Differential Approaches to Treat Deep Intracerebral Hematomas
- 1. Department of Neurosurgery, Hufeland Klinikum GmbH, Germany
Abstract
Surgical treatment of deep intracerebral hematomas remains a difficult problem to solve. One of its greatest difficulties is the lack of a clear differentiation into subtypes, that could benefit from the use of specific surgical approaches. There is also no agreement yet on which surgical techniques might be the most appropriate. This paper summarizes the current scientific knowledge on both limitations, and proposes a therapeutic algorithm based on the author’s experience, and on the possibility of appropriately treating associated problems.
Keywords
Deep intracerebral hematomas, Treatment possibilities
Citation
Nievas MC (2023) Differential Approaches to Treat Deep Intracerebral Hematomas. Arch Emerg Med Crit Care 7(1): 1055.
INTRODUCTION
Although significant deep intracerebral hematoma (DIH) related death and severe neurological deficits arise from hematoma mass effect and intraventricular extension, further clinical deterioration related to rebleeding and/or perihematomal brain edema development often occurs. Today it is well known that the level of disability and mortality after DIH, depends on the Glasgow Coma Scale (GCS) score at admission, hemorrhage size and patient age [1]. One of the commonly used indications for surgery is neurological deterioration, but this is also a predictor of poor outcome.
Even in cases with a clear indication for surgery, there is also no valid agreement on the technique to be used to remove the clot. Likewise, in the surgical planning, it should also not be forgotten that the clot has different consistency during its evolution, and that these in turn, are accompanied by a growing cerebral edema.
The aim of this paper is to provide clear statements concerning the surgical management of spontaneous intracerebral hematoma patients based on a detailed analysis of the literature and on own experience-based data.
CURRENT STATE OF CLINICAL RESEARCH
It is now accepted that patients with an intracerebral hemorrhage (ICH) and a neurological deterioration or with a tendency to expand the clot, present a real need for surgery if their lives are to be saved. As shown in previous studies, patients with deep hematomas are more prone to these complications, especially when compared to lobar hematomas [2-4].
It would be useful to recall that such hemorrhages are always associated with a high mortality rate. Approximately 30% to 55% of patients die within 1 month after onset, and the people who survive largely have different degrees of neurological sequelae [5,6].
In non-life-threatening patients, the surgical indication should be considered only in cases where a proven improvement in their outcome is certain. Usually, a good functional outcome six months after treatment is defined as with a Glasgow Outcome Scale GOS ≥ 4 or a Barthel Index ≥ 55 [7].
However, and particularly in patients with DIH, significant differences are observed in their outcomes. A multivariable logistic analysis showed that hematoma volume and his location at the posterior limb of the internal capsule were independent predictors of poor outcomes in the right hemisphere; while hematoma volume, the posterior limb of the internal capsule and thalamus were independent predictors of poor outcomes in the left hemisphere [8].
Other authors suggest that therapeutic results could be improved if the surgical indication is performed using a modified intracerebral hemorrhage (MICH) score [9,10]. The components of the score are: The Glasgow coma scale (GCS) score 15–13= 0, 12–5= 1, 4–3= 2; ICH volume, mL ≤20= 0; 21–50= 1, ≥ 51= 2; IVH or hydrocephalus No 0 Yes 1. Total MICH score 0–5. In this scoring system, a MICH score of 0 or 1 indicates that conservative treatment is better than surgical treatment to preserve neurologic function. Surgery is recommended for patients with midlevel MICH scores to obtain better functional outcomes (MICH = 2) and to reduce mortality (MICH = 3 or 4). There are no indications for surgery in patients with a MICH score of 5.
Beyond these possible guidelines about a surgical indication, it is necessary to reconsider whether conservative treatment is superior to surgical treatment in terms of functional improvement if initial muscle strength is low[11].
Despite these observations, surgical treatment for intracerebral hematomas remain controversial, due to the findings from the STICH and STICH II trials [7,12], which suggest that when compared with conservative treatment, surgical treatment cannot improve the prognosis of patients with neurological dysfunction.
However, the results of STICH trials may not be generalizable, because of the high rates of patients’ crossover from medical management to the surgical group. Without these high crossover percentages, the rates of unfavorable outcome and death with conservative management would have been higher.
It should also not be forgotten that in the centers where these trials were conducted, minimally invasive surgical techniques were not used, and additionally, comatose patients and patients at risk of cerebral herniation were not included. In such cases, surgery may be lifesaving, which prevented those patients of being enrolled in such trials [13].
Regarding the surgical technique to be used, a conventional craniotomy can help remove the hematoma. However, the brain may be more exposed to damaged. Hence, the benefits obtained with it use, must outweigh the risks of the employment this technique [14,15].
On the other side, it is not recommended to undertake conventional craniotomy for patients with a minor hematoma (25-40 ml) in the basal ganglia. A recent study showed that an open craniotomy in such cases, might induce worse long-term functional outcomes than the conservative treatment [16].
Today, only minimally invasive techniques (MIT) are recommended for the evacuation of DHI.
These techniques such as microscopy, stereotactic lysis drainage, endoscopy, and navigation facilitate and improve neurosurgical outcomes by reducing surgical trauma. Such advantages have already been confirmed by several studies [17- 22].
In the following pages, an individual and up-to-date analysis of the use of each of them will help us to determine their possible advantages and disadvantages. For example, two studies conducted in recent years [23,24], highlight the advantages of using MIT compared to conventional craniotomies.
2019, Shi et al examined the efficacy of this treatment using a stereotactic implanted catheter drainage (SCD) in patients with severe intracerebral hemorrhage (Glasgow Coma Scale, GCS) score ≤ 8 and with a hematoma volume ≥ 30 ml. These authors compared his results with that of a group of patients with similar characteristics, who were treated with conventional craniotomy; and found that the SCD surgery group had fewer complications and better clinical outcomes, than the conventional craniotomy group [23].
The other study [24], reviewed the results obtained in five hundred and sixteen patients, who received stereotactic aspiration, endoscopic aspiration, or craniotomy. For the entire cohort, the 6-month mortality in the endoscopic aspiration group was lower than that in the stereotactic aspiration and that in the craniotomy group. A further subgroup analysis was stratified by hematoma volume. In this, the mortality in the endoscopic aspiration group was significantly lower than in the stereotactic aspiration, which should rethink the need for rapid evacuation of hematomas with high volume.
Perhaps for the same reason, The MISTIE (“Safety and efficacy of minimally invasive surgery plus alteplase in intracerebral hemorrhage evacuation”), an open-label trial in phase 3 study, showed no benefit in the primary efficacy endpoint [25].
Already in 2004 [18], we had reported the combined employment of MIT techniques and the results obtained during a period of 7 years in 95 consecutive patients with DIH. Thirty-six deteriorating patients with DIH under 30 ml volume associated to intraventricular bleeding, were treated early (first 24 hours after bleeding) with navigation-guided-stereotactic lysis, using multiplanar targets (1 to 3). Microsurgical clot aspiration through an enlarged burr-hole was frequently combined with endoscope- or navigation-assisted evacuation within the first 6 hours after bleeding for the rest of the deteriorating patients with a clot volume larger than 30 ml. A 1.2 cm narrow surgical corridor assured the least injury to vital cortical areas, tracts, and blood vessels. In 86 cases the clots were adequately removed (non-measurable rest) with a reduced percentual morbid mortality (13.8 and 8.6 as well as 23.3 and 16.9 for stereotactic and microscopic MIT, respectively). In our experience, the use of combined MIT adapted to the surgical urgency of the individual patient, reduced the operative trauma, and improved the accuracy for the access to the clot, allowing an adequate hematoma evacuation and a satisfactory outcome in most of the cases.
When the use of these techniques is also added a preselection of cases (89), the assessment from residual volume of postoperative clots, and the clinical outcome of these patients compared with that of previously operated unselected cases (138) in which other procedures were used; statistical differences can then be demonstrated, which end up favoring the use of such techniques [19].
Despite in this study we also included lobar hematomas compressing eloquent areas; we found a significant difference p ‹ .001 in the volume of residual clots, as well as p ‹ .01 in the clinical outcome at the first evaluation three months after the patients’ discharge. For this reason, it can be stated that adapting minimally invasive techniques to case selection, improves the effectiveness of clot removal and the outcome of the patients with an intracerebral hemorrhage.
It is important to note that, when I referred to adapting the technique to the patient and the type of hematoma to be treated, I try to make it clear that not all hematomas can be adequately extracted using the minimally invasive techniques already described.If this point is not made clear, mistakes may even be made in assessing the individual effectiveness of each of them.
Regarding these observations, a recent study comparing the clinical efficacy of endoscopic neurosurgery with that of microsurgery with small window craniotomy, erroneously favors the widespread use of endoscopy, for the removal of hematomas from the basal ganglia [20].
The microsurgical approach used in the study of these authors, is not the one I would recommend for the vast majority of DIH. This is described as performed through a temporal bone window of approximately 4 × 5 cm, exposing the insula, and evacuating the hematoma through the latter.If we consider that the larger DIH, mostly present an ellipsoidal shape with a marked anteroposterior orientation, it is easy to predict the difficult microscopic exposure caused by the approach used by these authors.
For this reason, we use to perform a frontal approach in such cases and restrict the endoscopy procedures to those hematomas located laterally (Putamen and External Capsule), which are usually of a more spherical shape, and therefore better evacuated through a temporal approach (Figure 1)
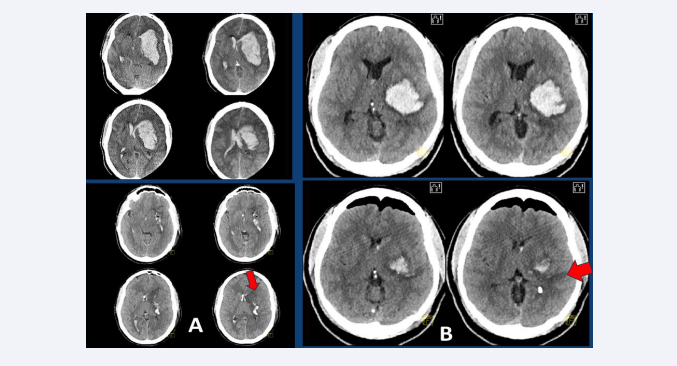
Figure 1: (A) Upper left: CT-scan of a 42-year-old patient with an ellipsoidal left DIH of 74 ml volume and bilateral ventricular filling, who is admitted to hospital in a coma and with a dilated left pupil, after suffering a generalized seizure. Bottom left: Postoperative CT scan showing almost complete evacuation of the clot using a microscopic MIT frontal approach (red arrow), with additional placement of two ventricular drains. (B) Upper right: CT-scan of a 51-year-old patient with a left spherical DIH of 56 ml volume, who was admitted to hospital with right hemiparesis and aphasia. Bottom right: Postoperative CT scan showing almost complete evacuation of the clot performed through an endoscopic temporal approach (red arrow).
Regarding the approach to be used in deep ellipsoidal giant hematomas of anteroposterior longitudinal axis, a recent study highlights the usefulness, efficacy, and safety achieved with the use of a modified transfrontal puncture drainage [26].
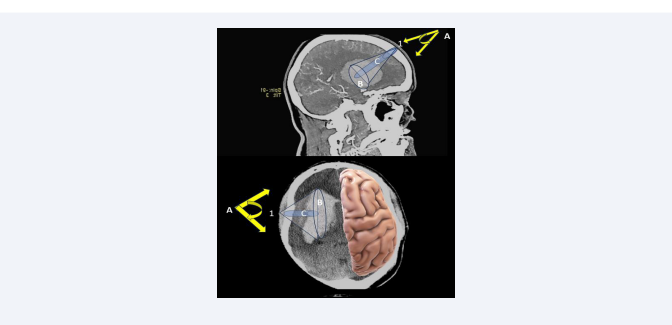
This study just confirms our analysis, on microsurgical approaches performed in patients treated with large basal ganglia hematomas carried out 2005 [27]. In that study, 15 cases were approached along the major axis of the hematoma (frontal approach), and 15 cases laterally (temporal approach), in which the point of the hematoma closest to the cerebral cortex was chosen, as the external point of the evacuation axis. Both approaches were performed through a slightly enlarged drill hole, and therefore can be considered as true keyhole surgeries and not as microsurgical performed small window craniotomy. To establish the differences between invasiveness and efficacy of both approaches, brain retraction angles and the volume of the brain exposed to surgery were calculated using a navigation device. A decrease in the invasiveness of the approach was associated with a decrease in the measured values. Efficacy was assessed by comparing the clot volume and the largest bleeding diameter exposed with each of these approaches. An increase in efficacy was associated with an increase in these parameters. All these estimates were finally compared with the residual clot volume detected on postoperative computed tomography. The comparison between the two approaches revealed a significant reduction in invasiveness (reduced brain retraction angles [P ‹ .001], reduced brain exposure [P ‹ .001]); as well as an increased efficacy in the removal of the clot (increase in visualized clot volume [P‹ .001] and greater bleeding diameter exposed to surgery [P‹ .001], for hematomas addressed along its major axis and through the frontal pole (Figure 2).
Figure 2: Schematic drawing demonstrating the differences between frontal (upper) and temporal (lower) keyhole approaches. 1: entry point; A: angle of brain retraction; B: (grey cone) brain exposed area; C: (blue cylinder) volume of microscopically visualized hematoma without additional brain retraction. Using a temporal keyhole approach, the volume of hematoma microscopically visualized without excessive brain retraction is rather reduced.
These patients also showed fewer postoperative residual clots (P ‹ .05).
Residual clots were correlated with greater need for retraction (P ‹ .001) and greater volume of exposed brain (P ‹ .001).
This analysis clearly demonstrated that the frontal approach to deep hematomas with an anteroposterior expansion axis has the advantage of less invasiveness and greater efficacy compared to those performed laterally, and at the same time leave fewer residual postoperative clots.
Another factor to consider about what should be managed concurrently in patients with deep intracerebral hematoma is the presence of accompanying intraventricular hemorrhage (IVH).
Previous studies [28,29], showed that DIH-patients with IVH may have worse outcomes due to the following mechanisms: acute obstructive hydrocephalus, delayed chronic hydrocephalus, and toxicity of the blood-breaking product. Furthermore, several studies found that DIH patients with IVH who received external ventricular drainage (EVD) presented better outcomes [30-33].
Two other studies also demonstrated that EVD placement in conjunction with thrombolytic drugs are associated with reduced mortality and better outcomes [34,35].
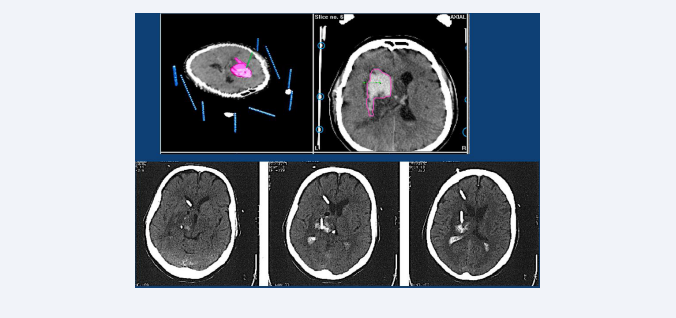
As described in some of our previous studies [18,19], we have often employed this last option in patients with DIH around 30 ml volume associated to intraventricular bleeding. Such cases were always treated early (first 24 hours after bleeding) with a ventricle drainage and in cases with a ventricular tamponade (cast ventricle), with a navigation-guided-stereotactic lysis, using multiplanar targets (Figure 3).
Figure 3: Top row: CT-scan with stereotactic frame of a 55-year-old patient with a right located DIH of 35 ml volume and bilateral partial ventricular filling, who was admitted to hospital with left hemiparesis and drowsiness. Lower row: Postoperative CT-scan, 24 hours after the first lysis (Actilyse) was performed through the stereotactic implantation of two catheters. One in the intracerebral clot, and the other in the right ventricle.
We found that the risk for intracranial infection and the rate of rebleeding did not significantly increase by employing both options.
Despite our positive experience with the use of ventricular drains, the CLEAR III trial (“Clot Lysis: Evaluating Accelerated Resolution of Intraventricular Hemorrhage”), which was a randomized controlled trial that assessed whether pharmacological disruption of IVH via intraventricular alteplase improves outcomes [36], found no improvement in 90-day functional outcomes in the patients who received intraventricular alteplase. However, there was a statistically significant reduction in shunt dependency if the treatment was supplemented using a lumbar drainage [37].
Other points that must be carefully analyzed before performing a surgical indication or an interventional procedure are the radiological characteristics of the hematoma. These include the volume, location, shape, and homogeneity of the hematoma, as well as the reaction produced in the surrounding brain.
These will be of great value to predict or rule out further expansion of the hematoma, as well as to calculate the effectiveness of the possible surgical treatment to be employed.
Li et al. described a CT finding called the “blend sign” to predict hematoma expansion [38]. The “blend sign” was defined as blending of a hypo-attenuating area within the hyperattenuated ICH with a well-defined margin. The “blend sign” also has comparative positive predictive value to the spot sign for predicting neurologic deterioration, making it a useful CT marker when CTA is not available [39]. Analogous to the “blend sign”, the “black hole sign” is another CT sign to predict hematoma expansion. The “black hole sign” is defined as a hypo-attenuating area encapsulated within the hyperattenuating ICH with a clearly defined border [40]. Notably, both signs are reflections of heterogeneity within the ICH bed.
More recently, Morotti et al. [41], have shown that using a combination of the CTA spot sign and identification of any hypodensity within the hematoma on CT is superior to predicting hematoma expansion than either sign alone.
Added to these data, there is also a nine-point prediction score for hematoma expansion created by Brouwers et al. [42], which also considers the use of Warfarin, the time to initial CT, and the volume of ICH.
Finally, and although some authors also recommend the use of decompressive craniotomy accompanied or not by evacuation of the clot [43-45], this measure should not be considered a first option in deeply localized hematomas.
Almost while the studies on the use of decompressive craniotomies were conducted, other authors compared the possible additional advantages of using minimally invasive techniques over conventional surgery and decompressive craniotomy. They found that the latter provide faster access to the hematoma and reduce the duration of surgery and anesthesia, which is extremely important in patients with clinical deterioration and elevated intracranial pressure ICP [46-48].
RECOMMENDATIONS, PERSPECTIVES, AND COMMENTARIES
In patients with rapidly deteriorating clinical condition, it is advisable to evacuate the clot by microscopic or endoscopic techniques. Regarding frontal approaches for microscopic MIT techniques, although many neurosurgeons are concerned about the depth of such approaches, and the possible difficulties in hemostasis under microscopic; in our patients such disadvantages were never an impediment to proper clot removal, nor were they associated with residual hematomas or new postoperative bleeding.
These disadvantages are offset, when the clot is aspirated early and before it acquires a firmer consistency. The consistency of the clots operated in the first six hours of formation through a canal that does not exceed 15 mm in diameter, allows the pressure of these clots to be released along its longitudinal axis, forcing the hematoma to move towards the surgical suction device.
With the necessary patience and care, only the hematoma will be sucked out, thus avoiding secondary lesions to the cerebral parenchyma.
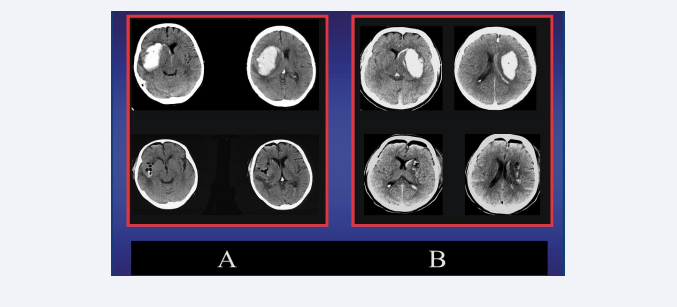
Once most of the clot has been evacuated, a small spatula can be inserted into this suction channel to help with careful irrigation, to detach the deeper residues of the hematoma. The mini tunnel thus created is maintained without collapsing by a very fine cerebral retractor, which allows a viewing angle of up to 15 degrees, when another micro instrument is additionally employed to perform the final hemostasis. The immediate pre and post-surgical results are shown in the figure 4.
Figure 4: A Left (upper): preoperative CT-scan of a patient with 64 ml DIH on the right side and small intraventricular hemorrhage. Left (lower): Postoperative CT scan following a right microscopic MIT-frontal approach revealing no residual hematoma. B Right (upper): preoperative CT-scan of a patient with 59 ml DIH on the left side and small intraventricular hemorrhage. Right (lower): Postoperative CT scan following a left microscopic MIT-frontal approach revealing no residual hematoma.
Usually, the small lenticulostriate artery responsible for bleeding has stopped bleeding. In the rare cases where the removal of the clot causes a new bleed, it can be perfectly visualized with the microscope, at the bottom of the tunnel.
The stereotactic introduction of a catheter along the axis of the hematoma before performing a small frontal opening, can also help the surgeon not yet familiar with this type of technique, to suck the clot from its most medial part.
If you want to further simplify the exact location of the suction channel, you can also use a navigator; virtually extending the tip of the pointer by 10 centimeters or more and inserting the catheter closely parallel to the direction and angle established by this instrument.
Regarding the volume of the hematomas to be treated surgically, of course, most of the deep spontaneous intracerebral hematomas of low volume, not only do they not require, but also do not show a clear benefit to be evacuated.
For this reason, a patient who will admitted awake, with a more lateralized hematoma limited to a part of the basal ganglia, will usually only need general and circulatory care, aimed at preventing new bleeding, as well as to avoid respiratory, thrombotic, and infectious complications of all kinds. In the algorithm (Table 1),
Table 1: Algorithm of treatment in deep intracerebral hematomas.
Legends: IC Vol: intracerebral clot volume; IVB: intraventricular bleeding; RPOP: radiologic predictor of poor outcome; ARPOP: Absence of radiologic predictor of poor outcome; H: hydrocephaly; CV: cast ventricles; SCC: stable clinical condition; RDCC: rapid deteriorating clinical condition; MEAPA: medial ellipsoidal anteroposterior clot axis; LSA: lateral spheric clot axis. CT: conservative treatment; CLD: clot lysis drainage; VD: ventricle drainage; VLD: ventricle lysis drainage; MFR: microscopic MIT-Frontal removal; ETR: Endoscopic temporal removal.
they remain in the group to be treated conservatively.
The algorithm presented in this manuscript does not mention an age limit for the use of certain surgical treatments. However, it is obvious that if the treating physician considers the survival of the patient as his primary goal of treatment, he will often obtain more satisfactory results treating younger patients with a lower degree of brain atrophy and accompanying diseases, than older patients with poorer health conditions.
Finally, surgical treatment should be avoided if it is assumed that functional improvement will not be achieved with clot evacuation, and if the hematoma presents radiological features that are predictive of a poor outcome (e.g., homogeneity, location, clot fragmentation, presence of other lesions presuming a different bleeding etiology or associated cerebrovascular disease).
The latter does not exclude the need for surgical treatment of secondary complications such as intraventricular bleeding (Figure 5).
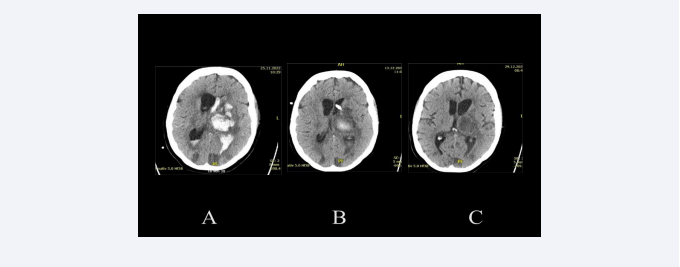
Figure 5: Overweight 50-year-old patient with hypertonic crisis, who as a car driver, had slowly driven into a ditch. She had previously noticed numbness in her right fingers, then in her right arm and right leg. At the admission to the hospital, she presented a hemiparesis and aphasia. The CT scan upon admission to the hospital (A) shows a thalamic bleeding of about 30 ml, with clot fragmentation and significant left intraventricular bleeding. In this CT-scan can also be observed various hypodensities, which confirm a more diffuse cerebrovascular disease. The CT-scan two weeks later (B) shows a reduction in intraventricular bleeding, which was treated only with a drainage. After more than two weeks and having also been treated with a lumbar drainage, the control CT-scan (C) shows the absence of hydrocephalus and the residual clot in the process of resorption. The patient was shortly after referred to a rehabilitation clinic.
REFERENCES
1. Carvi y Nievas MN, Pöllath A, Hoellerhage H. Influence of preoperative clinical condition and surgical technique on the postoperative results of intracerebral haemorrhage patients. In: Böker D-K, Deinsberger W, Winking M (eds). Therapeutic concepts in spontaneous intracerebral hemorrhage. Bierman Verlag GmbH, D-53909 Zülpich. 1997: 122- 126.