Curvularia and the Brain: Case Demonstration of Optimal Management
- 1. Department of Neurosurgery, University of Florida, USA
- 2. Department of Pathology, Immunology and Laboratory Medicine, University of Florida, USA
- 3. Department of Otolaryngology, University of Florida, USA
Abstract
Background: Curvularia is a ubiquitous fungus found in tropical climates and has been reported to grow on marijuana leaves. Rarely, it can infect humans and propagate from the nasal sinuses into the brain.
Case: A 28-year-old immunocompetent patient presented with history of nasal polyps, headache, and subtle visual deficits on the right. Imaging revealed what appeared to be an invasive mass growing through the ethmoid and sphenoid sinuses into the anterior cranial fossa.
Results: Otolaryngology performed an endoscopic nasal biopsy with pathology and cultures consistent for Curvularia (figure 6). A combination case with neurosurgery and otolaryngology was planned. Surgeons used a bifrontal craniotomy and endonasal approach for gross total resection. Following resection, the patient was placed on 4 weeks of amphotericin treatment followed by 12 months of voriconazole based on recommendations by infectious disease. The patient has been stable since surgery.
Conclusion: Curvularia is a rare but potentially life threatening central nervous system infection that can be acquired from inhalational marijuana use. This illustrative case shows the importance of aggressive debridement followed by broad spectrum antifungal treatment to optimize outcome. With marijuana’s increasing popularity, Curvalaria should be included on the differential diagnosis.
Keywords
• Fungus; Debridement; Anti-fungals; Multidisciplinary care; Nasal polyps
Citation
Goldman M, Reddy R, Lucke-Wold B, Barpujari A, Cameron M, et al. (2022) Curvularia and the Brain: Case Demonstration of Optimal Management. Arch Emerg Med Crit Care 6(1): 1051.
BACKGROUND
Curvularia is a filamentous, dematiaceous, ubiquitous fungus that causes rare, but often fatal CNS phaeohyphomycotic infections [1]. Phaeohyphomycotic infections generally have poor prognosis and are caused by dematiaceous fungi, identified by elevated levels of melanin in their cell walls [2]. Thus far, there have been only a few reports of patients with Curvularia infections, but the most common strain is C. lunata (others include C. pallescens, C. clavate, and C. geniculate) [3-9].
Curvularia infections can present acutely, but more commonly, present as gradual, chronic neurologic changes over several months [10]. Patients may present with chronic sinusitis, keratitis, cutaneous infection, onychomycosis, respiratory infection, endocarditis, and cerebral abscesses [1,10]. Abscesses in soft tissues (pulmonary, paravertebral, or cerebral abscesses) seem to be correlated especially with the C. lunata species [4,5,7,8]. Optic nerve atrophy may occur depending on the location of the cerebral abscess [1]. Although immunocompromised patients might be more prone to Curvularia, it is not necessary for development of the infection [10].
Pathological findings show infiltrates that can include lymphocytes and multi-nucleated giant cells, as well as fungal hyphae with terminal globose dilatation that are often characteristic of Curvularia (seen via Grocott’s Methenamine stain (GMS) [1]. If the patient presents with a long or chronic history, this often suggests that the mechanism of infection is via inhalation of spores, colonization of sinuses, followed by local invasion into the frontal lobe and/or skull base [11]. Although no singular treatment protocol has been determined for Curvularia infections, it is known that general phaeohyphomycotic infections respond poorly to typical antifungals (e.g.: fluconazole) but patients respond better to combinations of antifungals and complete surgical excision of the destructive mass [2]. The period of disease-free follow-up from intracranial Curvularia infection has been reported to be 3 years in 2013 [1]. The patient case mentioned above followed the use of antifungals and antibacterial pre-op, followed by surgical excision, and post-op antibiotics [1]. Serial MRIs are used for follow-up [1]. If significant erosion of facial bones and/or skull base is present, pericranial grafts may be used for reconstruction [1]. Below we present our unique case and use the features to highlight important teaching points.
CASE
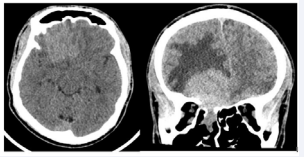
A 28-year-old immunocompetent African American male presented to the emergency room following a motor vehicle collision where a Computed Tomography (CT) scan of the head was obtained. Which revealed an incidental large sinonasal lesion with intracranial extension was noted as seen in Figure 1.
Figure 1: CT scan with axial and coronal slices of the brain showing a hyperdense mass in right anterior cranial fossa with extension into the right ethmoid. The mass measured 5.2 x 3.8 x 3.8 cm (AP, transverse, craniocaudal). There is surrounding vasogenic edema present (R>L), with minimal lesional extension across midline into the left mesial frontal lobe. Approximately 3mm right-to-left shift is present at the level of the third ventricle
Upon further questioning, it was discovered that he had developed persistent headaches, photophobia, chronic rhinosinusitis, and right sided vision loss over the course of three months. Of note, he had endoscopic sinus surgery for polyp while incarcerated approximately 10 years aprior. Due to the presence of the lesion, and unknown origin, Otolaryngology was consulted for and performed Endoscopic Sinus Surgery (ESS) with biopsy. Upon opening the maxillary and ethmoid sinuses, the performing surgeon noted a rubbery, firm mass that appeared to have morphed the present sinus tissues and was extending through the cirbiform, and planum into the intracranial space. Tissue removal was stopped at the skull base and focused on the sphenoid and ethmoid sinuses. Surgical pathology and tissue cultures were sent, and the patient had an otherwise uncomplicated course.
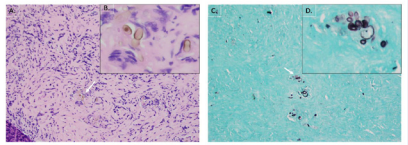
Final pathology revealed chronic granulomatous invasive fungal sinusitis and chronic polypoidal sinusitis consistent with Curvularia species (Figure 6).
Figure 6: A) 20x H&E showing the Culvularia buds in association with giant cells. B) 40x view of the pigmented organisms with their characteristic bulbous morphology. C) 20x GMS stain showing the Culvularia. D) 40x GMS stain showing classic grouping.
The patient was started on Amphotericin B and voriconazole.
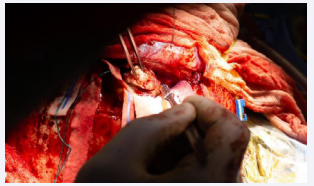
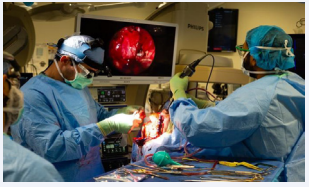
A combined endoscopic and open approach was then planned with otolaryngology and neurosurgery, with plan for endoscopic resection of the fungal mass down to the skull base and clearance of the sinuses, followed by open resection via a bifrontal craniotomy. Endoscopic resection was taken to the anterior cranial fossa dura, removing the bony skull base and exposing the dura adjacent the superomedial orbit, posterior to the planum, medially along the olfactory cleft, and anteriorly to the extent of the cribriform plate, exposing the area where the mass had penetrated the intracranial space with a wide dural margin. At this point bifrontal craniotomy was performed by the neurosurgical team. The dura was opened, and lesion was debulked. The anterior aspect of the sagittal sinus was ligated. The remainder of the lesion extending into the optic canal was then removed with Rhoton curretes. The lesion was then dissected from the frontal lobe with preservation of anterior cerebral artery branches (Figure 2).
Figure 2: The lesion is seen here through a bifrontal craniotomy exposure. The fungal lesion is photographed being removed with aggressive debulking using a fifteen blade. Tissue from this region was sent to pathology.
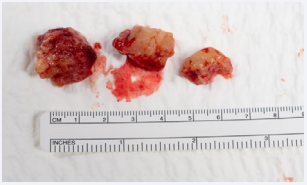
Attention was then transitioned to the lesion abutting the olfactory tracks with complete resection (Figure 3).
Figure 3: Intraoperative fungal specimen photographed here prior to being packaged and sent to pathology. Internal debulking was done in a piecewise fashion and select specimens from frontal lobe lesion are photographed here.
Frontal lobe encephalomalacia was noted and abnormal brain tissue was resected. The defect was then closed with abdominal fat graft, pericranial flap, and lyoplant. Once this was complete, attention was repaid to the ethmoid roof and the skull base, and reconstruction was then performed using a posterior septal artery pedicle nasoseptal flap to ensure successful dural closure (Figure 4).
Figure 4: Adequate closure was simultaneously evaluated superiorly by neurosurgery and inferiorly by otolaryngology.
Once this was performed, the bifrontal cranial incision was closed.
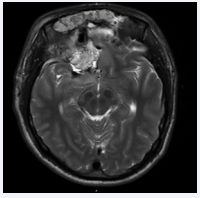
The procedure took approximately sixteen hours in total and was completed without complications. Immediately post operatively the patient was neurologically stable with preservation of pre-operative vision despite extensive optic nerve involvement. The post-operative magnetic resonance imaging (MRI) showed good resection of lesion (Figure 5).
Figure 5: Post operative T2 MRI sequence taken four days after bifrontal craniotomy and endonasal resection of right frontal abscess. No evidence of residual macroscopic disease with improving right frontal edema
Post operatively the infectious disease recommended 4 weeks of amphotericin treatment followed by 12 months of voriconazole. Patient was discharged with near term follow up for planned repeat MRI. At the patient’s most recent otolaryngology follow up, there was no sign of cerebrospinal fluid (CSF) leak, however the patient did complain of significant nasal crusting due to his persistent avoidance of saline lavage post operatively.
DISCUSSION
Epidemiology
Curvularia is a pigmented filamentous fungus found in tropical and subtropical regions [12]. Infection has been reported secondary to skin abrasions, catheter infections, and ocular trauma [13]. As in this case, nasal polyps have long been suggested as a predisposing risk factor [14]. Our patient additionally had chronic sinusitis, which is similar to prior reported cases by Ebright and Gadgil [1,15].
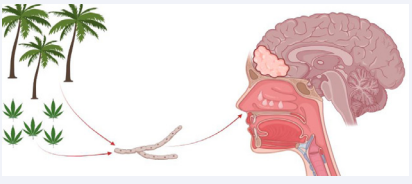
Potential infection sites include the heart [16], lungs [17,18], and urinary tract. Rarely, infection occurs in the central nervous system, and when it does it is often fatal [1]. There is a theorized association between marijuana smoking and Curvalaria infection [22]. Two prior case reports have detailed immunocompetent patients using marijuana who acquired CNS curvalaria infections [7,13]. Similarly, our patient reported occasional marijuana use, which may have been his exposure source. Due to the rarity of this infection, the influence of marijuana on fungal inoculation/ susceptibility is based off ancetodal evidence and needs further validation [13]. Skovrlj suggested a strong relationship may be present due to growth on marijuana leaves [12]. This important factor of the social history should therefore not to be overlooked (Figure 7).
Figure 7: This diagram highlights commonly accepted risk factors including marijuana smoking, living in a tropical/sub-tropical climate, and history of nasal polyps. All three risk factors were present in this reported case.
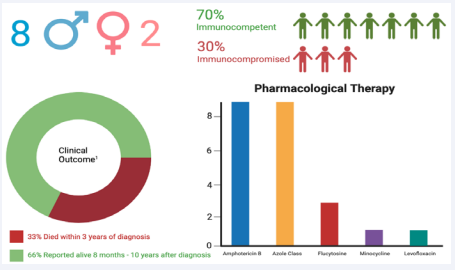
To our knowledge only nine cases have been reported involving the central nervous system since 1977 [12]. An updated infographic on previously reported CNS infections since 1977 including our most recent case is presented in Figure 8.
Figure 8: This infographic shows updated epidemiology, pharmacological management, and last reported outcomes of case reports since 1977. The study detailed in this paper was incorporated into the data presented. 1: Clinical outcome was based off previously reported long-term follow up. Our case was excluded from this calculation as long- term follow up is not yet available.
In prior case reports, CNS infections have been most commonly in the frontal and parietal lobe [13]. Cases have also been documented in deeper brain structures including the basal ganglia [7], and sellar region [20]. Fungal infections of the CNS generally affect immunocompromised patients (e.g. cryptococcal meningitis in HIV patient), but most cases of CNS Curvalaria have been reported in immunocompetent hosts. Including our report, 7/10 case reports were in immunocompetent patients.
Management
To date there is no standardized treatment regimen for Curvularia infections and prior published cases were used to help guide clinical decision making. Since 1977 there have been only nine reported cases of CNS infections [12]. A case with striking resemblance to ours was reported by Gadgil in 2012. Similarly, they reported an immunocompetent patient with history of previous sinus surgery presenting with a symptomatic CNS infection from Curvalaria. Although other cases proved to be fatal even with surgical and pharmacological treatment [19], Gadgil documented freedom from recurrent disease three years following discharge when complete resection is achieved [1]. With aggressive surgical resection and dual antifungal therapy, mortality rates are reported to be as high as 73% [19]. Including our most recent case mortality rates have been reduced to 33% (Figure 8) [12].
Upon reviewing recent published cases, amphotericin B and azole drugs were most reported being used. Nine prior reported since 1977 used Amhotericin B [12]. All cases reported after 2004 included voriconazole in their regiment [12] (Figure 8). Smith and Singh were the two prior studies using the same regiment presented in this cacse, Amphotericin B and Fluconazole. Gadgil’s reported management was handled in a similar fashion to ours, with a combination of aggressive surgical debridement and pharmacological therapy. This strategy offers the best chance for success. Curvularia often affects anatomical structures adjacent to the eye, which can sometimes limit debridement. Many cases within the literature report keratitis [14]. In this case, surgical decompression of the optic nerve was warranted. Fortunately, vision remained stable immediately following surgery. Aside from ocular complications, Curvularia has commonly been reported to infect the lungs [7]. The multi-organ involvement necessitates collaboration amongst specialties. In our case, we acquired imaging of the chest and a full ophthalmologic evaluation both before and after surgery to rule out any further systemic involvement. Failure to check for pulmonary involvement could potentially cause disease recurrence and sub-optimal outcomes. Future cases should be reported to provide additional insight on epidemiology and management of CNS Curvalaria infections.
SUMMARY
CNS curvularia responds poorly to standard antifungal therapy and no standardized regiment exists [13]. Like prior reported cases [19-21], amphotericin B and voriconazole were chosen to eradicate fungus. This approach combined with aggressive resection offers the best chance for disease free progression.