Mechanisms Mediating Increased Exercise Capacity, Aerobic Capacity and Healthful Aging Induced by Adenylyl Cyclase Type 5 Inhibition
- 1. Department of Kinesiology and Health, Rutgers University, USA
- 2. Department of Cell Biology & Molecular Medicine, Rutgers University, USA
Abstract
The mouse with disruption of adenylyl cyclase type 5 (AC5 knockout, KO) is a longevity model, as it lives a third longer than littermates. More importantly, the AC5 KO is a model of healthful longevity, as reflected by enhanced exercise performance and protection, not only against obesity, diabetes, and metabolic syndrome, but also Alzheimer’s disease, cancer and myocardial ischemia and heart failure. All these salutary features are mediated by oxidative stress protection and enhanced mitochondrial function. The mechanisms are mediated by the decreases in cAMP and PKA and increases in Raf 1, and the MEK/ERK pathway, along with increases in SIRT1, FoxO3A, and MnSOD. The AC5 KO model of healthful longevity shares much in common with the Calorie Restriction model of healthful longevity. However, the longevity is not additive; actually, when calorie restriction is applied to the AC5 KO model, the result is deleterious, and survival is reduced from 3 years to one month. The purpose of this review is to highlight the unique features of the AC5 KO mouse with focus on mechanisms that are relevant not only to healthful aging, but for enhancing exercise performance and aerobic capacity. The AC5 KO model’s ability to enhance exercise performance is paradoxical, since exercise capacity is usually mediated by increased beta-adrenergic receptor stimulation and increased cAMP, whereas cAMP and beta-adrenergic receptor signaling are reduced in the AC5 KO model. The AC5 KO’s protection against oxidative stress and enhanced mitochondrial signaling and enhanced aerobic capacity and the microbiome mediate the improved exercise performance and offset the need for an increase in cAMP. Thus, the AC5 KO model is uniquely designed for clinical translation of its beneficial effects, by using a pharmacological analog, which inhibits AC5.
Keywords
Oxidative stress, Cardiovascular system, SIRT1 , MnSOD, cAMP
Citation
Campbell SC, Zhang J, Vatner DE, Vatner SF. (2023) Mechanisms Mediating Increased Exercise Capacity, Aerobic Capacity and Healthful Aging Induced by Adenylyl Cyclase Type 5 Inhibition. Ann Sports Med Res 10(2): 1206.
INTRODUCTION
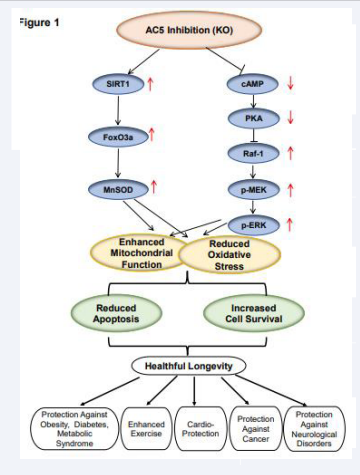
Since longevity is increasing in the human population, there is increasing interest in understanding longevity, clinically as well as in animal models. Longevity, per se, is not always desirable, since it is accompanied by diseases of aging and the lack of strength and sturdiness of young adults. Accordingly, there is an even greater interest in healthful longevity, that prolongs lifespan without the accompaniment of the diseases and disability of normal aging. The current review focuses on a model of healthful aging, inhibition of type 5 adenylyl cyclase (AC), induced in mice by disruption of adenylyl cyclase type 5 (AC5) (Figure 1) [1-7].
Figure 1: Signaling diagram for AC5 KO leading to increased mitochondrial function and reduced oxidative stress leading to reduced apoptosis and increased cell survival as mechanisms mediating healthful longevity
Adenylyl cyclase and its isoforms
Adenylyl cyclases (ACs) are a family of enzymes that catalyze the conversion of adenosine triphosphate (ATP) into cyclic adenosine monophosphate (cAMP), a ubiquitous second messenger, required for energy production and exercise [8,9]. The enzymes are expressed throughout most organisms and play key roles in the diversity and complexity of cAMP signaling pathways [10]. ACs enhance cAMP from ATP, G protein coupled receptors (GPCRs), and protein kinase A (PKA) [11]. Other GPCRs preferentially activate the inhibitory G protein alpha subunit, Gα?, which can inhibit AC activity and reduce cAMP synthesis [11].
Studies have shown that AC-cAMP signaling plays crucial roles in various biological functions, most importantly in mediating beta-adrenergic receptor (β-AR) signaling, including exercise [6,7], lipolysis [12], gluconeogenesis [13], respiration [14] and cytoskeletal organization [15], and its dysregulation, and in pathophysiological states including memory [16] and neurodegenerative disorders [17], tumorigenesis [18], and heart disease [19]. The diverse actions of cAMP are mediated through the cAMP-dependent activation of PKA, cyclic nucleotide-gated ion channels, and cAMP-activated exchange proteins.
Mammals express nine isoforms of transmembrane ACs, termed AC1–9, and one soluble mammalian adenylyl cyclase (often termed sAC or AC10). Cloning of these isoforms reveals not only highly conserved catalytic domains, but also significant sequence diversity outside these domains [20]. The structure of the adenylyl cyclase molecule is subdivided into seven domains: the N-terminal cytoplasmic domain; M1, the first six transmembrane-spanning domain; C1a, the first cytoplasmic catalytic domain; C1b, the first cytoplasmic linker domain; M2, the second six transmembrane-spanning domain; C2a, the second cytoplasmic catalytic domain; and C2b, the second cytoplasmic noncatalytic domains [21]. Most cell types express multiple isoforms, but abundant expression of certain isoforms in particular tissues is notable. The AC isoforms mediate function of all organs in the body [22], whereas AC5 and AC6 are the most important isoforms in the heart [23,24].
Healthful Aging
It has been recognized for some time that aging and longevity are regulated by evolutionarily conserved molecular pathways [25], many of which also affect metabolism. Factors affecting basal metabolism (e.g., growth hormone/insulin-like growth factor 1 (GH/IGF-1) signaling pathway or downstream effectors such as FOXO transcription factors) are all involved in the regulation of lifespan [26,27]. Sirtuins (Sir2), an NAD-dependent histone deacetylase, plays an essential role in mediating lifespan extension in diverse organisms [28-32]; it is required for longevity induced by calorie restriction in yeast and the fruit fly [32], and elevated activity of Sir2 increases lifespan in yeast [30], C. elegans [31], and the fruit fly [32]. Although the direct effects of Sir2 on longevity need to be determined in mammals, mammalian Sir2 has shown favorable effects on longevity in mice [33]. FOXO family transcription factors act as stress resistance factors that also control lifespan [34,35].
Interestingly, there is an interaction among these molecular mechanisms that regulate longevity; for example, in C. elegans, elevated Sir2 expression leads to an increase in lifespan that is dependent on DAF-16, a FOXO transcription factor that is regulated by insulin signaling (31). Mutant mice with GH deficiency, including Ames [36], Snell dwarf mice [36], and Growth Hormone mutant mice [37], all live longer and have delayed appearance of aging-associated phenotypes. Many of these mechanisms are shared by the AC5 KO mouse model of healthful longevity. IGF-1 receptor knockout (Igf1r+/–) mice [38], p66shc–/– mice [39], fat-specific insulin receptor knockout (FIRKO) mice [40], and hormone Klotho overexpression mice [41], also extend lifespan significantly. The studies with these longevity models also noted the association with increased stress resistance, especially resistance to oxidative stress and apoptosis [38-41], as seen in the AC5 KO mouse [1,4].
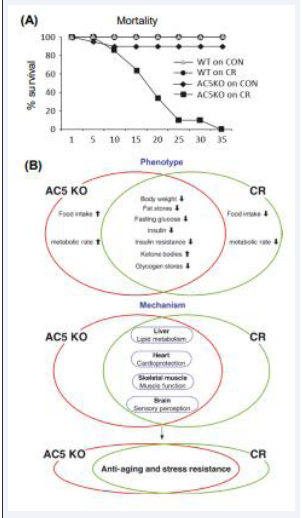
Calorie Restriction is one of the most frequently studied models of healthful aging [42,43]. Interestingly the AC5 KO mice share many of the same pathways and genes as calorie restriction in mediating longevity and stress resistance, and protection against cardiovascular stress, with a unifying feature of altered regulation of metabolism, resulting in lower body weight, a key feature of healthful aging [1,3]. Because of these similarities between the AC5 KO and calorie restriction models we examined whether calorie restriction would extend AC5 longevity. We actually found that when calorie restriction was applied to the AC5 KO, it did not extend lifespan, but actually radically reduced lifespan [3] (Figure 2).
Figure 2: (A) The effects of calorie restriction (CR) superimposed on AC5 KO on survival. CR superimposed on AC5 KO was lethal within a month. (B) Summarized differences and similarities between adenylyl cyclase type 5 knockout (AC5 KO) and calorie restriction (CR) mice in metabolism and the common pathways in liver, heart, skeletal muscle, and brain with respect to anti-aging and stress resistance.
Adenylyl cyclase 5 (AC5): General Overview
AC5 is broadly expressed in mammalian organs [44,45], and thus it is not surprising that AC5 plays important roles in the regulation of multiple organ functions. AC5, as noted above, is found in the brain, skeletal muscle, and heart, but also in the intestine and vasculature [45,46]. It is known that PKC/Raf kinase and Gα? confer direct stimulatory effects, while Gβ/γ exert conditional stimulatory effects on AC5 [47-49]. Once stimulated, AC5 localizes to a lipid raft domain where it initiates its physiological effects [50,51]. An important consequence of the localization of cAMP signaling complexes, as with AC5, is that it allows cAMP to be produced locally within a cell and have specific signaling effects, as opposed to globally diffusing throughout the cytosol and triggering all possible downstream signals [51-53]. Conversely, inhibition of AC5 is regulated by Gα?, Ca2+, PKA, nitric oxide, RGS proteins, and Annexin A4 [54- 56]. While AC5 stimulation has been studied more extensively, its inhibition or deletion has generated more interest. It has been shown that in metabolism and some organs such as the heart and kidney AC5 inhibition is protective [1,2], and could be therapeutically valuable [57]. However, in other organs such as the brain AC5 inhibition may be deleterious and cause multiple neuropsychiatric problems [58,59].
AC5 and Aspects of Healthful Aging
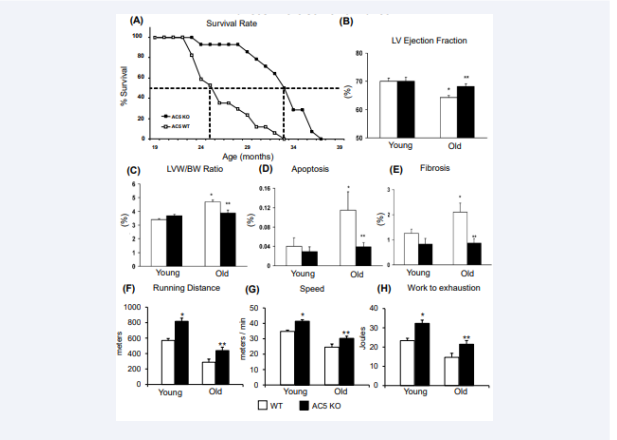
AC5 and the Cardiovascular System: Initially our laboratory was primarily involved in cardiovascular regulation. Therefore, our interest was in disrupting one of the two major isoforms in the heart, AC5, to see how regulation of cardiac function is altered. We first examined chronic pressure overload and found that the AC5 KO protected against the deleterious effects of chronic pressure overload observed results in WT mice, which leads to heart failure [60]. In the same study we examined aspects of cardiomyopathy of aging (e.g., the heart dilates and hypertrophies and there is increased apoptosis and fibrosis, which impairs cardiac function). This was observed in WT mice, but not in the AC5 KO, where normal cardiac function was preserved (Figure 3) [1]. Next, we set out to understand chronic catecholamine stress, since desensitization of β-adrenergic signaling can help preserve heart function. Our results showed that AC5 KO were protected compared with WT and exhibited enhanced β-AR desensitization and better preservation of myocardial function and architecture [61].
However, even more interesting, and potentially more important were our other findings of the salutary effects of AC5 inhibition, not necessarily related to the heart. Mice with disrupted AC5 live one-third longer than WT littermates (Figure 3). Kaplan-Meier statistics demonstrate not only that maximal survival is prolonged in AC5 KO, but also that the 50% survival point is extended from 26 months to 33 months, in both females and males (Figure 3).
Figure 3: Disruption of AC5 (AC5 KO) protects against cardiac stress. (A) AC5 KO showed increased longevity in AC5 KO. Effects of AC5 KO on cardiac function and exercise capacity in young (3-6 months old) and old (20-30 months old) AC5 KO (solid bars) vs WT (open bars). The old WT developed cardiomyopathy as reflected by reduced LV ejection fraction (B), increased LVW/BW (C), increased LV apoptosis (D), and increased LV fibrosis (E), whereas the old AC5 KO were protected from getting the cardiomyopathy of aging. in AC5 WT and KO, young and old. There were no significant differences between young WT and young AC5 KO mice. However, all of the four parameters were significantly different in old WT compared with young WT (∗p < 0.05), characteristic of aging cardiomyopathy. In contrast, none of the four parameters were different in old versus young AC5 KO, but all four parameters were significantly different in old AC5 KO versus old WT (∗∗p < 0.05). Exercise capacity was enhanced in both young and old AC5 KO mice, compared to WT, as reflected by running longer distances (F), achieving higher speed (G), and doing more work (H).
There are many similarities between the AC5 KO mouse and a more commonly studied model of longevity, Calorie Restriction, with one important difference: The AC5 KO mouse exhibits enhanced exercise capacity, whereas the Calorie Restriction model diminishes exercise capacity [3]. The unique aspects of the AC5 KO healthful aging model outside of the cardiovascular system that we have uncovered during our experimental pursuits are outlined next.
AC5 and Metabolism (Figure 4):
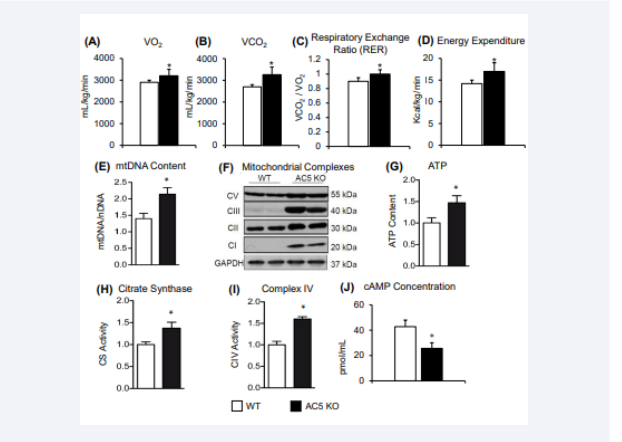
Figure 4:The mice were placed in a metabolic chamber for 48 h. The average oxygen consumption (VO2) (A) and CO2 production (VCO2) (B) and calculated Respiratory Exchange Ratio (C) and Energy Expenditure (D) were significantly higher in AC5 KO compared with WT mice. Increased mitochondrial content and activity in skeletal muscle of AC5 KO mice. (E) Increased mtDNA/nuclDNA ratio in AC5 KO gastrocnemius. (F) Western blotting showing the measurement of the four complexes of the mitochondrial electron transport chain in quadriceps muscles of mice. (G) Skeletal muscle of AC5 KO mice contains more ATP. (H) Higher citrate synthase activity was present in skeletal muscle of AC5 KO mice. (I) Higher complex IV activity was present in the gastrocnemius muscle of AC5 KO. (J) AC5 KO mice had a decrease in cAMP concentration when compared to WT. *p < 0.05 vs. WT.
Our group has established that AC5 deficiency results in increased longevity with reduced weight gain similar to that observed in calorie-restricted mice, noted above [1,3]. In fact, the mechanisms mediating improved metabolism in the Calorie Restriction and AC5 KO models are so close that when calories are restricted in the AC5 KO mice, they no longer live longer and actually die within a month (Figure 2) [3]. Thus, AC5 KO represents a unique opportunity to observe metabolic function in the absence of calorie-restriction, to determine the effectiveness of this model in application to obesity and diabetes. We showed that despite a 100-day high fat diet, AC5 KO mice had lower body weights, weight gain efficiency, adiposity index, adipocyte size, and smaller visceral fat pads [5]. Furthermore, biochemical analysis revealed lower triglycerides, total cholesterol, and fasting glucose. Additionally, AC5 KO mice demonstrated better glucose control and energy expenditure compared to their WT littermates. Finally, AC5 KO mice had improved insulin sensitivity and protection from high fat diet induced insulin resistance [5]. The findings also showed that AC5 KO animals have an increase in genes related to browning of white adipose tissue without the concomitant increase in thermogenesis [5]. However, we also found that AC5 KO mice had improved mitochondrial function, which could be responsible for the enhanced energy expenditure in the absence of higher thermogenesis [4,5]. These results highlight the novelty and importance of AC5 KO being a target for obesity and diabetes [5].
AC5 and Oxidative Stress: Increased longevity may be adversely affected by the burden of concomitant debilitating diseases, e.g., obesity, diabetes, heart disease and cancer and their accompanying morbidity, which reduce enjoyment of life.Most of these debilitating diseases are mediated, in part, by increased oxidative stress [62-70], which is considered a major mechanism limiting healthful longevity [71,72]. In contrast, in the AC5 KO model, healthful longevity and reduced oxidative stress enhances exercise capacity, with reduced beta-adrenergic signaling and protection from obesity, diabetes, cardiovascular stress, and cancer. Oxidative stress induced aging seems to be multifactorial, where mitochondrial damage and impaired function play a significant role. It is well established that both resting and contracting skeletal muscles produce reactive oxygen species (ROS). Exercise improves the antioxidant defense system, and provides increased resistance of tissues against ROS damage [73], and endothelium dependent vasodilation through increased production of nitric oxide [74].
Increased cAMP and PKA, negatively regulate the MEK-ERK signaling pathway, thereby increasing oxidative stress [75]. In contrast, cAMP and PKA are reduced in the AC5 KO model, which activates the Raf/MEK/ERK pathway [1], increases MnSOD and protects against oxidative stress (Figure 1). In addition, superoxide dismutase (SOD) has been reported as the downstream target of the Cyr1 (AC)/cAMP/PKA pathway in yeast, which induces protection [76]. MnSOD which protects against oxidative stress is increased in the AC5 KO model [4], and is regulated by several transcription factors, such as NF-????B [77,78], p53 [79], and FoxO3a [4,6,80]. Among them, FoxO3a is most closely related to the antiaging effects of MnSOD. The transcriptional activity of the FoxO3a factor can be activated by deacetylation. Sirtuin 1 (SIRT1), a member of the Sir2 family, is a deacetylase which can activate FoxO3a (Figure 1) [81]. Interestingly, we found that the SIRT1/FoxO3/MnSOD pathway is only activated by AC5 and not by AC6, and not by other AC isoforms, although it is known that increased oxidative stress is mediated by the other AC isoforms [82,83].
AC5 and Enhanced Exercise Capacity The most important physiological mechanism mediating enhanced exercise performance is increased sympathetic stimulation, mediated by β-AR tone and AC activity. In 2015, we were the first to report decreased AC activity mediating increased exercise performance [6]. We demonstrated that both young and old AC5KO mice have increased exercise performance (Figure 3). Furthermore, the mechanism for this enhanced exercise performance was in skeletal muscle, rather than in the heart. This was shown by comparing cardiac- and skeletal muscle-specific AC5 KO’s. Exercise performance was improved in the skeletal muscle-specific AC5 KO, as it was in the total body AC5 KO, but was not enhanced in the cardiac-specific AC5 KO (AC5 cardiac−/−) [6]. Within the skeletal muscle we found that mitochondrial biogenesis was a major mechanism mediating the enhanced exercise as SIRT1, FoxO3a, MEK, and the antioxidant, MnSOD were upregulated in AC5 KO mice [6]. In support of this mechanism, we noted that the improved exercise in the AC5 KO was blocked with either a SIRT1 inhibitor, MEK inhibitor, or by mating the AC5 KO with MnSOD hetero KO mice, confirming the role of SIRT1, MEK, and oxidative stress mechanisms [6].
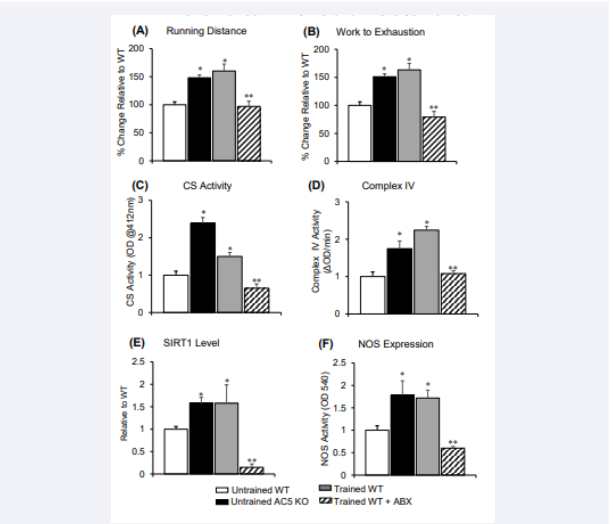
Exercise Training Since our initial study in 2015 examined acute exercise, e.g., a single bout, we then sought to understand how the AC5 KO would respond to chronic exercise, e.g., exercise training, and its effects on exercise performance. We found that exercise performance, reflected by maximal distance run and work to exhaustion, increased in exercise-trained WT to levels observed in untrained AC5 KO (Figure 5),
Figure 5: weeks of exercise training significantly increased maximal running distance and work to exhaustion in AC5 KO mice, compared to sedentary group. Ad libitum access to antibiotic (ABX) treated water was used to eliminate the gut microbiota resulting in abolition of the increases in maximal running distance (A), and work to exhaustion (B). All data in A and B are reflected by % difference from untrained WT. (C) Citrate synthase (CS) activity, (D) mitochondrial complex IV, (E) SIRT1, and (F) total nitric oxide synthase (NOS) were increased in untrained AC5 KO, and exercise trained WT mice compared to untrained WT mice, but reduced in trained WT + ABX mice. *p<0.05 to untrained WT. ABX significantly reduced effects of exercise training for all data. **p<0.05 to trained WT.
and furthermore, exercise training in AC5 KO was able to increase its exercise performance to greater levels in both exercised trained WT and untrained AC5 KO [7]. One major difference was the level of β-AR signaling, which was decreased in untrained AC5 KO compared to untrained WT, but was increased in WT with exercise training. Despite this key difference, untrained AC5 KO and exercise-trained WT mice shared similargene expression, determined by deep sequencing, in their gastrocnemius muscle with 183 genes commonly up or down-regulated, mainly involving muscle contraction, metabolism and mitochondrial function. Finally, SIRT1 and reductions in oxidative stress remain major mechanisms mediating the enhanced exercise performance observed both in the AC5 KO and WT with exercise training.
Paradox between AC stimulation improving exercise performance and inhibition of AC5 increasing exercise performance
As previously noted, exercise capacity is enhanced in young and old AC5 KO mice. These results seem paradoxical, since AC stimulation is an important physiological mechanism mediating enhanced exercise performance. There are some distinct features that can explain this issue. First, there were no significant differences between AC5 KO and WT in ether baseline or peak exercise in heart rate (baseline: 646 ± 23 vs. 700 ± 13 beats/min; peak exercise: 764 ± 18 vs. 729 ± 20 beats/min), stroke volume (baseline: 21.8 ± 1.1 vs. 20.7 ± 1.4 μL; peak exercise: 28.5 ± 1.4 vs. 26.2 ± 1.4 μL), and cardiac output (baseline:15.3 ± 1.0 vs. 13.2 ± 0.7 mL/min; peak exercise: 21.8 ± 1.4 vs. 19.0 ± 0.8 mL/min) [6]. These data indicate that heart function is not disrupted during exercise and can provide the necessary cardiac output and blood flow to exercising muscle. Second, we examined RNA levels in in cardiac?specific and skeletal muscle?specific (SKM) AC5 KO mice (AC5 skm−/−) [6]. The RNA levels were reduced in the heart of cardiac specific AC5 KO to the same extent as in the total body AC5 KO, but were not reduced in other organs, as they were in the total body AC5 KO. The RNA levels were reduced in the skeletal muscle of the skeletal muscle specific AC5 KO to the same extent as in the total body AC5 KO, but were not reduced in other organs, as they were in the total body AC5 KO. However, the cardiac?specific KO did not show complete loss of AC5, since the majority of cells in the heart are not myocytes. In contrast to the total body AC5 KO, the AC5 cardiac−/− did not run faster or longer than WT, but the AC5 skm−/− mice demonstrated 19% greater running distance than WT, p<0.01 vs. WT mice, indicating that the lack of AC5 in skeletal muscle mediates the enhanced exercise capacity [6].
Finally, the ratio between mitochondrial versus nuclear DNA and mitochondrial protein content were significantly increased in the skeletal muscle of AC5 KO mice [6]. ATP content, citrate synthase activity, and complex IV activity were increased by ~50% in skeletal muscle of total body AC5 KO (Figure 4), which was also observed in AC5 skm−/− mice. Consistent with enhanced mitochondrial number, mRNA levels of mitochondrial genes (Atp5 g1, Nrf?1, Ndufa2, citrate synthase, and Cox IV) were increased in the skeletal muscle of AC5 skm−/− mice [6]. Furthermore, knockdown (KD) of AC5 in myoblast cells in vitro showed significantly increased mRNA levels of several mitochondrial proteins. The mRNA levels of Nrf-1 regulating mitochondria DNA transcription and MnSOD, a mitochondrial enzyme involved in antioxidant defense, were also increased in AC5 KD myoblasts. This resulted in approximately a 20% increase in the oxygen consumption rate, which reflects the mitochondrial metabolic rate in AC5 KD cells [6]. Citrate synthase activity was also ~20% higher in the AC5 KD cells. These results indicate that mitochondrial biogenesis and function was the underlying mechanism in the enhanced exercise capacity of AC5 KO mice [6]. Taken together that despite AC5 inhibition there appears to be no limitation to the function of the heart, or skeletal muscle in the AC5 KO animal [6].
Aerobic Capacity
Aerobic capacity is commonly described as VO2 max, or maximal oxygen uptake. This measurement is an indication of both the ability of the cardiovascular system to provide oxygen to working muscles and also the ability of those muscles to extract oxygen for energy generation in the form of ATP. VO2 max can be tested in a variety of ways in both humans and animals, i.e., it can be measured directly or by using various proxies to identify capacity, e.g., running distance, maximum speed, and work to exhaustion. Exercise training can increase VO2 at baseline compared to sedentary conditions [84,85]. It is also critical to understand that exercise is central to longevity, and more importantly healthful aging, as it is well recognized to protect against diseases that limit longevity and compromise healthy lifespan (Figure 3). Since the AC5 KO animal was shown in earlier studies of ours to enhance longevity and many aspects of healthful aging [1]. We sought to determine if the aerobic capacity of this model was also enhanced.
Results from our early work show that untrained AC5 KO mice ran significantly longer, p<0.01, than WT littermates in distance and 17% faster in speed, resulting in 38% greater work to exhaustion (Figure 3F-H). Exercise capacity was also significantly improved, p<0.01, in older AC5 KO mice compared to WT (20 months) [6]. Follow up work analyzing chronic exercise training in untrained AC5 KO mice enhanced their exercise performance (running distance and work to exhaustion) even further, to levels exceeding those in WT mice with exercise training [7] (Figure 5A & 5B). The enhanced protection from oxidative stress helps to maintain the AC5 aerobic capacity.
Microbiota and Enhanced Exercise Capacity
While the study of microbiology has been around for centuries, only in the last few decades have scientists begun to aggressively pursue the role of gut microbes in human health. It is now widely accepted that these microorganisms play a powerful role in human health outcomes ranging from obesity and diabetes to anxiety and depression, and now exercise performance. While most of the research heavily focuses on diet, the growing niche of the exercise microbiome deserves recognition. It has been shown by our group and others that exercise alters the microbiome independent of diet [86,87]. In addition, studies have shown that depletion of the microbiome via antibiotics or the use of germ-free mice demonstrate reduced exercise tolerance, suggesting that an intact microbiome is essential for exercise capacity (Figure 5) [86-89]. Taken together, these studies not only illustrate the immense independent impact exercise has on microbial community structure, but that exercise tolerance and exercise training effects are indeed dependent on these microbes, highlighting a true symbiosis between host and its microbiome.
Recently, we showed that the gut microbiota composition of AC5 KO sedentary mice is similar to those in WT mice, with exercise training [90]. Additionally, we found that when AC5 KO mice are exercise trained, their gut microbiota differs compared to untrained AC5 KO mice. Specifically, Muribaculum intestinale, Parasutterella excrementihominis, two different Eubacterium sp., and Clostridium aldrichii were enriched in the AC5 KO mice after exercise training [90]. This observed species-level difference in gut microbiota profiles suggest that host factors (e.g., biological, or behavioral) are selecting for specific groups of bacteria within the gut and may ultimately contribute to critical aspects of the AC5 KO phenotype (e.g., improved longevity, increased glucose and insulin tolerance, enhanced exercise tolerance). Given the extensive research on the mechanisms that mediate enhanced exercise tolerance in AC5 KO mice, future studies should be aimed at understanding how these are influenced by the gut microbiota. This is particularly relevant given our recent work that shows significant reductions in mitochondrial function and blood flow in WT mice given antibiotics after 6-weeks of exercise training [86].
SUMMARY
In summary, the AC5 KO mouse is a model of healthful longevity. Not only do the mice live longer than WT controls but are protected by healthful longevity. They are protected against obesity, diabetes, and metabolic syndrome, but also Alzheimer’s disease, cancer and, myocardial ischemia and heart failure. Most importantly, they have enhanced exercise performance. All of these features are mediated by mitochondrial signaling, the microbiome, increased aerobic capacity and protection against oxidative stress.
Author Contributions Design of the Study:
S.C.C. D.E.V. & S.F.V.
Writing of the Manuscript: S.C.C., J.Z., D.E.V., & S.F.V.
All authors approved the final version of the manuscript.
ACKNOWLEDGEMENTS
This study was supported by the Department of Kinesiology and Health Grant (to S.C.C.).
This study was also supported by the National Institutes of Health grant R21AG075656 (to S.F.V.).
REFERENCES
36. Bartke A, Brown-Borg H. Life extension in the dwarf mouse. Curr Top Dev Biol. 2004; 63: 189-225.
50. Cooper DM, Tabbasum VG. Adenylate cyclase-centred microdomains. Biochem J. 2014; 462: 199-213.
53. Antoni FA. New paradigms in cAMP signalling. Mol Cell Endocrinol. 2012; 353: 3-9.
62. Cervantes Gracia K, Llanas-Cornejo D, Husi H. CVD and Oxidative Stress. J Clin Med. 2017; 6.