Decompression Sickness Associated with Neurological Sequalae and Marked Blood Test Changes. A Case Report in Athens Naval Hospital
- 1. Department of Hyperbaric & Diving Medicine, Athens Naval Hospital, Greece
Abstract
Decompression Sickness (DCS) is a condition consisting of complications/symptoms after diving. Main pathophysiological event involves formation of inert gas bubbles in tissues and microcirculation (usually Nitrogen or Helium), that target multiple organs and tissues. Identification of lesions, among other investigations, is associated with biochemical and enzymic changes as a result of cellular damage. We report a case of DCS causing neurological damage associated with significant changes in blood tests. Treatment with Hyperbaric Oxygen Therapy (HBO2) led to resolution.
Case report: A 37-year-old male diver was transferred to the Athens Naval Hospital with symptoms after diving. He developed neurological deficit manifesting as asymmetrical nystagmus, reduced motor power in the right lower extremity (4/5) and a rash of upper extremities and torso. Treatment strategy involved initial management of symptoms with normobaric oxygen 100% and fluid resuscitation, followed by recompression therapy and HBO2 combined with medication to resolve neurological injury. Ten days later following remarkable clinical improvement he was discharged.
Keywords
• Decompression Sickness
• Capillary leakage syndrome
• Venous gas emboli
• Hyperbaric Oxygen Therapy
Citation
Athanasiou K, Kalentzos VN (2025) Decompression Sickness Associated with Neurological Sequalae and Marked Blood Test Changes. A Case Report in Athens Naval Hospital. Arch Emerg Med Crit Care 9(1): 1068.
ABBREVIATIONS
DCI: Decompression Illness; DCS: Decompression Sickness; AGE: Arterial Gas Embolism; CLS: Capillary leakage syndrome; VGE: Venous gas emboli; HBO2: Hyperbaric Oxygen Therapy; O2: Oxygen; N2: Nitrogen; He: Helium; DHDM: Department of Hyperbaric & Diving Medicine; ANH: Athens Naval Hospital; CT: Computed Tomography
INTRODUCTION
Decompression Illness (DCI) is a term that describes two diving disorders, Decompression Sickness (DCS) and Arterial Gas Embolism (AGE) [1]. DCS is a disease related to complications/symptoms after diving. The disease originates from bubble formation in blood and/or tissues during ascent, which causes various complications in the human body and damages various areas through different mechanisms. Bubbles consist of inert gas contained in the breathing mixture, which is usually Nitrogen (N2 ) or Helium (He) or both [2]. DCS is classified in 2 main categories. Type 1 refers to the mild form with symptoms of musculoskeletal pain (joint pain), a cutaneous form (skin marbling and/or rash) and a lymphatic form with lymphatic occlusion and oedema. Type 2 includes severe or even life-threatening conditions and is usually divided into 4 subcategories depending on the affected organ, which are brain, spinal cord, inner ear and lung/heart [3]. We report a case of DCS leading to neurological damage associated with significant biochemical and enzymic changes in blood tests.
CASE PRESENTATION
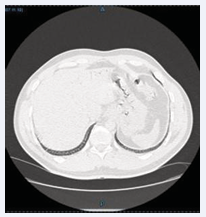
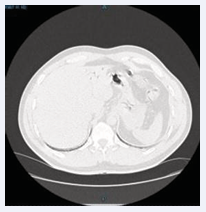
A 37-year-old male diver was transferred from a provincial hospital to the emergency department of the Athens Naval Hospital (ANH) due to symptoms after diving. The dive was carried out with air; Maximum depth of the dive was 40 meters with 5 minutes time at depth (reported by diver), slow ascent with decompression stop at 5m for 5’ and then surfaced. 10’ later, while transporting the SCUBA equipment to his vehicle, he felt numbness in his lower extremities and stomach upset, so he visited the nearest emergency department. After receiving first aid, telephone contact was made to the Department of Hyperbaric & Diving Medicine (DHDM) and he was transferred to the ANH 6 hours later. Microbiological examinations (Table 1), electrocardiogram (normal), blood gases (PO2 : 70, PCO2 : 26,5, Ph: 7,42 on room air) and chest C/T (large amount of air in the portal vein, Figure 1,2) were performed.
Figure 1: Presence of intravascular air in the portal vein
Figure 2: Presence of intravascular air in the portal vei
Table 1: Blood Test Results on days 1–3
|
|
Before HBO2 |
After 1st HBO2 |
After 2nd HBO2 |
After 4th HBO2 |
After 10 HBO2 |
|
Hct (40,0-52,0%) |
65,5 |
61,9 |
56,8 |
39,1 |
43,7 |
|
WBC (4,0-10,0 K/ Μl) |
31,34 |
23,01 |
19,83 |
11,16 |
14,47 |
|
PLT (150-450 K/μL) |
204 |
178 |
166 |
151 |
316 |
|
Troponin hs-T (<14 pg/ml) |
137,7 |
54,5 |
38,9 |
25,2 |
5,7 |
|
Glu (70-110 mg/dl) |
168 |
253 |
116 |
90 |
105 |
|
LDH (80-248 U/l) |
348 |
350 |
433 |
401 |
224 |
|
CPK (10-171 U/l) |
268,0 |
729,0 |
999,0 |
344,0 |
113,0 |
|
CK MB (0-25 U/L) |
33,1 |
30,1 |
38,5 |
20,0 |
17,6 |
|
CRP (0-7,0 mg/l) |
9,20 |
|
|
29,5 |
6,20 |
Vital signs were within normal range. He was alert, oriented in time-space (GCS 15/15), and neurological assessment revealed asymmetrical nystagmus (mainly left-gaze evoked), with horizontal and torsional movements, reduced motor function of the right lower extremity (4/5), numbness of the lower extremities and gait instability. Also, rash of the torso and arms. The patient reported increased fatigue, generalized weakness and also vomiting for 2 times. Intravenous hydration, cortisone and gastroprotection were administered and before entering the hyperbaric chamber an urinary catheter was placed. DCS was probable, and medical management was based on that as a working diagnosis.
Recompression treatment initially involved air compression at 2.5ATA during which the rash disappeared but intense pain appeared of the upper extremities, while numbness in the lowered extremities persisted. Afterwards, 100% O2 was administered as the breathing gas (Hyperbaric Oxygen Therapy – HBO2 ) and a modified US treatment table 6 was used at 2.5ATA. 5 hours after the end of the initial treatment, he underwent a 2nd HBO2 session. Clinical improvement was noted with return of appetite, improved mobility of lower extremities but dizziness persisted with profuse sweating in the supine position. A 3rd HBO2 session was conducted later within first 24 hours. An attempt was made for the patient to stand up which was achieved with difficulty (asymmetrical strength of the lower limbs and gait instability). Dizziness gradually improved, and nystagmus and pain of the upper extremities disappeared. After each session blood was drawn for blood tests (Table 1).
Patient underwent further 9 HBO2 sessions (12 in total), with gradual improvement. A new CT was performed on day 6, which was normal, with elimination of air of the portal vein as evident on initial CT scan. No free air or other lesions of the thoracic cavity. Further clinical improvement with normalization of limbs’ muscle strength, ability to stand and walk. Bladder catheter was removed with satisfactory urinary function and subsequent assessment by a Neurologist reported near normal findings with slight asymmetry in motor function of the limbs (right < left). The patient was discharged with medical instructions and a referral for physical therapy, including exercises aiming to strengthening and enhancement of stability and balance.
DISCUSSION
The main pathological event in the development of DCS is the formation of intravascular bubbles [4]. Inhalation of air under increased environmental pressure (during descent and at depth), causes dissolution of additive amount of inert gas (N2 when breathing air) reaching saturation of body tissues in extreme diving schedules.
As the diver emerges, this extra inert gas amount must be released through the respiratory system. Depending on the amount of additional dissolved gas and the rate of ascent, inert gas can become supersaturated in tissues and separate from the body liquids, forming bubbles [5]. Bubbles form mainly in venous vasculature (Venous Gas Emboli – VGE). They are trapped as they pass through the pulmonary vessels and inert gas is eliminated from the body. VGE are initially asymptomatic («silent» bubbles). However, their excessive formation in various tissues has been recognized as the main cause of DCS. Occasionally, VGE can pass into the arterial systemic circulation, especially in cases of patent foramen ovale (PFO) or other shunts and cause undeserved / unexpected DCS or major neurological damage [6].
Vascular bubbles cause problems through mechanical effects directly (occlusion or distortion of vessels/tissues) or indirectly from the associated inflammatory response, which is stimulated by various factors [7]. Endothelial injury stimulates coagulation factors and inflammatory cascade with platelet aggregation and leukocyte activation, events that lead to worsening ischemia, hypoxia, edema and tissue damage [8,9]. Inflammatory activation and responses of the human body are very important because they can lead to severe or fulminant DCS. They are also the reason for persistence of clinical manifestations after bubble elimination [10]. Increased capillary permeability to proteins across the cell membrane leads to loss of fluid (rich in proteins) from the intravascular to the interstitial space, a phenomenon described in other human illness as ‘capillary leak syndrome’ (CLS) [11]. Patients with severe or fulminant type of DCS may develop severe hemoconcentration and shock, secondary to vascular endothelial leakage [10]. It appears that bubbles created during decompression and passing through systemic circulation can cause diffuse intravascular damage, resulting in increased vascular permeability with subsequent fluid extravasation to interstitial space [12]. A clinical study has correlated the degree of hemoconcentration to the severity of CNS damage in divers with DCS [13].
HBO2 involves placing a patient in a hyperbaric chamber administering 100% O2 at a pressure greater than ambient pressure (minimum acceptable pressure of 1.5ATA) [14]. Benefits of HBO2 in the treatment of diving disorders vary and are achieved through two main mechanisms of action, a) reduction of size of tissue and vascular bubbles, as a result of Boyle’s law and b) hyperoxygenation (re-oxygenation) of hypoxic tissues by increasing the concentration of O2 in blood and plasma, as a result of Henry’s law [15]. HBO2 as a treatment modality is indicated for 15 different clinical conditions by UHMS [14]. At the biochemical and cellular level, HBO2 seems to affect the healing process by reversing tissue hypoxia and reducing oxidative stress, promotion of neo-angiogenesis, reduction of edema, enhancement of action of leucocytes against pathogens, bacteriostatic action against microorganisms and enhancement of healing potential [16,17]. Clinical management of DCS initially includes provision of pre-hospital first aid (normobaric O2 , airway management, hemodynamic stability, fluid resuscitation and patient supine position) and then recompression therapy & HBO2 depending on the severity of the incident [18].
In addition, pharmacological treatment is used, and supportive therapy may be needed in compromised patients. Medical procedures may be necessary after appropriate assessment (bladder catheterization in para-paresis/plegia, chest tube insertion in pneumothorax etc) before initiation of recompression therapy.
Although there is no single diagnostic test for DCS, biochemical and enzymic changes have been reported in various case studies in human divers following diving accidents [19-22]. Smith et al., in 1994 observed an increase in creatine kinase in divers after AGE, as a result of skeletal muscle injury [19].Viecelli et al., in 2014 reported increase in liver enzymes and impaired renal function (acute kidney injury) in a diver after DCS, who recovered fully after HBO2 [20]. Other case studies reported statistically significant differences (p<0,05) in various blood parameters such as WBCs, prothrombin time, blood sugar (hyperglycemia), very-low-density lipoprotein and blood urea nitrogen after decompression [21,22].
CONCLUSION
The embolic nature of DCS has multiple target organs and may affect more than one. Clinical examination of the patient remains the main diagnostic criterion, in relation to the schedule and conditions of diving. Blood testing can help determine the extent of injury, monitoring of the condition and treatment effects both in the initial assessment and during hospitalization. The incident we are referring to, was a severe DCS type II case, and it could evolve to shock. Significant changes in hematocrit and white blood cells as well as enzyme changes were evident and dealt with successfully. Although we didn’t face the typical spectrum of CLS manifestations (hypotension, hypoalbuminemia, extravascular fluid accumulation), blood test abnormalities similar to ours have been found in diving related CLS cases [12]. DCS management with fluid resuscitation, medical therapy and repetitive HBO2 treatments were efficient as judged by the patient’s clinical course and led to resolution.
ACKNOWLEDGEMENTS
All authors have reviewed and approved the manuscript’s contents
REFERENCES
- Mitchell SJ. Decompression illness: a comprehensive overview. Diving Hyperb Med. 2024; 54: 1-53
- Cooper JS, Hanson KC. Decompression Sickness (DCS, Bends, Caisson Disease). Nih.gov.StatPearls Publishing; 2019.
- Kamtchum Tatuene J, Pignel R, Pollak P, Lovblad KO, Kleinschmidt A, Vargas MI. Neuroimaging of diving-related decompression illness: current knowledge and perspectives. Am J Neuro Radiol. 2014; 35: 2039-2044.
- Ljubkovic M, Dujic Z, Møllerløkken A, Bakovic D, Obad A, Breskovic T, Brubakk AO. Venous and arterial bubbles at rest after no- decompression air dives. Med Sci Sports Exerc. 2011; 43: 990-995
- Scuba diving: Decompression illness & other dive-related injuries. Cdc.gov. 2025.
- Ljubkovic M, Marinovic J, Obad A, Breskovic T, Gaustad SE, Dujic Z. High incidence of venous and arterial gas emboli at rest after trimix diving without protocol violations. J Appl Physiol. 2010; 109: 1670- 1674.
- Papadopoulou V, Eckersley RJ, Balestra C, Karapantsios TD, Tang MX. A critical review of physiological bubble formation in hyperbaric decompression. Adv Colloid Interface Sci. 2013; 22-30
- Alcock J, Brainard AH. Gene–environment mismatch in decompression sickness and air embolism. Med Hypotheses. 2010; 75: 199-203.
- García E, Mitchell S. Bubbles in the skin microcirculation underlying cutis marmorata in decompression sickness: Preliminary observations. Diving Hyperb Med. 2020; 50: 173-177.
- Mitchell S. Decompression Sickness: pathophysiology. In: Edmonds C, Bennett M, Lippmann J, Mitchell S. Diving and Subaquatic Medicine 5th Edition. Taylor & Francis Group. Boca Raton London New York; 2016; 125-140
- Siddall E, Khatri M, Radhakrishnan J. Capillary leak syndrome: etiologies, pathophysiology, and management. Kidney Int. 2017; 92: 37-46.
- Gempp E, Lacroix G, Cournac JM, Louge P. Severe capillary leak syndrome after inner ear decompression sickness in a recreational scuba diver. J Emerg Med. 2013; 45: 70-73
- Boussuges A, Blanc P, Molenat F, Bergmann E, Sainty JM. Haemoconcentration in neurological decompression illness. Int J Sports Med. 1996; 17: 351-355.
- HBO indications (2020) - Undersea & Hyperbaric Medical Society. 2020.
- Researchgate.net.
- Gupta M, Rathored J. Hyperbaric oxygen therapy: future prospects in regenerative therapy and anti-aging. Front Aging. 2024; 5: 1368982
- Schottlender N, Gottfried I, Ashery U. Hyperbaric Oxygen Treatment: Effects on Mitochondrial Function and Oxidative Stress. Biomolecules. 2021; 11: 1827.
- Moon RE, Mitchell SJ. Hyperbaric oxygen for decompression sickness. Undersea Hyperb Med. 2021; 48: 195-203.
- Smith RM, Neuman TS. Elevation of serum creatine kinase in divers with arterial gas embolization. N Engl J Med. 1994; 330: 19-24.
- Viecelli A, Jamboti J, Waring A, Banham N, Ferrari P. Acute kidney injury due to decompression illness. Clin Kidney J. 2014; 7: 380-382.
- Jauchem JR, Waligora JM, Johnson PC Jr. Blood biochemical and cellular changes during decompression and simulated extravehicular activity. Int Arch Occup Environ Health. 1990; 62: 391-396.
- Jacey MJ, Heyder E, Williamson RA, Tappan DV. Biochemistry and hematology at decompression sickness: a case report. Aviat Space Environ Med. 1976; 47: 657-661.