infection (1 week after completion of vaccination), 100uL poly(I:C) (InvivoGen) (1mg/mL) or 100uL PBS was intravaginally administered to guinea pigs. This procedure was repeated on day 36 and 37 for groups with repeated (x3) doses.
On day 35 post-infection, 100uL of 1% R848 (InvivoGen) was intravaginally administered to guinea pigs. This procedure was repeated on day 39 and 41 using 100uL 0.5% R848 for groups with repeated doses.
HSV-2 Pathology
Guinea pig pathology was monitored daily and scored as follows; 0, no redness/disease; 1, redness and/or swelling; 2, a few small vesicles; 3, several large vesicles; 4, several large ulcers with skin breakages. At stage 4, guinea pigs were euthanised as a humane endpoint [12].
CD8 staining of reproductive tracts
At sacrifice, reproductive tracts (Vaginae and uterine horns) were collected and fixed in neutral-buffered formalin. Serial sections from tissues (3 animals per group) were cut and stained for CD8 cells using the guinea pig CD8-specific monoclonal antibody (MCA752S, BioRad, Australia) by the QIMR Histology Facility. CD8 cells per mm2-field were counted and quantitated using Aperio ImageScope software (Leica Biosystems, Mount Waverley, VIC, Australia). Results are presented as CD8 positive cells per mm2.
Statistical Analysis
Cumulative pathology scores were calculated by summing daily pathology scores for each group (n=6) and analysed using linear regression to calculate the slope of the curve or Rate of Pathology (ROP). ROP between groups were analysed using one-way ANOVA and Tukey’s HSD test for multiple comparisons. Differences between CD8 cell numbers were analysed by 2-way ANOVA with Tukey’s HSD test (GraphPad Prism V9). Statistical significance is denoted by *p<0.05, **p<0.01, ***p<0.001 and ****p<0.0001.
RESULTS
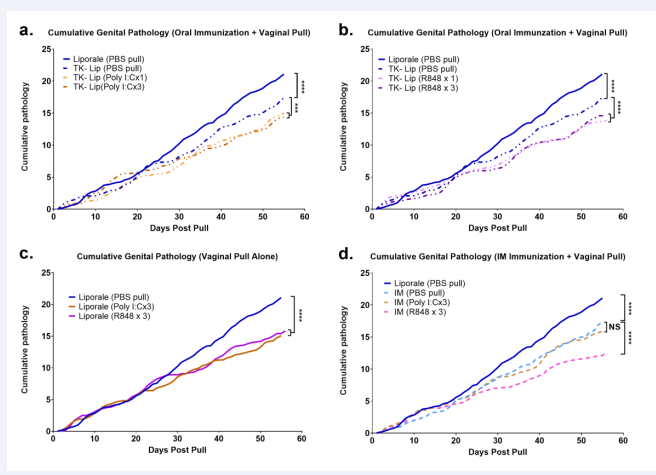
We investigated if oral immunisation of GP with attenuated HSV in LiporaleTM protected GP against recurrent pathology and if this protection was further enhanced by local vaginal application of poly I:C or R848. Figure 1a s
Figure 1: Guinea pigs were infected vaginally with WT HSV-2 (day 0) then orally vaccinated 4x (days 7, 14, 21 and 28) with thymidine kinase -ve HSV-2 in LiporaleTM. Starting 1 week after vaccination either poly I:C (Figure 1A) or R848 (Figure 1B) was applied intravaginally 1x (day 35) or 3 times (days 35-37) post-infection. Control guinea pigs immunised with PBS in LiporaleTM were treated 3x with either poly I:C or R848 (Figure 1C). Guinea pigs were also vaccinated intramuscularly on days 14 and 28 with killed HSV-2 in Alum/MPL adjuvant. Starting 1 week after vaccination either poly I:C (x3) or R848 (x3) was applied intravaginally (Figure 1D). Genital pathology was scored as described in materials and methods. Cumulative pathology scores were calculated by summing daily pathology scores for each group (n=6) and were analysed using linear regression to calculate the slope of the curve or Rate of Pathology (ROP). ROP were analysed using one-way ANOVA and Tukey HSD test for multiple comparisons. Statistical significance is denoted by *p<0.05, **p<0.01, ***p<0.001 and ****p<0.0001.
hows that oral immunisation alone significantly reduced the cumulative pathology score compared to non-immunised animals (P<0.0001). Intravaginal pull with poly I:C [Figure 1a] or R848 [Figure 1b], following oral vaccination, further reduced the incidence of cumulative pathology (P<0.001). There was no detectable difference in pathology between GP receiving either 1 or 3 intravaginal administrations of poly I:C or R848.
Interestingly, when looking at the rates of recurrent pathology in control non-immunised guinea pigs that received only the vaginal pulls [Figure 1c] we found that vaginal application of either Poly I:C or R848 alone also significantly (p<0.001) reduced the cumulative pathology score.
For comparison, GP were also immunised intramuscularly (IM) with killed virus combined with Alum/MPL adjuvant [Figure 1d]. IM immunisation also significantly reduced the cumulative pathology score (P<0.0001). Interestingly, protection was further enhanced by R848 (x3) intravaginal pull (P<0.0001) but not by a poly I:C (x3).
The two vaccines that elicited the greatest reduction in cumulative pathology were oral immunisation with attenuated virus in LiporaleTM followed by R848 (x3) and IM immunisation with killed virus followed by R848 (x3) [Table 1].
Table 1: Comparison of cumulative rates of pathology in “prime” and “pulled” Guinea pigs.
|
|
Oral TK- Liporale/R848 x 3 vs |
|
|
|
Significance |
P value |
|
Liporale/PBS pull |
**** |
<0.0001 |
|
TK- Lip/PBS pull |
**** |
<0.0001 |
|
Liporale/R848 x 3 |
*** |
<0.0004 |
|
IM/R848 x 3 |
NS |
0.73 |
|
|
IM/R848 x 3 vs |
|
|
|
Significance |
P value |
|
Liporale/PBS pull |
**** |
<0.0001 |
|
IM/PBS pull |
**** |
<0.0001 |
|
Liporale/R848 x 3 |
**** |
<0.0001 |
|
Oral TK-Lip/R848 x 3 |
NS |
0.73 |
Cumulative pathology scores were analysed using linear regression to calculate the slope of the curve or Rate of Pathology (ROP). ROP between experimental groups was compared using one-way ANOVA and Tukey HSD test for multiple comparisons. The ROP of animals receiving both immunisation (oral or IM) plus a R848 vaginal pull was significantly less than either the immunisation or pull alone.
When compared to all treatments, oral immunisation plus R848 vaginal pull was significantly more protective than either oral immunisation alone or R848 pull alone, although not significantly different than IM immunisation plus R848 pull. Similarly, IM immunisation plus R848 pull was significantly better than either IM immunisation alone or R848 pull alone but not significantly different to oral immunisation plus R848. Thus, in both cases, immunisation (either oral or IM) synergised with R848 vaginal pull to reduce the rate of pathology whilst only oral immunisation was enhanced by poly I:C pull [Figure 1a].
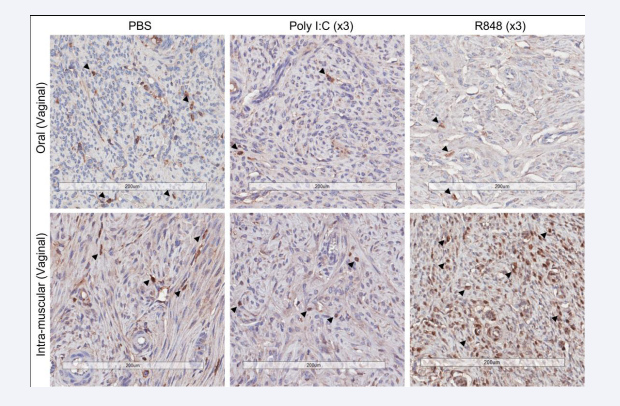
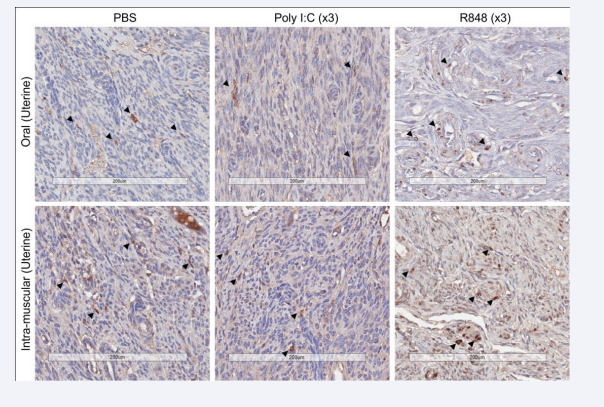
Protection against HSV infection has been associated with local CD8 Trm cells. Thus, CD8 cell numbers in were evaluated by immunohistochemistry at day 60. (Representative staining of vaginal and uterine tissues are shown in Supplementary Figures 1 and 2.
Supplementary Figure 1: Representative vaginal sections from orally and IM-immunised guinea pigs followed by vaginal pull with PBS, poly I:C or R848, stained with anti-GP CD8 as per M and M.
Supplementary Figure 2: Representative uterine horn sections from orally and IM-immunised guinea pigs followed by vaginal pull with PBS, poly I:C or R848, stained with anti-GP CD8 as per M and M.
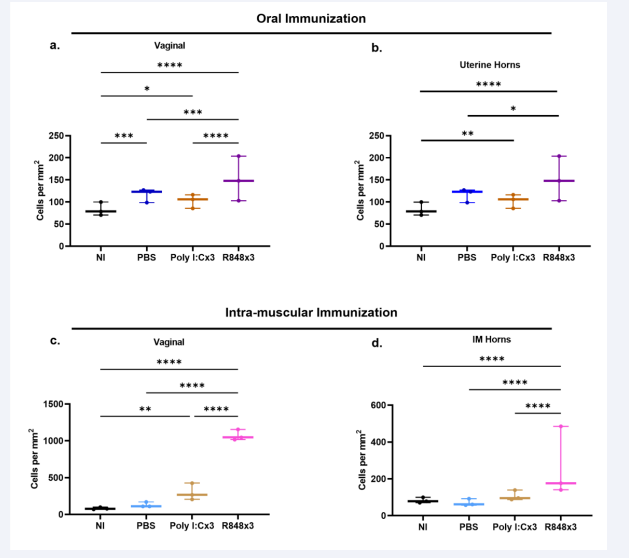
Non-immunised, infected Guinea pigs had ~75 CD8 cells per mm2, likely elicited by repeat infections. Oral immunisation significantly increased CD8 cells (~120 CD8 cells per mm2) in both vaginal and uterine tissues [Figures 2a and 2b].
Figure 2: At sacrifice (day 60) vaginae and uterine horns were collected and fixed in neutral buffered formalin. Serial sections were cut and stained for CD8 as described. CD8 cells were counted in 5 fields per section and expressed as cell per mm2 of tissue. Differences between CD8 cell numbers were analysed by Multiple Comparisons 2-way ANOVA (Graphpad Prism V9). Statistical significance is denoted by *p<0.05, **p<0.01, ***p<0.001 and ****p<0.0001. Panels 2a and 2b represent non-immunised animals (NI) and animals orally immunised with TK- HSV in Liporale with no pull (PBS), poly I:C pull (Poly I:C x3) or R848 pull (R848 x 3). Panels 2c and 2d represent non-immunised animals (NI) and animals immunised intramuscularly with inactivated TK- HSV in Alum/MPL with no pull (PBS), poly I:C pull (Poly I:C x3) or R848 pull (R848 x 3).
Oral immunisation followed by poly I:C pull did not further increase CD8 cell numbers in either vaginal or uterine tissues, whilst vaginal application of R848 following oral vaccination significantly increased CD8 cells in vaginal tissues and to a lesser extent in uterine horns. Following IM immunisation, vaginal tissues [Figure 2c] contained ~120 cells per mm2 and uterine horns [Figure 2d] ~50 cells per mm2. IM immunisation alone did not significantly increase CD8 cells in either tissue compared to non-immunised controls, however R848 vaginal pull significantly increased CD8 cells in both tissues, most notably in vaginal tissues where CD8 cell numbers increased 8-10-fold. Thus the highest numbers of CD8 cells, after both oral and IM immunisation, were seen in animals receiving topical R848, the groups with the greatest reduction in cumulative pathology.
DISCUSSION
Currently there are no licensed vaccines targeting genital herpes infections in humans. However, the recent success of two therapeutic vaccines for recurrent Varicella infections, (ZostaVax and Shingrix®), suggests that a therapeutic vaccine that reduces or prevents recurrent genital tract HSV-2 infections may be possible. We previously showed that oral immunisation of mice with attenuated HSV, followed by a vaginal pull with DNFB, protected mice against HSV infection [5]. Recurrent infections cannot be studied in the mice however as primary infection is lethal in non- vaccinated mice. Infection of guinea pigs, however, establishes a latent infection that reactivates resulting in recurrent genital HSV infections [12]. Using the guinea pig model, we now show that oral immunisation of guinea pigs with an attenuated HSV or IM immunisation with killed virus, followed by a vaginal pull, reduces the cumulative pathology of an established HSV infection in a therapeutic setting.
Using immunostimulatory agents to recruit vaccine induced T cells into the genital tract has been demonstrated previously [15]. This study used the TLR7 imidazoquinoline imiquimod to reduce HSV pathology in infected guinea pigs. We expand this work by using two immunostimulatory agents that target other TLR and are approved for human use. Poly I:C is a viral dsRNA mimic that
stimulates innate immunity primarily via binding to TLR3. Stimulation also occurs through binding to MDA-5 and RIG-1. It has been used as an adjuvant for many years and is undergoing testing as an immunotherapy in human glioblastoma trials [16]. Poly I:C induces secretion of type 1 interferons and multiple pro- inflammatory cytokines characteristic of an anti-viral response. Resiquimod (R848) is also an imidazoquinoline that activates TLR7 and TLR8. In clinical studies R848 has been used to successfully treat hepatitis C [17] and herpes simplex virus [18] infections, where it reduces viral shedding. R848 also suppresses HIV replication in monocytes [19]. R848 is available as a topical cream for treatment of anogenital warts and has been shown to induce disease regression in cutaneous T-cell lymphoma [20]. Interestingly, we detected a reduction in pathology with poly I:C after oral, but not intramuscular, immunisation. Conversely, topical R848 enhanced the protection elicited by both oral and intramuscular immunisations, indicating a relationship between route of immunisation and pull agents. Future studies investigating the local T cell chemokines elicited after intravaginal administration of immunogenic compounds would prove useful for matching immunisation route and pull compound for further translation into human studies [21].
Because tissue resident CD8 T cells have been shown to be important for clearance of HSV in mice we determined the numbers of CD8 cells in vaginal and uterine horn tissues of orally and intramuscularly immunised guinea pigs collected at sacrifice (day 60). Oral immunisation alone increased resident CD8 cells and R848 further increased CD8 cell numbers in vagina and uterine horns. Perhaps surprisingly, IM immunisation alone did not increase CD8 cells numbers compared to infected, non- immunised controls. Topical poly I:C did not further increase CD8 cells whereas R848 pull significantly increased CD8 cells, particularly in vaginal tissues. Topical application of both poly I:C and R848 to non-vaccinated guinea pigs did however reduce the cumulative rate of pathology [Figure 1C], indicating effects of both compounds that are independent of local CD8 cell numbers, most likely due to activation of type 1 interferon anti-viral immune mechanisms. Because our analysis used fixed tissues, we cannot rule out however that either poly I:C or R848 alters the phenotype of resident CD8 cells, possibly by increasing interferon gamma production or perforin/granzyme-mediated killing capacity, both characteristics of tissue-resident CD8 T cells [22]. It is also possible that CD8-expressing cells, other than conventional T cells, could be present in GP tissues as these have been identified in other species (CD8+ NKT-like cells [23], Innate CD8aa cells [24] and CD8 TCRgd cells [25]. Little is known about other CD8 cells in Guinea pigs. Our previous mouse studies demonstrated that genital CD8 Trm in mice secrete IFNg more rapidly than splenic CD8 cells following in vitro restimulation [5]. Another possible mechanism of protection that could be induced by either the “prime” or “pull” (or in combination) is the recruitment of non-HSV specific CD8 T cells with innate-like protective capacity, independent of TCR engagement [26]. This study found that immunisation of mice with an irrelevant antigen, prior to vaginal challenge with HSV-2, reduced the vaginal HSV- 2 burden due to early infiltration of antigen-non-specific CD8 T cells into the infected tissues. Local cytokine cues in the infected genital tissues were sufficient for bystander activation of these recruited memory CD8 cells.
One limitation of our study is that CD8 cell numbers were only evaluated at a single time point (day 60). Although HSV-specific Trm should remain in the genital mucosa and likely expand after each reactivation before returning to homeostatic levels [27,28], this single time point does not allow us to determine if bystander or antigen-specific CD8 cells were recruited following each reactivation of infection but may not have been retained. Studies of the kinetics of specific and non-specific CD8 T cell infiltration would be required to confirm these possibilities.
SUMMARY
In summary, combined oral immunisation with attenuated virus in LiporaleTM or IM immunisation with killed virus in Alum/MPL, followed by R848 (x3) pull significantly reduced cumulative rates of pathology. Oral immunisation followed by R848 vaginal pull was more protective than either oral immunisation alone or R848 pull alone. Similarly, the protection elicited by IM immunisation plus R848 pull was better than either IM immunisation alone or R848 pull alone. Thus, in both cases immunisation (oral or IM) synergised with R848 vaginal pull to reduce cumulative pathology.
ACKNOWLEDGEMENTS
We would like to thank Prof Nicolas King for the virus isolates, Queensland Institute of Medical Research animal house staff for assistance with the guinea pig experiments and Crystal Chang and Sang-Hee Park in the QIMR histology facility for assistance with CD8 cell staining. This work was supported by Immune Solutions Limited and Otago Innovation Ltd, Otago, New Zealand.
REFERENCES
- Groves MJ. Genital Herpes: A Review. Am Fam Physician. 2016; 93: 928-934.
- Shin H, Iwasaki A. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature. 2012; 491: 463- 467.
- Peng T, Phasouk K, Sodroski CN, Sun S, Hwangbo Y, Layton ED, et al. Tissue-Resident-Memory CD8(+) T Cells Bridge Innate Immune Responses in Neighboring Epithelial Cells to Control Human Genital Herpes. Front Immunol. 2021; 12: 735643.
- Mackay LK, Stock A, Ma JZ, Jones CM, Kent SJ, Mueller SN, et al. Long- lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc National Acad Sci U S A. 2012; 109: 7037-7042.
- Mulvey PBM, Trim LK, Aaskov JG, Bryan ER, Sweeney EL, Kollipara A, et al. Primary oral vaccination followed by a vaginal pull protects mice against genital HSV-2 infection. Am J Reprod Immunol. 2023; 89: e13668.
- Litt J, Booy R, Bourke D, Dwyer DE, Leeb A,McCloud P, Stein AN, et al. Early impact of the Australian national shingles vaccination program with the herpes zoster live attenuated vaccine. Hum Vaccin Immunother. 2020; 16: 3081-3089.
- Cunningham AL. The herpes zoster subunit vaccine. Expert Opin Biol Ther. 2016; 16: 265-271.
- Cunningham AL, Heineman T. Vaccine profile of herpes zoster (HZ/su) subunit vaccine. Expert Rev Vaccines. 2017; 16: 1-10.
- Cunningham AL, Levin MJ. Herpes Zoster Vaccines. J Infect Dis. 2018; 218: S127-S133.
- Low JS, Farsakoglu Y, Amezcua Vesely MC, Sefik, Kelly JB, Harman CCD, et al. Tissue-resident memory T cell reactivation by diverse antigen-presenting cells imparts distinct functional responses. J Exp Med. 2020; 217: e20192291.
- McDermott MR, Smiley JR, Leslie P, Brais J, Rudzroga HE, Bienenstock J. Immunity in the female genital tract after intravaginal vaccination of mice with an attenuated strain of herpes simplex virus type 2. J Virol. 1984; 51: 747-53.
- Hook LM, Friedman HM, Awasthi S. Guinea Pig and Mouse Models for Genital Herpes Infection. Curr Protoc. 2021; 1: e332.
- Aldwell FE, Tucker IG, de Lisle GW, Buddle BM. Oral Delivery of Mycobacterium bovis BCG in a Lipid Formulation Induces Resistance to Pulmonary Tuberculosis in Mice. Infection and Immunity. 2003; 71(1):101-108.
- 14. Aldwell FE, Baird M, Fitzpatrick CE, McLellan AD, Cross ML, Lambeth MR, et al. Oral vaccination of mice with lipid-encapsulated Mycobacterium bovis BCG: anatomical sites of bacterial replication and immune activity. Immunol Cell Biol. 2005; 83: 549-553.
- Bernstein DI, Cardin RD, Bravo FJ, Awasthi S, Lu P, Pullum DA, et al. Successful application of prime and pull strategy for a therapeutic HSV vaccine. NPJ Vaccines. 2019; 4: 33.
- De Waele J, Verhezen T, van der Heijden S, Berneman ZN, Peeters M, Lardon F, et al. A systematic review on poly(I:C) and poly- ICLC in glioblastoma: adjuvants coordinating the unlocking of immunotherapy. J Exp Clin Cancer Res. 2021; 40: 213.
- Thomas A, Laxton C, Rodman J, Myangar N, Horscroft N, Parkinson T. Investigating Toll-like receptor agonists for potential to treat hepatitis C virus infection. Antimicrob Agents Chemother. 2007; 51: 2969-2978.
- Mark KE, Corey L, Meng TC, Magaret AS, Huang M-L, Selke S, et al. Topical resiquimod 0.01% gel decreases herpes simplex virus type 2 genital shedding: a randomized, controlled trial. J Infect Dis. 2007; 195: 1324-1331.
- Nian H, Geng WQ, Cui HL, Bao M-J, Zhang Z, Zhang M, et al. R-848 triggers the expression of TLR7/8 and suppresses HIV replication in monocytes. BMC Infect Dis. 2012; 12: 5.
- Rook AH, Gelfand JM, Wysocka M, Troxel AB, Benoit B, Surber C, et al. Topical resiquimod can induce disease regression and enhance T-cell effector functions in cutaneous T-cell lymphoma. Blood. 2015; 126: 1452-1461.
- Smith JB, Herbert JJ, Truong NR, Cunningham AL. Cytokines and chemokines: The vital role they play in herpes simplex virus mucosal immunology. Front Immunol. 2022; 13: 936235.
- Behr FM, Chuwonpad A, Stark R, van Gisbergen K. Armed and Ready: Transcriptional Regulation of Tissue-Resident Memory CD8 T Cells. Front Immunol. 2018; 9: 1770.
- Wang C, Liu X, Li Z, Chai Y, Jiang Q, Wang Q, et al. CD8(+)NKT-like cells regulate the immune response by killing antigen-bearing DCs. Sci Rep. 2015; 5: 14124.
- Nazmi A, Hoek KL, Greer MJ, Piazuelo MB, Minato N, Olivares- Villagomez D. Innate CD8alphaalpha+ cells promote ILC1-like intraepithelial lymphocyte homeostasis and intestinal inflammation. PLoS One. 2019; 14: e0215883.
- Garcillan B, Marin AV, Jimenez-Reinoso A, Briones C, Ruiz MM, Leon MJG, et al. gammadelta T Lymphocytes in the Diagnosis of Human T Cell Receptor Immunodeficiencies. Front Immunol. 2015; 6: 20.
- Arkatkar T, Dave VA, Cruz Talavera I, Graham JB, Swarts JL, Hughes SM, et al. Memory T cells possess an innate-like function in local protection from mucosal infection. J Clin Invest. 2023; 133: e162800.
- Dave V, Richert-Spuhler LE, Arkatkar T, Warrier L, Pholsena T,Johnston C, et al. Recurrent infection transiently expands human tissue T cells while maintaining long-term homeostasis. J Exp Med. 2023; 220: e20210692.
- von Hoesslin M, Kuhlmann M, de Almeida GP, Kanev K, Wurmser C, Gerullis AK, et al. Secondary infections rejuvenate the intestinal CD103(+) tissue-resident memory T cell pool. Sci Immunol. 2022; 7: eabp9553.