Comparison of Intranasal Ketamine and Intranasal Fentanyl as Adjuncts to Intravenous Ketorolac for Pain Relief in Renal Colic: A Randomized Clinical Trial
- 1. Medical doctor, Shahid Sadoughi University of Medical Sciences, Iran
- 2. Department of Emergency, Shahid Sadoughi University of Medical Sciences, Iran
- 3. Department of Urology, Shahid Sadoughi University of Medical Sciences, Iran
Abstract
Background: Pain relief in patients with renal colic is a crucial aspect of emergency department (ED) management. Current analgesic options, such as opioids and nonsteroidal anti-inflammatory drugs (NSAIDs), are associated with various side effects and contraindications. This study aimed to investigate and compare the efficacy of intranasal ketamine/intravenous ketorolac and intranasal fentanyl/intravenous ketorolac combinations with intravenous ketorolac monotherapy in reducing pain among patients with renal colic.
Materials and Methods: This double-blind randomized clinical trial enrolled 120 patients with renal colic who met the inclusion and exclusion criteria. Participants were randomly assigned to three groups: Group A received 1 mg/kg intranasal ketamine and 30 mg intravenous ketorolac, Group B received 1.5 µg/kg intranasal fentanyl and 30 mg intravenous ketorolac, and Group C received only 30 mg intravenous ketorolac and nasal placebo. Vital signs, adverse effects, and pain intensity based on the visual analog scale (VAS) score were recorded at specified intervals.
Results: There were no significant differences in demographic variables and mean initial pain intensity among the groups. Following the intervention, both the ketamine/ketorolac and fentanyl/ketorolac groups exhibited faster pain reduction compared to the ketorolac group. However, there was no significant difference between the two combination drug regimens (P Value = 0.044, 0.906). The ketamine/ketorolac group demonstrated a quicker response to treatment compared to the other two groups (P Value = 0.004). Some side effects were more frequently reported in the combination drug group than in the single drug group.
Conclusion: The combined regimens of ketamine/ketorolac and fentanyl/ketorolac appear to provide faster pain control in patients with renal colic compared to the single-drug regimen of ketorolac, without increasing the risk of serious complications.
Keywords
• Intranasal administration
• Ketamine
• Ketorolac
• Fentanyl
• Renal colic
CITATION
Ashkezari FSZ, Jafari M, Ezzabadi AR, Zarehoroki A, Abarghouei SA (2023) Comparison of Intranasal Ketamine and Intranasal Fentanyl as Adjuncts to Intravenous Ketorolac for Pain Relief in Renal Colic: A Randomized Clinical Trial. Arch Emerg Med Crit Care 7(2): 1060.
INTRODUCTION
Renal colic, a common complication of urolithiasis, affects 5_15% of the global population [1], and is responsible for a notable percentage of emergency department visits [2]. Managing the intense pain associated with renal colic is a top priority for healthcare providers. Opioids, such as morphine, and nonsteroidal anti-inflammatory drugs (NSAIDs), like ketorolac, are commonly used analgesics for this purpose. However, opioids can be addictive [4] and have potential adverse effects [3], while NSAIDs may have side effects and contraindications in certain patient populations [5].
Intravenous administration of medication can be challenging in agitated patients, making intranasal administration through Kiesselbach’s plexus an attractive alternative due to its ease and speed [6]. Nonetheless, it remains unclear which intranasal analgesic is most suitable for patients with renal colic. Intranasal fentanyl is a popular choice, known for its high potency and bioavailability for acute pain relief [7-9]. However, like other opioids, it carries the risk of serious complications such as hypotension and bradypnea [10]. On the other hand, ketamine has demonstrated effectiveness in reducing acute pain by inhibiting NMDA receptors and affecting various other receptors involved in pain modulation [11,12]. Although it can cause mild and transient side effects such as dizziness and hallucinations [10].
This study aimed to compare the analgesic effects of intranasal ketamine and intranasal fentanyl, both in conjunction with intravenous ketorolac, for the management of renal colic pain. By evaluating the efficacy and safety of these combinations, we hope to provide evidence to guide clinicians in selecting the most appropriate intranasal analgesic regimen for patients with renal colic.
MATERIALS AND METHODS
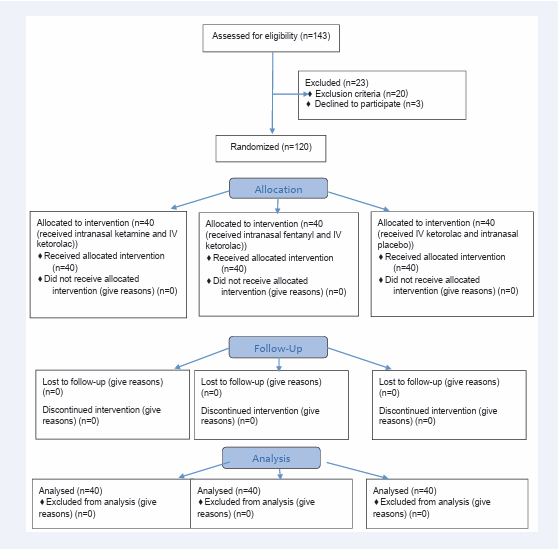
This study was a double-blind randomized controlled trial conducted on 120 patients who presented with acute renal colic to the emergency departments at Shahid Sadoughi Hospital and Shahid Rahnemoon Hospital (Figure 1).
Figure 1: Consort flow chart.
The study received approval from the ethics committee of Shahid Sadoughi University (ethics code: IR.SSU.MEDICINE.REC.1399.081) and was registered on www.irct.ir with trial number IRCT20201028049179N1. Prior to the commencement of the study, the patients were informed about the study goals, and their consent was obtained. The authors adhered to the principles outlined in the Declaration of Helsinki throughout the study.
Inclusion criteria consisted of patients aged between 15 to 64 years old with a diagnosis of renal colic based on history, physical examination, urinalysis, and imaging studies collected through ultrasound or computed tomography scans. Patients were excluded if they met any of the following criteria: pregnancy, addiction to drugs and opioids, allergy to fentanyl, Ketamine, or Ketorolac, body temperature over 38°C, suspension of pyelonephritis, systolic blood pressure over 180 or less than 90 mmHg, respiratory rate less than 12, heart rate less than 60 or over 140, history of serious liver, kidney or cardiovascular diseases, active peptic ulcer, brain tumor, glaucoma, psychosis, use of analgesics in the past 4 hours, and a Numeric Rating Scale (NRS) score less than 5.
NRS is a pain severity measuring tool that uses a scale from 0 to 10, with 0 indicating no pain and 10 indicating the worst pain imaginable.
Before the administration of medication, all patients were assessed for vital signs, excluding criteria, and NRS scores. They were then monitored for 60 minutes after treatment, during which they received 500 cc of intravenous fluid and nasal oxygen at a flow rate of 3-5 L/min. Participants were assigned to one of three groups based on a predetermined randomization list generated by https://www.random.org/lists/.
For the nasal ketamine spray, ketamine vials manufactured by Sterop Company were used. These vials contained ketamine hydrochloride injection parenteral 50 mg/1mL, 10 mL, and 50 mg of benzalkonium chloride as a preservative. Desmopressin nasal sprays from Raha Company were emptied, sterilized, and filled with the ketamine solution. Each spray puff contained 7 mg of ketamine, and the spray could be used up to one month after opening the vial.
For the fentanyl spray, fentanyl vials manufactured by Caspian Company were used. Each 10-cc vial contained 0.5 mg of fentanyl and 50 mg of benzalkonium chloride as a preservative. Desmopressin nasal sprays from Raha Company, with a volume of 10 cc, were emptied, sterilized, and filled with the prepared fentanyl solution. Each spray puff contained 7 μg of fentanyl, and the sprays could be used up to one month after opening the vial.
Placebo sprays were prepared using 10 cc vials of distilled water manufactured by Sunlife Company, along with 50 mg of benzalkonium chloride as a preservative, similar to the ketamine and fentanyl sprays. Participants were divided into three groups: - Group A received intranasal ketamine at a dose of 1 mg/ kg (maximum 50 mg, each puff containing 7 mg) and 30 mg of intravenous ketorolac. - Group B received intranasal fentanyl at a dose of 1.5 μg/kg (maximum 50 μg, each puff containing 7 μg) and 30 mg of intravenous ketorolac. - Group C received 30 mg of intravenous ketorolac and 6 puffs of placebo spray. The solvents used in all nasal sprays and syringes were identical. In case participants experienced nausea and vomiting, 4 mg of intravenous ondansetron was prescribed. A triage nurse, who was unaware of the medications being administered, prepared and prescribed the medications based on the random list. Neither the patients nor the assessing physicians were aware of the assigned medications. The physician recorded vital signs, pain scores, and side effects at baseline, as well as at 5, 10, 15, 30, and 60 minutes using a checklist. The checklist consisted of two parts: the first part captured demographic information such as age and gender, while the second part included pain scores, time to respond to treatment (with a reduction of 2 scores), time until pain subside (pain score below 5), request for a second dose, request for rescue analgesic, and side effects. Dissociative side effects were assessed using the Side Effects Rating Scale for Dissociative Anesthetics (SERSDA). If the pain score did not decrease by 2 scores after 15 minutes, a second dose (half of the first dose) was prescribed. If the pain intensity remained unbearable after the second dose and 15 minutes, rescue therapy was administered using 0.15 mg/kg of intravenous morphine. In case of serious complications such as hypo/hypertension, apnea, lethal arrhythmia, or loss of consciousness, the investigation was immediately halted, and appropriate treatment was provided. Other side effects, including nausea and vomiting, drowsiness, hallucinations, agitation, hot flashes, bradypnea, itching, dizziness, and chest tightness, were recorded in the checklist. Participant satisfaction was assessed using a rating scale from 1 to 10 (with 1 being the least satisfaction and 10 indicating complete satisfaction). After 60 minutes, participants were discharged if their pain was controlled, they did not experience nausea, had a normal gait, and did not require hospitalization. The secondary outcome measures included the mean reduction in pain scores at different time intervals, frequency of response to treatment and its time, frequency of patients whose pain scores reduced to 4 or less and the time it took, frequency of the need for a second dose and rescue analgesic, and side effects. Finally, the collected data were analyzed using SPSS (version 16).
FINDINGS
During the course of the study, a total of 143 patients were initially enrolled. However, 23 patients were subsequently excluded from the analysis due to various reasons: 2 patients had an allergy to ketorolac, 15 patients had used analgesics within the past 4 hours, 1 patient was pregnant, 2 patients were addicted to opioids, and 3 patients declined to participate. As a result, the final analysis included 120 patients who were randomly assigned into three groups of 40 participants each. In Group A, 67.5% of participants were men, while in Group B it was 70%, and in Group C it was 62.5%. The mean age range was comparable across all three groups (Table 1).
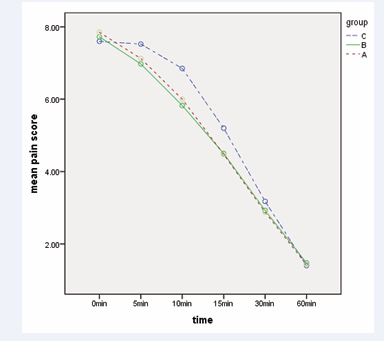
The mean ± SD NRS scores at baseline and at the specified time points are presented in Table 2. There was no significant variation in average pain scores among the three groups according to the Kruskal-Wallis test. However, Group C exhibited a slower rate of pain reduction compared to the other two groups, as determined by repeated measures analysis (P value of 0.044). No significant difference in pain reduction was observed between Groups A and B (P value of 0.906) (Figure 2).
Figure 2: The progression of pain intensity in the mentioned minutes within the three groups.
The study findings revealed no significant differences in the frequency of response to treatment, the need for a second dose, or the use of a rescue analgesic among the groups (Table 3). Additionally, all groups demonstrated a similar number of patients with pain scores reduced to 4 or less. However, Group A exhibited a shorter response time compared to the other groups (P value: 0.004) (Tables 4 and 5).
The mean satisfaction score was 7.65 ± 2.55 in Group A, 8.05 ± 2.35 in Group B, and 7.42 ± 3.02 in Group C, with no significant difference observed between them (P value: 0.848).
No instances of hypotension, bradypnea, apnea, chest discomfort, or loss of consciousness were reported by any of the patients. Nausea was the most common reported side effect, with no significant differences observed among the groups. Other side effects are outlined in Table 5. Dissociative side effects were reported in all groups, but they were all mild and transient.
DISCUSSION
Pain relief is of utmost importance for patients with renal colic, and analgesics such as opioids and NSAIDs are commonly used, usually administered intravenously [3].
In this study, we aimed to compare intranasal ketamine with intravenous ketorolac versus intranasal fentanyl with intravenous ketorolac for renal colic pain relief. The results show that the combination regimens of ketamine/ketorolac and fentanyl/ketorolac provide faster pain relief compared to ketorolac/placebo. However, these combination regimens also carry a slightly increased risk of minimal adverse effects.
Some studies have demonstrated the synergistic effect of ketorolac and opioids for renal colic pain relief. For instance, Hosseininejad et al., compared the efficacy of combination therapy with ketorolac and morphine to monotherapy with each drug in patients with acute renal colic. The study found that the balanced analgesia group had significantly lower pain intensity compared to the morphine or ketorolac alone groups. After 40 minutes, the mean pain score in the combination group was significantly less than in the other groups [13]. However, in our study, there was no significant difference in pain intensity after 60 minutes, possibly due to the longer follow-up period in our research.
Ketamine is an analgesic that can be administered through various routes and is a good choice for acute pain relief. Ketamine acts by inhibiting NMDA receptors and reducing pain through a different pathway than NSAIDs and opioids [11,12]. Some studies consider it a suitable option for renal colic pain management on its own, while others suggest its use as an additional therapy for controlling severe pain. For example, a study by Mozafari et al., compared the analgesic effect of intravenous fentanyl with intranasal ketamine in 120 renal colic patients. The findings indicated that ketamine is less effective than fentanyl in controlling renal colic pain and is associated with a higher prevalence of side effects. However, ketamine could be effective in combination with other medications for pain control [10]. In our study, we found that ketamine in combination with ketorolac is more effective than ketorolac alone, and the time needed to respond to treatment in the ketamine/ketorolac group was significantly shorter than in the other two groups. Farnia et al., also reported that intranasal ketamine is a good choice for renal colic and has no more adverse effects than intravenous morphine [12]. In a meta-analysis conducted by Dongxu Zhang et al., it was reported that compared with opioids, ketamine has a longer duration of analgesia in renal colic and a better safety profile. There were no significant differences between ketamine and opioids in terms of nausea, vomiting, and dizziness, but the risk of hypotension was significantly lower in the ketamine group [14]. Our study demonstrated that the combination of ketamine/ ketorolac had a faster onset of activity compared to the other groups. Moreover, Miller et al. (n.d.) reported in their study that low-dose intravenous ketamine has similar analgesic effects to intravenous morphine in acute pain management, but with a faster onset [15].
In a study by Abd El Motlb EA. et al., the analgesic effect of intramuscular ketorolac/ketamine and ketorolac/fentanyl was compared in 80 children undergoing bone marrow aspiration and biopsy under general anesthesia. The study concluded that these two regimens were equally effective as analgesics in the pediatric population undergoing the procedure, without adverse effects. The time to first analgesic request, CHOEP scale, emergence-agitation (EA) score, and the need for rescue analgesic did not differ significantly between the two groups (16), which is consistent with our findings.
LIMITATIONS OF THE STUDY
This study had a couple of limitations. Firstly, we only enrolled patients who presented to the emergency department at the same time as the assessing physician, which may introduce selection bias. It would have been beneficial to include a larger sample size and a more diverse group of patients to enhance the generalizability of the results. Additionally, we were unable to measure the blood levels of the intranasal medications, which could have provided valuable insights into their pharmacokinetics and correlation with pain relief. Furthermore, the patients were discharged after 60 minutes, so we did not collect data on possible delayed complications or pain recurrence. It is recommended that future studies include longer follow-up periods to assess for these outcomes. Lastly, we did not document certain side effects that may have been caused by the nature of the disease, such as nausea and vomiting, prior to medication administration. To capture a more comprehensive understanding of side effects, it is suggested that future studies evaluate these symptoms in longer follow-up periods and record all signs and symptoms before investigation.
CONCLUSION
In conclusion, our findings suggest that the combined regimens of ketamine/ketorolac and fentanyl/ketorolac are more effective in controlling pain in patients with renal colic compared to the monotherapy regimen of ketorolac alone. These combined regimens provide faster pain relief. However, it is important to note that the possibility of some side effects also increases with the use of these combined regimens. Further research is needed to optimize the dosages and explore the potential long term complications and recurrence of pain associated with these regimens.
ACKNOWLEDGMENT
The authors gratefully acknowledge the support and cooperation of the staff in the Emergency Departments of Shahid Sadoughi and Shahid Rahnemoon Hospitals in Yazd, Iran.