Aerobic Exercise Improves Subcutaneous Adipocyte Differentiation in Rats with Different Obesity Levels by BMP4/PPAR Signal
- 1. School of Sports and Health, Nanjing Sport Institute, China
- 2. Department Science Experiment Center, Nanjing Sports Institute, China
Abstract
Background: Aerobic exercise can promote lipolytic potential, however the effect of aerobic exercise on lipids metabolism in individuals with different obesity levels remain unclear.
Methods and results: Sprague Dawley rats were fed a high-fat diet to establish models of different obesity degrees, and they were subjected to 8 weeks of treadmill running. The weight and fat weight of the rats were collected, and blood lipids and leptin levels were measured. Subcutaneous fat samples were taken and stained with hematoxylin-eosin (HE) to observe the morphology of adipocytes. The expressions of adipocyte differentiation proteins were determined by Western blotting. The aerobic exercise group exhibited significant reductions in body weight, subcutaneous fat weight, and body fat percentage compared to the control group. Additionally, there were significant decreases in serum total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C) and leptin levels, as well as a decrease in adipocyte volume. Furthermore, high-density lipoprotein cholesterol (HDL-C) levels increased significantly along with the expression of adipocyte differentiation proteins Bone morphogenetic protein 4 (BMP4) and drosophila mothers against decapentaplegic protein 1 (SMAD1) proliferator activator receptor gamma (PPARγ), etc.
Conclusions: Aerobic exercise significantly improved the body fat and lipid status of obese rats, and the improvement increased with increasing obesity. Exercise significantly reduced the volume of adipocytes, and the effect increased with increasing obesity. The effect of exercise on proteins involved in differentiation of subcutaneous adipocytes was not affected by obesity level.
KEYWORDS
- Exercise
- Obesity
- High fat diet
- Adipocyte
- BMP4
CITATION
Liu X, Yu L, Feng W, Wang Z, Sheng L (2025) Aerobic Exercise Improves Subcutaneous Adipocyte Differentiation in Rats with Different Obesity Levels by BMP4/PPAR Signal. Ann Sports Med Res 12(1): 1231.
ABBREVIATIONS
TC: Total Cholesterol; TG: Triglyceride; LDL-C: Low- Density Lipoprotein Cholesterol; HDL-C: High-Density Lipoprotein Cholesterol; BMP4: Bone Morphogenetic Protein 4; SMAD1: Drosophila Mothers Against Decapentaplegic Protein 1; ZNF423: Zinc Finger Protein 423; PPARγ: Proliferator Activator Receptor gamma; FABP4: Fatty Acid- Binding Protein 4; LPL: Lipoprotein Lipase
INTRODUCTION
With the improvement of people’s living standards, the number of obese individuals has been increasing year by year, which has a serious impact on their physical health. Obesity is caused by excessive fat accumulation in the body, mainly through an increase in the size and/ or number of adipocytes [1-3].White adipocytes play a key role in regulating fat mass and energy balance. Obesity is the result of imbalanced differentiation of white adipocytes in the body. A high-fat diet affects the number and size of adipocytes. Poret et al. [4], found that long-term consumption of a high-fat diet in rats led to a significant increase in the number and size of adipocytes in various body regions.
The formation of white adipocytes is regulated by numerous factors. Bone morphogenetic protein (BMP4) plays a crucial role in the early formation of white adipocytes [5,6]. It sends signals through its receptors to form a catalytic cell surface complex, which phosphorylates the downstream signal, Drosophila Mothers Against Decapentaplegic Protein 1 (SMAD1). Subsequently, SMAD1 binds to Zinc finger protein 423 (ZNF423) to form a complex, which activates peroxisome proliferator-activated receptor gamma (PPARγ), a key regulator in the nucleus that enhances the differentiation of adipocyte precursor cells [7]. Once activated, PPARγ binds to fatty acid-binding protein 4 (FABP4), thereby promoting the formation of mature adipocytes. In the process of individual obesity, the differentiation level of adipocytes plays a crucial role. An appropriate level of white adipocyte differentiation is essential for maintaining metabolic homeostasis. It is well known that exercise is an important way to reduce body fat and can regulate the differentiation of white adipocytes. However, the impact of exercise on adipocyte differentiation in individuals with varying degrees of obesity is seldom documented.
Based on the above, in this study, adult rats with varying levels of obesity underwent a moderate exercise intervention for 8 weeks to determine if aerobic exercise can enhance the lipid profile of obese rats and whether the extent of improvement differs with increasing obesity. Whether exercise reduces the volume of fat cells (adipocytes) and if this effect varies with increasing obesity. Whether the effects of aerobic exercise on adipose differentiation BMP4/PPARγ signaling differ in rats with varying levels of obesity.
MATERIALS AND METHODS
Experimental animals
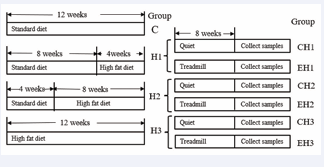
In this study, forty-four male Sprague-Dawley rats weighing approximately 200 grams and aged around 6 weeks were sourced from Zhejiang Weitong Lihua Experimental Animal Technology Co., Ltd. [SCXK 2021- 001] in Zhejiang, China. The rats were housed in controlled conditions in the animal facility for five days, with a temperature maintained at 25 ± 3°C and a 12-hour light/ dark cycle. The rats were given unrestricted access to standard chow and water in their cages. Initially, all rats were fed a standard diet for one week. Subsequently, 8 rats were randomly selected to form the standard control group (group C) and continued to receive the regular diet. Groups H1, H2, and H3 were fed high-fat diets for 4 weeks, 8 weeks, and 12 weeks, respectively. To ensure consistency in age among the experimental animals, specific feeding schemes are detailed in Figure1.
Figure 1: Experimental scheme diagram.
The criteria for successfully modeling different levels of obesity are as follows: the body weight of group H1, group H2, and group H3 exceeds 10% to 20%, 20% to 30%, and 30% to 50% respectively, of the body weight of the control group [8]. During the experiment, the rats’ growth was recorded daily, and their weight was measured weekly. The standard feed used in the experiment was purchased from SLACOM, with the product number P1101F-25. The high-fat feed was purchased from Research Diet, model D12451, with the specific feed formula detailed in Table 1. After successfully inducing various levels of obesity, the rats were divided into two groups: a quiet control group and an exercise group, named CH1 group, EH1 group, CH2 group, EH2 group, CH3 group, and EH3 group, each comprising 6 rats, as illustrated in Figure 1. Afterward, all groups were fed a high-fat diet. All procedures used in this study were approved by The Nanjing Sports Institute Animals Experiment Ethics Committee and adhered to the international ethical standards [9].
Exercise program
In the EH1, EH2, and EH3 groups, rats were first subjected to adaptive exercise for three days. They exercised for 15 minutes every day at an adaptive speed of 8 m/s. After the adaptation period, the rats underwent an eight-week treadmill training program. The training program was based on the experiment conducted by Fu Y et al. [10], and involved training five times a week. The exercise program for the rats is shown in Table 1.
Table 1: Eight-week treadmill training program
|
Mode of exercise |
Week |
Speed (m/s) |
Time (min) |
|
Treadmill running |
Week 1, week 2 |
8-10 |
60 |
|
Week 3, week 4 |
10-12 |
60 |
|
|
Week 5, week 6 |
12-14 |
60 |
|
|
Week 7, week 8 |
13-15 |
60 |
Serum index determination
After a successful modeling procedure, the rats were anesthetized, and blood samples were collected from the abdominal aorta. The serum was then separated by centrifugation at 3000 rpm, 4°C, for 15 minutes. Following the kit instructions, the levels of total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), insulin, and leptin were measured.
HE staining was used to detect the morphology of adipocytes
Theadiposetissuewasfixed with 4% paraformaldehyde, dehydrated, embedded in paraffin, and sectioned. After that, it was stained by HE. We analyzed the image using Image-Pro Plus 6.0 software. We measured the diameter of adipocytes and calculated the cell volume (V=4/3πr^3) based on the cell radius. To determine the total volume of subcutaneous fat, we utilized its mass and density. Finally, we calculated the total number of cells by dividing the total fat volume by the volume of a single adipocyte, following the specific calculation method outlined in Arnold’s literature [11]. Statistical analysis was performed to examine the data.
Western blot was used to detect the expression of adipose tissue protein
An appropriate amount of subcutaneous adipose tissue was collected and lysed at a ratio of 1:1 (weight/ volume). The mixture was homogenized in an ice bath and then placed on ice for 10 minutes. After centrifugation at 4°C for 10 minutes at a speed of 10000 revolutions per minute, the supernatant was aspirated, and the protein concentration was determined using the bicinchoninic acid (BCA) method. A 4% concentrated solution of SDS- PAGE separation gel was prepared. An appropriate amount of protein was sampled, electrophoresed, and transferred to a PVDF membrane. The membrane was then closed, incubated with the primary antibody, washed with TBST three times, incubated with the secondary antibody, washed with TBST three times, chemiluminescent signals were generated, and the results were photographed and analyzed using Image Lab software.
Statistical Analysis
All data were presented in the form of mean ± standard deviation. The data were analyzed and plotted using SPSS 26.0 and GraphPad Prism 5 software. We conducted an independent T-test between Group E and Group H. Additionally, a one-way ANOVA was performed to analyze the data among groups EH1, EH2, and EH3.
RESULTS
Effects of exercise intervention on body weight, subcutaneous fat weight, sebum ratio, and body fat ratio of rats
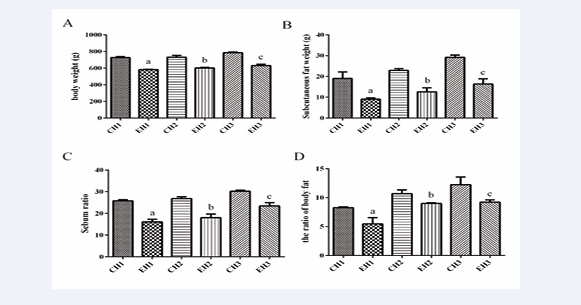
After 8 weeks of treadmill exercise, the body weight of rats in groups EH1, EH2, and EH3 showed a significant decrease compared to the corresponding control group (Figure 2A).
Figure 2: Exercise reduced the body weight, subcutaneous fat weight, sebum ratio and body fat ratio of rats with different levels of obesity. A body weight, B subcutaneous fat weight, C sebum ratio, D the ratio of body fat. Values are presented as mean ± SEM (n=8). a p < 0.05 (versus CH1 group); b p < 0.05 (versus CH2 group); c p < 0.05 (versus CH3 group); * p < 0.05 (versus EH1 group).
Moreover, the subcutaneous fat mass and sebum ratio were significantly reduced (Figure 2B,C), and the body fat ratio showed an extremely significant decrease (Figure 2D). Interestingly, after 8 weeks of exercise, the body weight, subcutaneous fat weight, and sebum ratio of the EH3 group were significantly higher than those of the EH1 group (Figure 2). However, no significant change in Lee’s index was observed among any of the exercise groups, in comparison to the control group.
Effects of exercise intervention on plasma lipoprotein levels, insulin and leptin in rats
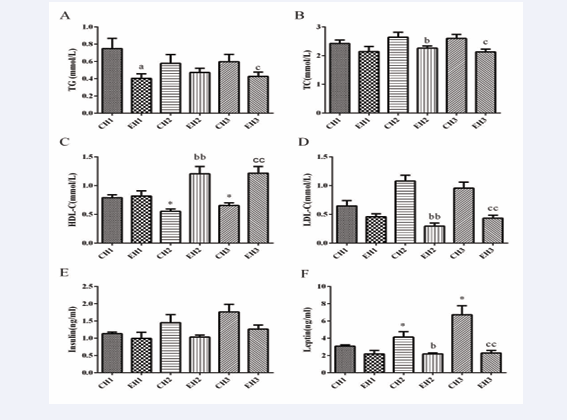
Significant differences were observed between the exercise intervention groups (EH1, EH2, EH3) and the quiet group in terms of serum total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglyceride (TG) levels (Figure 3). Compared with the quiet group, rats in the EH1 group showed a significant decrease in serum TG levels (Figure 3A).
Figure 3: Exercise decreased plasma lipoprotein and increased plasma leptin levels in rats with different levels of obesity. A the content of TG in serum, B the content of TC in serum, C the content of HDL-C in serum, D the content of LDL-C in serum, E the content of insulin in serum, F the content of leptin in serum, Values are presented as mean ± SEM (n=8). a p < 0.05 (versus CH1 group); b p < 0.05, bb p < 0.01 (versus CH2 group); c p < 0.05, cc p < 0.01 (versus CH3 group); * p < 0.05 (versus EH1 group).
Moreover, significant reductions in serum TC and LDL-C levels were observed in rats of the EH2 group, along with a notable increase in HDL-C levels (Figure 3B,C,D). In comparison, the EH3 group exhibited significant decreases in serum TG and LDL-C levels, accompanied by a significant increase in HDL-C levels (Figure 3A,C,D). Furthermore, rats in the EH3 group exhibited a significant increase in serum HDL-C levels compared to rats in the EH1 group (Figure 3C). Overall, the exercise intervention groups (EH1, EH2, EH3) showed significant improvements in serum lipid levels, including reductions in TC and LDL-C, as well as increases in HDL-C. These results demonstrate the beneficial effects of exercise on lipid metabolism.
The serum insulin concentration of rats in the EH1, EH2, and EH3 groups did not show any significant changes compared to the corresponding control group (Figure 3E). However, the serum leptin concentration in the EH2 group was significantly decreased, and the serum leptin concentration in the EH3 group was significantly reduced to an even greater extent (Figure 3F).
Influence of exercise intervention on adipocyte area and number
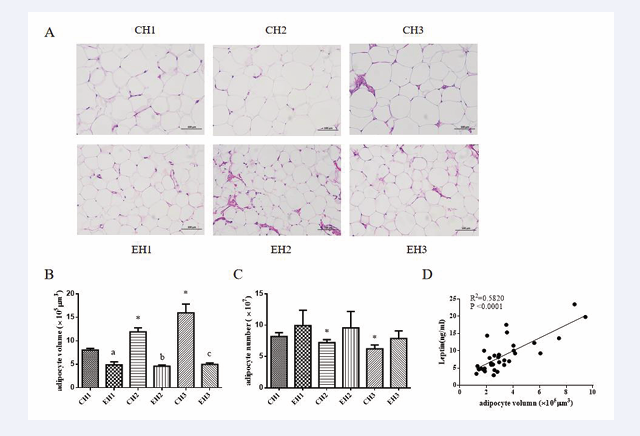
After 8 weeks of exercise training, approximately 300 mg of subcutaneous adipose tissue was collected from the rats. Subsequently, the tissue was photographed under a 200-fold field of view using a microscope (Figure 4A).
Figure 4: Exercise reduced the volume and size of adipocytes in rats with different levels of obesity. Representative image (A) and quantification (B, C) of Hematoxylin eosin staining (HE) of subcutaneous fat, correlation analysis of adipocyte volume and serum leptin (D). Values are presented as mean ± SEM. a p < 0.05 (versus CH1 group); b p < 0.05, c p < 0.05 (versus CH3 group); * p < 0.05 (versus EH1 group).
We found changes in the number and volume of adipocytes in the sections of rats with varying levels of obesity under both control and exercise conditions (Figure 4B). The area of subcutaneous adipocytes in the EH1, EH2, and EH3 groups showed a significant decrease (p < 0.05), while the number of adipocytes in the same visual field significantly increased (p < 0.05) (Figure 4C). Further analysis revealed a positive correlation between the size of adipocytes and the concentration of serum leptin (Figure 4D).
Western blot analysis detected fat-differentiated proteins after 8 weeks of exercise
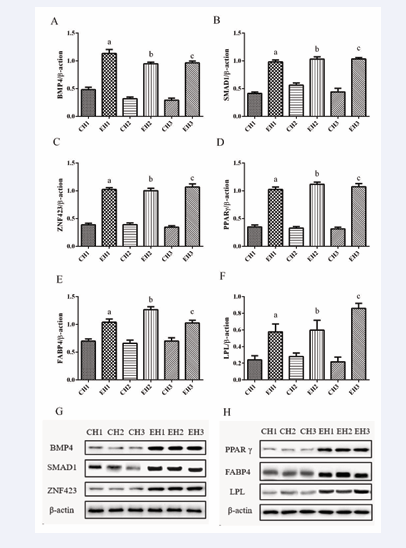
The results demonstrated that the expressions of fat- differentiated proteins BMP4, SMAD1, ZNF423, PPAR, FABP4, and LPL protein were significantly increased in the exercise group compared to the control group (p <0.05). Upon further examination of the results, it was noted that after 8 weeks of training, the expression of BMP4 was significantly higher in the EH1 group compared to the EH2 and EH3 groups (Figure 5A).
Figure 5: Exercise increased the expression of adipocytes differentiation-related proteins in rats with different levels of obesity. Representative image (G, H) and quantification (A-F) of western blot of BMP4 (A), SMAD1 (B), ZNF423 (C), PPARγ (D), FABP4 (E) and LPL (F) protein expression in the subcutaneous fat tissue of rats with different levels of obesity. Targeted bands were normalized by β-actin. Values are presented as mean ± SEM (n = 4). Values are presented as mean ± SEM. a p < 0.05 (versus CH1 group); b p < 0.05 (versus CH2 group); c p < 0.05 (versus CH3 group).
The expression of FABP4 in the EH2 group was significantly higher compared to the EH1 and EH3 groups (Figure 5E). However, the expression of LPL was significantly higher in the EH3 group compared to the EH1 and EH2 groups (Figure 5F).
DISCUSSION
An individual’s body weight depends on the balance of energy metabolism [12]. When anabolism exceeds catabolism, the body stores excess energy as fat, which leads to weight gain. Exercise accelerates metabolism and, with consistent energy intake, can lead to weight loss. A study on rats with varying levels of obesity showed a significant reduction in body weight after exercise, which is consistent with previous reports [13,14]. This indicates that exercise plays a role in weight control for obese rats. Additionally, exercise significantly reduces subcutaneous fat mass, sebum, and body fat percentage in rats, as shown in a previous study. Obese rats undergoing exercise experienced reduced levels of lipid TC, TG, and LDL-C, and an increased level of HDL-C, aligning with previous reports [15]. Notably, the EH2 and EH3 groups had particularly significant outcomes, suggesting that exercise promotes lipid metabolism. Reduced subcutaneous fat mass, sebum, and body fat percentage, along with improved blood lipid levels, contribute to a decreased risk of coronary heart disease and overall health improvements. Furthermore, compared to the obesity groups, the body fat percentage, LDL-C, and HDL-C levels in the rats of the CH2 and CH3 groups showed no significant differences. However, the body fat percentage and LDL-C level in the CH2 and CH3 groups were significantly higher, and the HDL-C level was significantly lower than those in the CH1 group before exercise. This suggests that exercise has a more significant impact on lipid metabolism in the CH2 and CH3 groups of rats. Overall, the findings of this study suggest that exercise intervention has a positive effect on plasma lipoprotein levels in rats. Specifically, it leads to a reduction in TC and LDL-C levels and an increase in HDL-C levels. These findings offer valuable insights into the potential benefits of exercise in maintaining a healthy lipid profile.
In this study, it was found that the serum insulin and leptin levels of rats increased with obesity. Leptin, a hormone primarily secreted by adipocytes, directly acts on them and inhibiting cell synthesis in adipose tissue. With the increase in obesity, the volume of adipocytes also increases, leading to higher levels of leptin. This indicates the presence of leptin resistance in obese rats. However, exercise has been shown to significantly reverse this phenomenon. Exercise helps improve metabolic disorders in the body by affecting adipocytes directly and inhibiting adipose tissue synthesis. Our study detected an increase in adipocyte volume in the context of obesity, resulting in an elevated leptin level. This indicates the presence of leptin resistance in obese rats. Exercise can help improve the metabolic imbalance in the body. Furthermore, previous studies have shown inconsistent results regarding the effect of leptin concentration on adipocyte differentiation, emphasizing the need for further investigation to determine the specific reasons.
After 8 weeks of treadmill exercise, the volume of adipocytes in obeseratswassignificantlyreducedcompared to the control group. The volume of adipocytes is closely related to intracellular lipid droplets. As the capacity for fat storage and the number of lipid droplets increase, the size of adipocytes also increases. After exercise, the catabolic rate of rats exceeded the anabolic rate, resulting in the breakdown of lipid droplets in adipocytes and a reduction in adipocyte volume. Therefore, exercise improved the expansion of adipocytes caused by a high-fat diet, which is consistent with the findings of Pflugradt et al. [16], who observed a reduction in adipocyte volume after treadmill running and swimming exercises. Mendona et al. [17], also found a decrease in the volume of white adipocytes in obese mice undergoing aerobic training. These studies have shown that the reduction in adipose tissue weight is more closely related to adipocyte size rather than the number of adipocytes or the level of triglycerides [18], which supports the results of this study. Additionally, previous studies [19, 20] have shown that the proliferation ratio of adipocytes reflects the increase in the number of adipocytes to some extent. The proliferation ability of adipocytes increased after a high-fat diet in rats, indicating that a high-fat diet could promote adipocyte proliferation and increase the number of adipocytes. However, the proliferation of adipocytes in rats on a high-fat diet did not show significant changes after 2, 4, and 6 weeks of exercise but increased after 8 weeks. This indicates that exercise could reverse the adverse effects of a high-fat diet. The response of adipocyte proliferation to exercise factors required a certain period of time to accumulate, which aligns with the results of this experiment [19,20].
In this experiment, the relative expressions of adipose differentiation-related proteins BMP4, SMAD1, ZNF423, PPARγ, and FABP4 increased after exercise intervention in rats with varying levels of obesity. This suggests that exercise can enhance the differentiation of white adipocytes, aligning with previous reports [21,22]. Our previous study revealed a significant decrease in the expression of BMP4, SMAD1, and ZNF423 with increasing obesity. In this study, the expression levels of adipose differentiation-related proteins significantly increased in rats with varying degrees of obesity after exercise intervention. This suggests that exercise can reverse the effects of obesity on adipose differentiation and inhibit the adverse effects of obesity.
BMP4 is an important regulator of the differentiation of pluripotent stem cells into adipocytes [23]. During the obesity modeling, the expression of BMP4 in rats in the CH1 group was significantly higher than that in the CH3 group. After an 8-week exercise intervention, the expression of BMP4 protein in rats in the CH1 group was significantly higher than that in the CH2 and CH3 groups. This indicates that during the process of directional differentiation of pluripotent stem cells into adipocytes, the effect of exercise on the differentiation of the CH1 group was more significant. BMP4 can bind to downstream SMAD1 and translocate to the nucleus along with ZNF423 to regulate PPARγ, a marker of adipocyte differentiation, and control the late differentiation of adipocytes [24]. During the obesity modeling, the expression of the SMAD1 protein was higher in the CH2 group compared to the CH1 and CH3 groups. After 8 weeks of exercise intervention, there was a significant increase in SMAD1 protein expression in all three exercise groups, with no significant difference among the three exercise groups. ZNF423 is a significant factor in determining the differentiation of adipose stem cells into adipose precursor cells. After the 8-week exercise intervention, ZNF423 protein expression significantly increased in all exercise groups, with no significant difference among the three exercise groups. PPARγ is the central regulatory protein involved in the differentiation of adipose precursor cells. In this study, exercise promoted the expression of PPARγ protein in rats, which was consistent with findings from several researchers [25- 27]. In summary, exercise promotes the differentiation of adipose stem cells into adipose precursor cells, and it has a more significant effect on adipocyte differentiation in the CH1 group. During the differentiation into mature adipocytes, exercise had little impact on adipose cell differentiation in rats with different levels of obesity.
Lipoprotein lipase (LPL) is a marker of precursor fat cells [28]. After the exercise intervention, the relative expression of LPL protein in the adipose tissue of rats in different obesity groups significantly increased. This suggests that exercise can significantly enhance the expression of LPL protein in rats, indicating an increase in the number of fat precursor cells in the adipose tissue of rats following exercise intervention. Leptin is a crucial indicator of mature adipocytes. After exercise, there was a significant decrease in serum leptin content, indicating a reduction in the number of mature adipocytes in the rats. This finding aligns with the observation that the total number of adipocytes in the rats remained relatively constant after exercise. We also observed a positive correlation between leptin levels and the volume of fat cells. Following aerobic exercise intervention, there was a significant reduction in both fat cell volume and leptin levels, with this effect being more pronounced in individuals with higher degrees of obesity.
CONCLUSIONS
Exercise significantly reduced body weight and body fat percentage in rats with varying levels of obesity. It also improved the status of blood lipids and leptin, with enhancements increasing as obesity levels increased. Exercise significantly reduced the volume of adipocytes, and the reduction effect increased with higher levels of obesity. Exercise significantly increased subcutaneous adipocyte differentiation BMP4/PPAR signal, but this was not affected by the level of obesity. These findings further illustrate the importance of exercise in managing weight, regulating health, and differentiating fat.
ACKNOWLEDGEMENTS
This study was funded by projects of National Nature Science Foundation of China under Grant Number 31900844 and the Key Laboratory Open Project of Nanjing Sport Institute (SYS202106).
AUTHOR CONTRIBUTIONS
Zhaoxin Wang and Wanyu Feng were involved in the feeding and training of experimental animals. Li Yu was involved in the detection of experimental data. Lei Sheng was involved in the experimental design and supervision. Xiujuan Liu was involved in writing—original draft preparation and submission.
ETHICAL APPROVAL
The study was conducted in accordance with the Declaration of Helsinki and approved by the Nanjing Sports Institute Animals Experiment Ethics Committee (SYDW-2022-003).
REFERENCES
- Song T, Kuang S. Adipocyte differentiation in health and diseases. Clin Sci (Lond). 2019; 133: 2107-2119.
- Stenkula KG, Erlanson-Albertsson C. Adipose cell size: importance in health and disease. Am J Physiol Regul Integr Comp Physiol. 2018; 315: R284-R295.
- Tang QQ, Lane MD. Adipogenesis: from stem cell to adipocyte. Annu Rev Biochem. 2012; 81: 715-36.
- Poret JM, Souza-Smith F, Marcell SJ, Gaudet DA, Tzeng TH, Braymer HD, et al. High fat diet consumption differentially affects adipose tissue inflammation and adipocyte size in obesity-prone and obesity- resistant rats. Int J Obes (Lond). 2018; 42: 535-541.
- Hammarstedt A, Hedjazifar S, Jenndahl L, Gogg S, Grünberg J, Gustafson B, et al. WISP2 regulates preadipocyte commitment and PPARgamma activation by BMP4. Proc Natl Acad Sci U S A. 2013; 110: 2563-8.
- Bowers RR, Lane MD. A role for bone morphogenetic protein-4 in adipocyte development. Cell Cycle. 2007; 6: 385-9.
- Bjorntorp P, Bengtsson C, Blohme G, Jonsson A, Sjöström L, Tibblin E, et al. Adipose tissue fat cell size and number in relation to metabolism in randomly selected middle-aged men and women. Metabolism. 1971; 20: 927-35.
- Nie C, Yu H, Wang X, Xiahong Li , Zairong Wei , Xiuquan Shi, et al. Pro-inflammatory effect of obesity on rats with burn wounds. Peerj. 2020; 8: e10499.
- Tanisawa K, Wang G, Seto J, Verdouka I, Twycross-Lewis R, Karanikolou A, et al. Sport and exercise genomics: the FIMS 2019 consensus statement update. Br J Sports Med. 2020; 54: 969-975.
- Fu Y, Gao Y, Wang L, Chun-Jie G , Ya-Xuan L , Liang Y, et al. [Effects of alternate-day modified fasting combined exercise on fat reducing and the FNDC5/Irisin-UCP1 pathway]. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2022; 38: 577-583.
- Arnold SE, Lucki I, Brookshire BR, Carlson GC, Browne CA, Kazi H, et al. High fat diet produces brain insulin resistance, synaptodendritic abnormalities and altered behavior in mice. Neurobiol Dis. 2014; 67: 79-87.
- Melzer K, Kayser B, Saris WH, Pichard C. Effects of physical activity on food intake. Clin Nutr. 2005; 24: 885-95.
- Gorostegi-Anduaga I, Corres P, MartinezAguirre-Betolaza A, Pérez- Asenjo J, Aispuru GR, Fryer SM, et al. Effects of different aerobic exercise programmes with nutritional intervention in sedentary adults with overweight/obesity and hypertension: EXERDIET-HTA study. Eur J Prev Cardiol. 2018; 25: 343-353.
- Villareal DT, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento- Villareal R, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011; 364: 1218-29.
- Smith AC, Mullen KL, Junkin KA, Nickerson J, Chabowski A, Bonen A, et al. Metformin and exercise reduce muscle FAT/CD36 and lipid accumulation and blunt the progression of high-fat diet-induced hyperglycemia. Am J Physiol Endocrinol Metab. 2007; 293: E172-81.
- Pflugradt K, Hartmann N, Voss C. [Properties of adipocytes (diameter, volume, triglyceride content, cell number) of certain types of fatty tissue as a function of age in male Wistar rats]. Nahrung. 1978; 22: 219-28.
- de Mendonca M, Rocha KC, de Sousa E, Beatriz M V P, Oyama LM, Rodrigues AC, et al. Aerobic exercise training regulates serum extracellular vesicle miRNAs linked to obesity to promote their beneficial effects in mice. Am J Physiol Endocrinol Metab. 2020; 319: E579-E591.
- Bluher M, Michael MD, Peroni OD, Kohjiro U, Carter N, Kahn BB, et al. Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Dev Cell. 2002; 3: 25-38
- Sasoh T, Kugo H, Kondo Y, Miyamoto K, Minami M, Higashihara M, et al. Different effects of high-fat and high-sucrose diets on the physiology of perivascular adipose tissues of the thoracic and abdominal aorta. Adipocyte. 2021; 10: 412-423.
- Ellis JR, McDonald RB, Stern JS. A diet high in fat stimulates adipocyte proliferation in older (22 month) rats. Exp Gerontol. 1990; 25: 141-8
- Yuksel OB, Demiral I, Zeybek U, Celik F, Buyru F, John Y, et al. Effects of Irisin Compared with Exercise on Specific Metabolic and Obesity Parameters in Female Mice with Obesity. Metab Syndr Relat Disord. 2020; 18: 141-145.
- Majerczak J, Filipowska J, Tylko G, Guzik M, Karasinski J, Piechowicz E, et al. Impact of long-lasting spontaneous physical activity on bone morphogenetic protein 4 in the heart and tibia in murine model of heart failure. Physiol Rep. 2020; 8: e14412
- Wan DC, Shi YY, Nacamuli RP, Quarto N, Lyons MK, Longaker MT, et al. Osteogenic differentiation of mouse adipose-derived adult stromal cells requires retinoic acid and bone morphogenetic protein receptor type IB signaling. Proc Natl Acad Sci U S A. 2006; 103: 12335-40.
- Nohe A, Hassel S, Ehrlich M, Neubauer F, Sebald W, Henis YI, et al. The mode of bone morphogenetic protein (BMP) receptor oligomerization determines different BMP-2 signaling pathways. J Biol Chem. 2002; 277: 5330-8.
- Liu Q, Chen L, Liang X, Yuqing C, Xinyue Z, Wang S, et al. Exercise attenuates angiotensin-induced muscle atrophy by targeting PPARgamma/miR-29b. J Sport Health Sci. 2022; 11: 696-707.
- De Carvalho FG, Brandao C, Batitucci G, Anderson de Oliveira SA, Ferrari GD, Alberici LC, et al. Taurine supplementation associated with exercise increases mitochondrial activity and fatty acid oxidation gene expression in the subcutaneous white adipose tissue of obese women. Clin Nutr. 2021; 40: 2180-2187.
- Zheng F, Cai Y. Concurrent exercise improves insulin resistance and nonalcoholic fatty liver disease by upregulating PPAR-gamma and genes involved in the beta-oxidation of fatty acids in ApoE-KO mice fed a high-fat diet. Lipids Health Dis. 2019; 18: 6.
- Liu Y, Sun WL, Sun Y, Hu G, Ding GX. Role of 11-beta-hydroxysteroid dehydrogenase type 1 in differentiation of 3T3-L1 cells and in rats with diet-induced obesity. Acta Pharmacol Sin. 2006; 27: 588-96.