Dyslipidaemia and Oxidative Stress in Primary Knee Osteoarthritis: Analysis of Clinical and Serological Features
- 1. School of Life Sciences and Biotechnology, Chhatrapati Shahu Ji Maharaj University Kanpur, India
- 2. School of Biotechnology, Gautam Buddha University, India
- 3. Amity Institute of Neuropsychology and Neurosciences, Amity University Uttar Pradesh, India
- 4. School of Biotechnology, Madurai Kamaraj University, India
Abstract
Background: Osteoarthritis (OA) is a prevalent and debilitating joint disorder with complex etiology, leading to substantial morbidity worldwide. This study aimed to provide a comprehensive and integrative analysis of the clinical and serological features of OA, focusing on primary knee OA as a representative model.
Methods: A large and diverse cohort of 100 OA patients and 120 healthy controls from India were recruited and subjected to rigorous assessments, including radiological, clinical, functional, and biochemical measurements. Serological evaluations encompassed the quantification of creatinine, uric acid, lipids, and MDA as markers of lipid peroxidation and oxidative stress. Additionally, antioxidant enzyme activities, CRP levels, and inflammatory markers were assessed.
Results: Statistical analyses revealed significant differences between OA patients and controls, indicating dyslipidemia and increased oxidative stress in OA. Furthermore, significant correlations between some parameters and OA severity or pain scores were found, suggesting potential prognostic roles.
Conclusion: This study provides valuable insights into the clinical and serological features of OA, highlighting the potential for novel biomarkers and personalized interventions for OA management and prevention.
Keywords
• Clinical and serological features
• Dyslipidemia and oxidative stress
• Biomarkers
• Antioxidant enzymes
• Inflammatory markers
• Comprehensive analysis
• Integrative assessment
CITATION
Chandra V, Ashraf MT, Yadav P, Raghuvanshi V (2023) Dyslipidaemia and Oxidative Stress in Primary Knee Osteoarthritis: Analysis of Clini cal and Serological Features. Ann Virol Res 8(1): 1039.
ABBREVIATIONS
ACR: American College of Rheumatology; ANOVA: Analysis of Variance; BMI: Body Mass Index; BP: Blood Pressure; CAT: Catalase; CRP: C-Reactive Protein; ESR: Erythrocyte sedimentation rate; GSH: Reduced Glutathione; GST: Glutathione S-transferase; HDL: High-Density Lipoprotein; HNE: 4-Hydroxy 2-nonenal; K-L: Kellgren-Lawrence; LDL: Low-Density Lipoprotein; MDA: Malondialdehyde; MEC: Molar extinction coefficient; OA: Osteoarthritis; SOD: Superoxide Dismutase; VAS: Visual Analog Scale; WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index
INTRODUCTION
Osteoarthritis is a prevalent and debilitating joint disorder that affects millions of people worldwide [1]. The exact causes and mechanisms of OA remain elusive, despite extensive research, hindering the development of effective treatments and preventive measures. Therefore, unravelling the complex interplay of factors that contribute to OA onset and progression, such as genetic [2], environmental, biomechanical [3,4], inflammatory [5], and oxidative stress components [6], is an urgent need. This study provided a comprehensive and integrative analysis of the clinical and serological features of OA, focusing on primary knee OA as a representative model. A large and diverse cohort of 100 OA patients and 120 healthy controls from Kanpur and nearby regions, India, was recruited and subjected to rigorous assessments of their radiological, clinical, functional, and biochemical status. Various validated tools and scales were employed to quantify OA severity, pain, disability, and quality of life, such as the K-L score, WOMAC, VAS, and ACR classification. Relevant clinical parameters, such as BMI and BP, were also measured, and detailed information on patient history, physical activity, disease awareness, and treatment adherence was collected through specific questionnaires. Serological assessments were conducted to investigate the biochemical alterations associated with OA using advanced colorimetric and enzymatic methods. Statistical analyses were performed on the obtained data using GraphPad Prism and STATISTICA software. One-way ANOVA was used to compare values between groups, and Pearson correlation analysis was used to assess associations among variables. The results revealed significant differences in clinical and serological parameters between OA patients and controls, indicating dyslipidemia and increased oxidative stress in OA patients. Significant correlations between some of these parameters and OA severity or pain scores were also found, suggesting potential causal or prognostic roles. The implications of the findings for the identification of novel biomarkers and therapeutic targets for OA management and prevention are discussed. By combining comprehensive clinical assessments with state-of-the-art analytical techniques, this study contributed significantly to the understanding of this complex joint disorder and provided valuable insights into the clinical and serological features of OA. This study highlights the potential for personalized and multidisciplinary interventions based on individual clinical and serological status, aiming to improve joint health and function. The identification of novel biomarkers and therapeutic targets may pave the way for innovative approaches in OA management and prevention. While acknowledging some limitations, including the cross-sectional design, restricted study population, and reliance on self-reported measures, this study lays the groundwork for future longitudinal and interventional investigations with larger and diverse samples. The findings underscore the importance of continued research in elucidating the complex pathogenesis of OA, facilitating improved patient care and quality of life.
CLINICAL AND SEROLOGICAL ASSESSMENT
Inclusion and exclusion criteria
We enrolled 120 healthy controls and 100 OA patients in this study. Controls were medical college or departmental staff, free of OA or other systemic diseases, and matched with patients for sex, age, weight, and height. Patients were diagnosed with primary knee OA according to the ACR criteria and recruited from outpatient departments of various hospitals in Kanpur and nearby regions, India. We excluded participants with trauma, joint disease, smoking, obesity, hypersensitivity, cardiovascular disease, ligament instability or other forms of arthritis. We obtained written informed consent from all participants and ethical approval from the institutional committee. We assessed the radiological, clinical, and functional status of OA patients using the K-L score, WOMAC, VAS and ACR classification. We also collected data on patient history, physical activity, treatment history, relevant clinical parameters (BMI and BP), disease awareness and treatment adherence using a HAQ and a modified DAS-28 with CRP score questionnaire.
Investigating parameters and analytical methods
We collected 5.0 ml of blood from fasting participants using a standard clinical procedure. We isolated DNA from heparinized blood and measured serum creatinine, uric acid, and lipids from plain blood. We used a colorimetric method to determine serum creatinine levels by measuring the absorbance of an orange coloured complex formed by the reaction of creatinine with picric acid in an alkaline environment [7]. We used the formula creatinine (mg/dl) = Δ???????? X 2 Δ???????? and converted the values to SI units (µmoles/L) by multiplying by 88.42. We used another colorimetric method to measure serum uric acid levels by measuring the absorbance of a red-coloured quinoneimine dye formed by the reaction of uric acid with a phenolic compound and 4-aminoantipyrine, catalysed by uricase and peroxidase [8]. We used the formula: Uric acid (mg/dl) = AbsorbanceT X 8 Absorbance???? . We estimated LDL cholesterol using the equation LDL cholesterol = Total cholesterol – (HDL cholesterol + Triglycerides). We estimated HDL cholesterol using the PEG precipitation method and measured the absorbance of the supernatant after centrifugation. We used the formula HDL cholesterol (mg/dl) = Absorbance of T X 200 Absorbance of ???? . We measured plasma lipids, including cholesterol and triglycerides, using a coupled enzyme assay and separated HDL and LDL/VLDL using a precipitation buffer [9]. We measured the absorbance of the reaction mixture after incubation and used the following formula: Total lipids (mg/dl) = ???????????????????????? ???????? ???????????????????????? X 2 total volume of sample. We estimated cholesterol using the CHOD POD method by measuring the absorbance of a red-coloured quinoneimine complex formed by the oxidation of cholesterol and its reaction with phenol and 4-aminoantipyrine, catalysed by cholesterol esterase, cholesterol oxidase, and peroxidase [9,10]. We used the formula Cholesterol (mg/dl) = Absorbance of T X 200 Absorbance of ???? . We estimated MDA using the method described by Ohkawa by measuring the absorbance of a pink coloured complex formed by the reaction of MDA with TBA in an acidic medium [9]. We used the formula MDA (µmol/l) = OD532 x 1.75/0.156 and expressed the results as µmol/l/mg protein by an MEC of 1.56 x 105 M-1 cm-1[11]. We performed a qualitative analysis of CRP using a latex agglutination test. We observed the presence or absence of visible agglutination after mixing serum samples with CRP-latex reagents on a test slide and rotating it for 2 minutes. We interpreted agglutination as a positive result indicating a CRP concentration equal to or greater than 6 mg/L. We estimated triglycerides using the GPO-POD method by measuring the absorbance of hydrogen peroxide formed by the hydrolysis of triglycerides and their subsequent phosphorylation and oxidation, catalysed by lipoprotein lipase, glycerokinase, and glycerol phosphate oxidase [12]. We used the formula: Serum Triglycerides (mg/dl) = Absorbance of T X 200 Absorbance of ???? . We determined SOD activity using the method described by Mishra and Fridovich by measuring the absorbance of adrenochrome formed by the oxidation of epinephrine in the presence of SOD [10]. We expressed SOD activity as U/gHb, with one unit defined as the amount of enzyme required to exhibit 50% inhibition in the conversion of epinephrine to adrenochrome under specified conditions. We calculated the specific activity of the enzyme (SOD) = units per ml enzyme Hb gm/dl and percentage inhibition (%) = ????×100 ????, where x represents the absorbance change in the experimental reaction minus the absorbance change in the control/blank reaction, and A represents the absorbance change in the experimental reaction. We determined catalase activity using the method described by Sinha by measuring the absorbance of chromic acetate formed by the reaction of dichromate/acetic acid reagent with residual H2 O2 after catalase action [12]. We expressed catalase activity as U/gmHb, with one unit defined as the amount of enzyme that decomposes 1 µmole of hydrogen peroxide per minute under specified conditions. We prepared a standard curve using different amounts of H2 O2 ranging from 40-160 µmoles and plotted it between O.D. and the amount of H2 O2 . GSH activity was quantified by the Teitze method, which involved spectrophotometric detection of TNB at 412 nm as an indicator of the total reduced and oxidized glutathione in the sample [13]. TNB was formed by the oxidation of GSH by DTNB, followed by the reduction of GSSG by glutathione reductase using NADPH as the reducing equivalent [14]. GST activity was measured by the Habdous method, which involved spectrophotometric detection of NADPH consumption at 340 nm as a reflection of GST catalysis in the sample [15]. GST activity was assayed using CDNB as the standard substrate and reduced glutathione as the substrate. We expressed GST activity in U/L, corresponding to the transformation of one µmole of substrate per minute at room temperature. We compared the values of three independent groups using one-way ANOVA. We tested the homogeneity of variance among groups using Ψ2 tests prior to ANOVA. We performed Pearson correlation analysis to assess the association among variables in both groups. We compared the proportion of males and females among the three groups using the Ψ2 test. Data analysis was performed using STATISTICA (version 6.0) and GraphPad Prism (version 3.0) software [16,17].
RESULTS
Demographic Studies
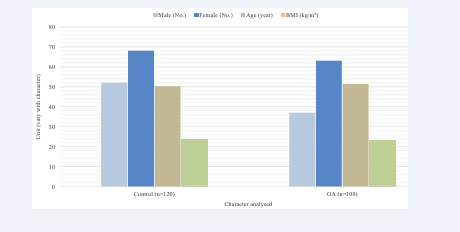
We obtained approval from the institutional ethical committee and written informed consent from the participants. We enrolled 100 OA patients (37 males and 63 females) and 120 controls (52 males and 68 females) in this study (see Figure 1).
Figure 1: Graphical representation of demographic characteristics of healthy control and patients.
Patients were diagnosed with primary knee OA according to the ACR criteria and without any comorbidities. Controls were asymptomatic and recruited from the neighbourhoods. X-ray examination showed that 78 patients had OA grade II and 22 had grade III changes (see Table 1). Their range of motion was 0-140/142 degrees with pain (see Table 1 Their pain on the visual analogue scale in squatting was 4.7±1.4 (see Table 1). Their total mean WOMAC score was 44.2±9.8 (see Figure 1) (WOMAC score 0-100 with 0 as the worst and 100 as the best). Figure1 show demographic parameters such as sex ratio, age, and BMI of the participants.
Serological Parameters
The serological parameters of 28 OA patients and 36 healthy controls were measured and are summarized in Table 2 [18]. OA patients showed lower uric acid and HDL levels and higher LDL, total lipids, cholesterol, MDA, CRP, and triglyceride levels than controls. Serum creatinine was also significantly increased but within the normal range in OA patients (p0.05) [19].
Status of Anti oxidative enzymes
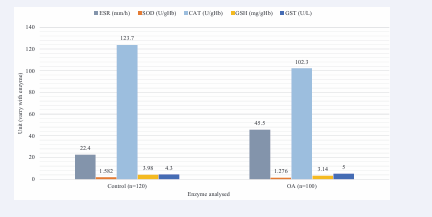
Lipid peroxidation, as indicated by MDA production and ESR, was elevated in OA patients (Table 2), suggesting increased ROS production during chronic inflammation. Lipid peroxides are formed at the site of tissue injury due to inflammation and diffuse into blood, where they can be measured in serum or plasma [20]. The changes occurring in OA may be due to alterations in the balance of oxidants and antioxidants in the system. ROS may play a major role in the pathogenesis of OA due to their generation and inefficiency of the system to eliminate them. We observed decreased enzymatic activity of SOD in OA patients, suggesting inappropriate quenching of superoxide anions (O2-) that may play a role in pathogenesis. We also observed downregulation of catalase and a reduction in GSH levels in OA patients and increased activity of GST (see Table 2 and Figure 2).
Figure 2: Graphic representation of key enzymes expression in healthy control and OA patients.
DISCUSSION
OA is a joint disease with unknown aetiology. Aging-induced degenerative changes have been considered to play an important role in OA. OA was previously regarded as a degenerative and non inflammatory disease. However, increasing evidence supports the role of inflammation, dyslipidaemia, and oxidative stress in the pathophysiology of OA. OA patients had high WOMAC scores, and high VAS scores compared to controls, indicating that they had problems and pain in their knees. The present study aims to evaluate the changes occurring in OA by serological analyses, inflammatory markers, oxidative stress, scavenging enzymes, and proteome profiling using mass spectrometry and NMR. The study also evaluates the association of TGF-β and MTHFR genes with OA by genome-wide association studies and gene expression analysis through microarray.
Serological component analyses
Serum uric acid levels did not differ significantly (p>0.05) between OA patients and controls, indicating no alteration in uric acid metabolism in OA. Previous studies have found a positive association between serum uric acid and generalized OA [18], but not with knee OA. Serum creatinine, a marker of kidney function, was significantly elevated (p<0.05) in OA patients compared to the control group. However, the levels were still within the normal physiological range (~44-106 µmolesl-1) in OA patients, suggesting mild effects on kidney function. Further studies are needed to confirm whether the changes are related to OA [19]. NMR analyses of metabolite concentrations also revealed increased serum creatinine levels in OA patients compared to controls. Thus, both serological and NMR analyses showed consistent changes in creatinine concentration in OA subjects. Furthermore, OA patients exhibited pathological levels of inflammation and had considerably higher CRP levels than controls.
Lipid and MDA profile analyses
Inflammation affects lipids, which might be responsible for other complications in OA. Our study shows high total cholesterol, LDL, and triglycerides, which were significantly higher (P <0.05) in OA patients. Earlier associations have been observed between high serum cholesterol and both generalized and knee OA [21]. In our study, HDL levels were significantly decreased in OA patients compared to controls. In OA, an increase in the severity of disease might be related to higher triglyceride and cholesterol levels and lower HDL. These patterns of lipids indicate dyslipidemia. Altered lipids, such as high cholesterol, may induce oxidative stress, resulting in free radical formation, which promotes lipid peroxidation [22]. In hypercholesterolemia, high amounts of lipids and phospholipids accumulate, leading to high release of arachidonic acid along with prostaglandins with the help of cyclooxygenase enzymes and phospholipase A2. Our NMR data also revealed a low HDL/LDL ratio, confirming the serological findings that lipids are altered in OA. Dyslipidemia is the result of inflammatory changes occurring in OA. MDA, the product of lipid peroxidation, provides indirect evidence of LDL oxidation. Oxidative stress results in a high increase in aldehyde levels, which participate in numerous pathological conditions, such as arthritis, cancer, arthrosclerosis, and cardiac diseases [23]. MDA levels were high in patients with OA (p<0.05) compared to controls. Previous studies have shown that synoviocytes from OA patients have high synthesis of HNE and MDA [19,24].
Oxidative stress analyses
Our results of high oxidative stress and dyslipidemia are in line with the report by Pawlowska et al. that OA has immunological involvement [24]. The first reactive oxygen radicals are usually superoxide radicals (O2-), produced by superoxide dismutase, which may be further converted to hydroxyl radicals (OH) and hydrogen peroxide (H2 O2 ) [25]. Knee joint destruction is mediated by ROS, which target both invading pathogens and host cartilage [26]. ROS degrade aggrecan, a major ECM component that maintains cartilage hydration and integrity [27]. ROS also modify collagen, the fibrillar network that confers mechanical strength and resists swelling pressure, by direct cleavage or by increasing its susceptibility to proteases [28]. Hydroxyl radicals can directly cleave collagen into small peptides in the presence of oxygen. Interestingly, ROS may also activate matrix metalloproteinases (MMPs), the enzymes that participate in the catabolism of matrix macromolecules [29]. Glutathione reductase, which forms reduced glutathione from oxidized glutathione, has a main role in the scavenging and detoxification of oxygen free radicals [30]. The study by Ostalowska et al. showed increased SOD, glutathione peroxidase and glutathione reductase activity in the synovial fluid of patients with primary or secondary OA of the knee joint [26]. Superoxide can degrade collagen as well as synovial fluid, depolymerize hyaluronic acid, inactivate antiproteinases, and convert arachidonic acid into active components, which further cause joint tissue injury and associated clinical symptoms [31]. Regan and colleagues showed that joint fluid obtained from OA patients had decreased SOD activity and reduced glutathione levels [32]. Our findings are consistent with their findings, as our study also showed reduced activities of SOD and catalase. Afonso studied the role of SOD in scavenging the production of free radicals that may have a role in joint inflammation [33]. Kalpakcioglu reviewed the interactions between antioxidants and free radicals in patients with osteoarthritis [34]. Antioxidative enzymes such as glutathione reductase, catalase, glutathione peroxidase, and superoxide dismutase are known to scavenge free radicals. In our study, the serum of OA patients had significantly decreased levels of SOD and glutathione [32]. The synovial fluid (SF) of affected joints had reduced activity and levels of antioxidants with intense localized inflammatory reactions occurring in the active stage of osteoarthritic joints. These reactions may lead to variable clinical signs and symptoms (pain, swelling, cripitus, etc.) experienced by osteoarthritic patients. Higher GSH levels are supposed to be beneficial for good health; the significance of low GSH status in elderly individuals has been revealed [35].
CONCLUSION
This study provided a comprehensive and integrative analysis of the clinical and serological features of primary knee osteoarthritis (OA), a prevalent and debilitating joint disorder. The findings indicated dyslipidaemia and increased oxidative stress in OA patients, implying the involvement of inflammation and free radicals in OA pathogenesis. Serological markers, such as MDA, CRP, and lipid profiles, may complement radiological and functional assessments for enhanced diagnosis and monitoring. These results underscore the potential for personalized and multidisciplinary interventions based on individual clinical and serological status, aiming to improve joint health and function. The identification of novel biomarkers and therapeutic targets may pave the way for innovative approaches in OA management and prevention. Despite some limitations, this study laid the groundwork for future longitudinal and interventional investigations with larger and diverse samples, emphasizing the importance of continued research in understanding OA pathogenesis for improved patient care and quality of life.
DECLARATIONS
Ethical Approval and Consent to Participate
The study adhered to the ethical standards of India and internationally, with approval from the Institutional Ethical Committee (CSJMU/BSBT/BT/EC-20) and written informed consent from all patients, including controls and OA subjects.
Guarantor
The article’s full responsibility lies with PY, who is the corresponding author and the third author in the list.
Authors’ contributions
Dr. VC was responsible for manuscript conceptualization, writing - original draft, ethical approvals, consent, and sample collection. VR participated in writing - review & editing. PY contributed to manuscript writing, formatting, revision, and communication with all authors. Dr. TA supervised all authors and wrote, reviewed, and edited the final manuscript.
ACKNOWLEDGEMENTS
The authors thank the Institute of Biosciences and Biotechnology, Chhatrapati Shahu Ji Maharaj University, Kanpur - 208024, India for providing the laboratory facilities and the Department of the Community Health Centre, Ganesh Shankar Vidyarthi Memorial Medical College (GSVM) medical college, Kanpur – 208002, India for recruiting the control and subjects.
Availability of data and materials
Data and materials are available upon request.
Consent for publication
All authors consented to manuscript publication.