Acrania-Exencephaly Associated with Amniotic Band Syndrome in Newborn of a Post-Bariatric Pregnancy : Maternal Disabsorption?
- 1. Departament of Pediatrics, University Hospital Gaffrée e Guinle, Brazil
- 2. Department of Neonatology, University Hospital Gaffrée e Guinle, Brazil
- 3. Department of Obstetrics, University Hospital Gaffrée e Guinle, Brazil
Abstract
We described a cranial vault defect associated with amniotic band syndrome of a post-bariatric pregnancy. Obstetric ultrasonography with gestational age of 14 weeks and six days showed fetal acrania and at 21 weeks, revealed also a tortuosity of right radius and ulna. Case report: newborn, vaginal delivery, preterm (35 weeks and three days of gestation), Apgar 6/6, presented at birth a diagnosis of acrania and amniotic constriction band in the left upper limb without radial pulse in this limb. Transferred after birth to a Neonatal ICU, she died after 9 days. This rare lethal anomaly has important implications for obstetric management and counseling. Although there is no consensus in the literature regarding the risks of gestations after malabsorption procedures, gestations after bariatric surgery should be accompanied with extreme care aiming at an adequate nutritional intervention at risk of affecting the intrauterine environment and the developing fetus.
Keywords
• Acrania
• Amniotic band syndrome
• Congenital malformations
• Bariatric surgery
Citation
Perlroth NH, de Almeida Di Maio Ferreira FCP, da Silva ASV, de Oliveira VA, do Nascimento YS, et al. (2017) Acrania-Exencephaly Associated with Amniotic Band Syndrome in Newborn of a Post-Bariatric Pregnancy: Maternal Disabsorption? Arch Paediatr Dev Pathol 1(1): 1005.
ABBREVIATIONS
MRI: Magnetic Nuclear Resonance Imaging; ABS: Amniotic Band Syndrome; RYGB: Roux-En-Y Gastric Bypass; BMI: Body Mass Index; USG: Ultrasound; HIV: Antibody to Human Immunodeficiency Virus; VDRL: Venereal Disease Research Laboratory; NB: Newborn; NICU: Neonatal Intensive Care Unit; RT-PCR -Reverse Transcription - Polymerase Chain Reaction
INTRODUCTION
Acrania is a rare congenital anomaly with a reserved prognosis and still unclear etiology [1-6]. According to researchers, it is also known as exencephaly [7-9]. It is a cranial vault defect characterized by the partial or total absence of the cranial bones and the covering skin, with complete but abnormal development of the chondrocranium and the presence of brain tissue that is exposed [1 2,3,10].
In healthy fetuses during the embryological development, after the closure of the anterior neuropore around the fourth week, occurs the migration of the mesenchymal tissue (membranous neurocranium) under the ectoderm, underlying the future cerebral hemispheres [3]. The neurochranium is composed of the chondrocranium (cartilaginous base of the developing skull) that forms the base bones and the membranous plane bones that surround the brain [11], while the ectoderm forms the skin and scalp [3].
In the acrania, the normal migration of mesenchymal tissue (cells that develop the bone and cartilage of the skull) under the calvarial ectoderm (which in turn remains membranous instead of forming the epidermis) does not occur [9]. Because of this, the dura mater, skull, and all involved muscles (known as the neurocranium) are absent and brain tissue, even is present and disorganized. Since that the neurocranium is not present to mold the hemispheres, they will not differentiate and will morph into a single mass that will not be able to sustain life [3,4,11]. The bones of the base and face are present and normal [12].The cerebellum, brain stem and cranial nerves develop normally, but the diencephalon and outer globe are abnormally small [11,12].
Maranha’s research group refer that there is histological evidence that the onset of mineralization of flat bones of the skull occurs at the tenth gestational week and, therefore, in this period ultrasonography shows the hyperechogenic skull in relation to the underlying tissues [4]. In this sense, the diagnosis of acrania can be made in the second trimester of gestation, when bone mineralization is complete. Ultrasonographic findings are: development of brain tissue with no evidence of calvaria (in partial acrania, parts of the skeleton can be observed); brain tissue may appear with irregularly diffuse echogenicity; presence of prominent brain sulci [13].
The acrania appears to result from a multifactorial process involving developmental genes and environmental factors [5]. It is believed that the defect may be related to specific genes involved in folate metabolism [14,15]. Thus, folic acid supplementation may be a possible protective factor to the acrania, and its effect only occurs if the supplementation is initiated before gestation. The environmental factors involved in the pathology include hyperthermia, uncontrolled pre-gestational diabetes, antiepileptic drugs, lead exposure, maternal obesity and folate deficiency [5].Studies have shown that prenatal sonographic diagnosis of acrania is established by the following criteria: the fetus should have a normal facial bone and a normal cervical column but without fetal skull and a volume of brain tissue equivalent to a third of the expected size of the brain [7,16].
According to literature, acrania differential diagnosis should be made with acalvaria [3,17-19], anencephaly [17,20- 23], encephalocele [4,24-26], osteogenesis imperfecta [27-29], congenital hypophosphatasia [4,29], congenital cutis aplasia [12,30-32] and amniotic band syndrome [2,4,17,33-37]. Although the amniotic band syndrome is classified as a differential diagnosis of acrania, studies reveal its association with both acrania [6,13] and with acalvaria [17].
During the prenatal period, maternal serum alpha protein level may be a predictor of neural tube malformations, as well as biochemical and cytological examination of amniotic fluid, but such tests are non-specific [38], being the gold standard diagnostic confirmation for congenital malformations the obstetric ultrasonography, especially in the second trimester, when the mineralization of the bones of the skull is already complete [2,6,39,40]. Magnetic nuclear resonance imaging (MRI) may be indicated in the suspicion of other associated malformations [41], and is as a complementary modality to 2D/3D Ultrasound in diagnosis of fetal central nervous system anomalies [42].
Even though there are no reports of cases of acrania associated with maternal malabsorption or after bariatric surgery, some studies have reported the occurrence of acrania with fetal genes, such as Cart 1 which is suppressed by folate (vitamin B9), which is why supplementation of folic acid may interfere in the number of occurrences of this pathology and its deficiency increases the number of cases [14]. Women who have undergone procedures that impair nutrient absorption may possibly present deficiencies of iron, calcium, thiamine and malabsorption of fats, fat-soluble vitamins and vitamin B12 [41].
Due to these deficiencies, both the pregnant woman and the concept may be subject to serious health implications such as preterm birth, low birth weight, maternal osteomalacia, mental retardation and neural tube defects [43,44]. Recent studies suggest a possible relationship between maternal malabsorption problems after bariatric surgeries and fetal malformations [45,46].
CASE PRESENTATION
Maternal history
Patient reports having undergone bariatric surgery eight years ago using the Fobi-Capella technique or Roux-en-Y gastric bypass (RYGB) when she presented approximately 120 kg and 1.65 cm in height with Body Mass Index (BMI) = 44.0 kg / m2 , and abdominoplasty 7 years ago. Before conception weighed 75 kg (BMI = 27.5 kg / m2 ), she had depressed mood, followed by a psychiatrist, having made use of clonazepam and amitriptyline, both suspended on her own six months before gestation. She used oral hormonal contraceptives. No follow-up by gynecologist or nutritionist. In October of 2015, even during oral contraceptive use, the gestation was evidenced.
Gestational history
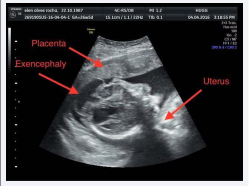
Patient, 28 years of age, high school degree, first pregnancy, white, born in Rio de Janeiro, with no comorbidities. Date of last period of menstruation on 09/30/2015. The first obstetrical ultrasound (USG) was performed on 11/09/2015 and dated a gestational age of six/seven weeks. She started prenatal care at a family clinic nearby her residence, where they prescribed oral folic acid and ferrous sulphate, according to doses recommended by the Ministry of Health, with 0.4 mg of folic acid and 150 mg of ferrous sulfate. Maternal tests were negative for hepatitis B, toxoplasmosis, cytomegalovirus, antibody to human immunodeficiency virus (HIV) and Venereal Disease Research Laboratory (VDRL) and were immune to Rubella. The obstetric ultrasonography of 01/07/2016 with gestational age of approximately 14 weeks and six days, evidenced the absence of a skull cap with cerebral exposure. On 02/18/16, with approximately 21 weeks’ gestation, it was evidenced by the USG a fetus with acrania and left radius and ulna tortuosity.
On 04/11/2016 the pregnant woman with 28 weeks of gestation (by the USG), single, live, female fetus, began to be followed up at the obstetrics service of a university hospital in Rio de Janeiro. The ultrasound 2D report showed: cephalic polo: partial acrania with hypoplasia of frontal, temporal and occipital bones and absence of parietal bones (Figure 1),
Figure 1 USG in 2D: partial acrania with hypoplasia of frontal, temporal and occipital bones and amorphous and disorganized encephalic mass
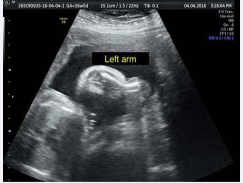
malformed left forearm with growth axis deviation in the distal third at an angle of approximately 45 degrees, and bone slope point visualized strangling soft tissue - suggestive of amniotic constriction band (Figure 2).
Figure 2 USG in 2D: bone slope point visualized strangling soft tissue - suggestive of amniotic constriction band.
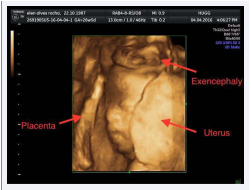
Ultrasound in 3D showed: an amorphous, disorganized and enlarged encephalic mass, externalizing through bone failure, without definition of the cerebral hemispheres, with enlargement of the ventricles (Figure 3).
Figure 3 USG in 3D: encephalic mass, externalizing through bone failure
After the completion of laboratory tests, it was decided to maintain the folic acid dosage and to replace iron intravenously, due to normocytic/normochromic anemia. She was referred to a nutritionist for protein supplementation, but was unable to consult. After counseling, the patient decided to carry the gestation to term.
Day 02/06/2016 - Newborn (NB), vaginal delivery, preterm (35 weeks and 3 days by USG), female, Apgar bulletin 6/6, birth weight 2.510g, adequate for gestational age, meconium amniotic fluid. The baby had no short umbilical cord.
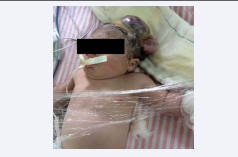
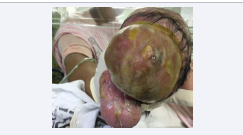
Examination of the NB: preterm NB, weak crying, whining, irregular breathing and hypotonia. Presence of complex malformation in the fronto-parieto-occipital region with acrania (Figure 4),
Figure 4 NB in dorsal decubitus with exposure of brain tissue
abnormal and disorganized structure and vascularization of the discovered brain (Figure 5)
Figure 5 NB with exposure of brain tissue with loss of its normal anatomy beyond the cranial limits.
associated with the amniotic band in the left upper limb, with absence of radial pulse (Figure 6).
Figure 6 Lateralized NB showing externalized encephalic mass with loss of its normal anatomy and highlighted fibrotic compressive cord in upper left limb.
She was referred to the Neonatal Intensive Care Unit (NICU), maintained in an incubator, monitored, in Oxy Hood, fed by oro-enteral catheter and with peripheral venous access in the upper right limb.
Echocardiography revealed patent foramen ovale, persistent moderate ductus arteriosus, and small-moderate apical ventricular septal defect. The funduscopic examination of the baby eye showed normal formation of the vascular arches, but peridiscal atrophy with optical deviation elongated vertically and pale, in both eyes and macules with no apparent impairment. Due to alterations in the optic nerve, it was suggested by the ophthalmology group, subsequent follow-up of the small patient to investigate glaucoma. Due to the epidemic of arbovirus in the city of Rio de Janeiro, it was performed research for zika virus with direct tests of Polymerase Chain Reverse Transcriptase (RTPCR) in serum samples was negative. The condition of the patient was informed to the mother who was already aware of the clinical condition of her daughter. She died 9 days after birth. The mother did not allow microscopic study of lesions and abnormalities found in the newborn.
DISCUSSION
The terminology used in the scientific literature to describe the defects of the cranial vault, among them the acrania, still has little precision [19]. According to the literature, acrania is a developmental anomaly characterized by a partial or complete absence of calvarium and presence of the exposed cerebral hemispheres with loss of its normal anatomy. [1,2,3,10]. With a reserved prognosis, its etiology has not yet been clarified. Multiple associated anomalies were observed in an inconsistent way, such as cardiac anomalies, club foot, cleft palate, tethered cord, omphalocele and Cantrell pentalogy [11].
In medical literature, acrania and acalvaria often have the same definition, although reports indicate them as differential diagnoses [3,11,18,19]. These two abnormalities of the skull differ in the sense that in the acalvaria (also an uncommon congenital anomaly) there is partial or total absence of calvary bones [12], with preservation of condocranium and a thin layer of epidermis that covers cerebral hemispheres [12,19], whereas the cerebral surface of the acrania is exposed [11,17]. In addition, acalvaria can be compatible with life while acrania is essentially lethal [11]. The acrania is then the diagnosis compatible with our study report, because the baby presented absence of the skull cap, and macroscopically the brain presented disorganization mainly of the left hemisphere. And as described in the literature, the NB presented in addition to the acrania other problems such as congenital heart disease and peridiscal optical atrophy.
Although the association between acrania and anencephaly is a recognized entity [40], the acrania-exencephaly (with partial preservation of the cerebral hemispheres) is a rare anomaly with only a few reports in the world literature, making it impossible to evaluate its incidence [1,11,16,17].
Anencephaly is a severe anomaly resulting from failure of neural tube closure during the first month of embryogenesis. Due to the absence of the skull cap, there is progressive destruction of the encephalic mass, due to exposure to mechanical and chemical aggressions of the amniotic fluid, leading to its degeneration [17,21-23]. Interactions between genetic (higher incidence in twins and familial aggregates) and environmental factors, like drugs use (valpropoic acid interferes with folic acid metabolism), socioeconomic status, infections and diet (deficiency of folic acid during gestation [47], especially before neural tube closure) are described [48]. Clinical and epidemiological researches, in some studies, suggest that teratogenic agents (radiation), maternal factors (age, parity and ethnic origin), chromosomal abnormalities and gene mutations are causes of this malformation [23,48] but not in others, perhaps because of the small numbers involved in each study. In anencephaly the brain tissue around the orbits is absent, giving the fetus a frog-like appearance with protruding eyes. Although a third of the fetuses are born alive, they are not viable, nor conscious, and can quickly die [15]. CDC estimates that each year, about 3 pregnancies in every 10,000 in the United States will have anencephaly [49]. In our case report the concept had normal face, so we discard this anomaly as a diagnosis.
Exencephaly can be defined as a large amount of disorganized brain tissue with loss of its normal anatomy [10], arising from the base of the skull where most of the embryo’s brain is exposed or extruded through the skull [20]. Because of the abnormal structure and vascularization of the discovered brain, the nervous tissue undergoes degeneration. It is considered that exencephaly is situated in the spectrum between anencephaly and encephalocele and is considered an unusual and lethal anomaly [20]. In the case reported, the NB had exposed brain tissue, with loss of its normal anatomy compatible with the diagnosis of acrania-exencephaly, as described in the research groups of Casellas [10] and Renuka [20].
The encephalocele, as well as the anencephaly and the exencephaly, is associated to acrania even in partial acrania. The encephalocele is a congenital anomaly due to neural tube defect [4,24]. It is categorized according to its content, exit site through the skull/bones of the face, and the pathway through bone failure. It is classified as occipital, parietal and sincipital, the latter of which is subclassified as frontoethmoidal, interfrontal, and those associated with craniofacial clefts [24]. In 75% of the cases, the lesion is occipital, but it can be located in the frontoethmoidal or parietal region [4,25].
Occipital encephaloceles are the most frequent type in North America and Western Europe [50]. In Southeast Asia [51], parts of Russia, and Central Africa, frontal encephaloceles are more common than occipital type [50]. According to studies approximately 1 in 12,200 babies born in the United States each year will have encephalocele [52], of women non-hispanic black [53]. Forty percent of the encephaloceles cases have other chromosomal anomalies, in addition to the association with microcephaly, hydrocephalus, spina bifida and Meckel-Gruber syndrome [4,25,26]. As the skull-forming cells fail to migrate, the brain and the meninges herniate through a hole in the skull cap that is usually covered with skin [4,25,54]. In our patient, there was absence of the skull cap and presence of exposed brain tissue, without meninges herniation which distinguished it from the diagnosis of encephalocele.
Distinction must also be made from conditions characterized by lack of skull mineralization such as congenital hypophosphatasia or osteogenesis imperfecta [11,55,56]. Hypophosphatasia is a hereditary metabolic disorder that results in the loss of function of the gene coding for tissuenonspecific alkaline phosphatase (TNSALP) and is characterized by clinical and radiological features similar to rickets [29,57]. In congenital hypophosphatasia, ultrasonography demonstrates poorly mineralized bones with multiple fractures. Important ultrasonographic areas to be researched are the bones of the hand, which are translucent in the patient with hypophosphatasia, but echogenic in the patient with osteogenesis imperfecta [4].
Osteogenesis imperfecta is a group of genetic disorder of the connective tissue characterized by the presence of poorly mineralized skull bones and curved and short long bones, which may show signs of fracture. Most of the cases are due to a mutation in the gene that encodes amino acid chains (type I collagen), which may reduce the synthesis and secretion of this collagen synthesized by osteoblasts [27]. As collagen is an important structural component of bones, without this protein the bones become abnormally fragile and brittle. At ultrasound, the limbs are hypoechogenic, with posterior acoustic shadow. It is a rare clinical disease occurring at a rate between 1/10,000 and 1/25,000 worldwide [28]. At birth, severe and lethal forms should be differentiated from other types of short-limbed dwarfism, and particularly from congenital hypophosphatasia and campomelic dwarfism [29].
As suggested Vergani’s group research [58], in these dysplasias of the skeleton, the brain is normal and surrounded by a thin ridge of soft tissue representing non-ossification of the skull and scalp. Curvature, fractures, and shortening of the long bones are generally present. Both congenital hypophosphatasia and osteogenesis imperfecta were discarded as a diagnosis due to the brain tissue exposure of the concept and no bone mineralization failure.
Another diagnosis discarded from our clinical case was aplasia cutis congenita, a rare condition in which the skin is absent mainly in the cranial region. This anomaly differs from our case report because the cerebral hemispheres are completely formed and normal [4,32]. The most affected site is the skull, and rarely occurs in the upper body and limbs, occurring alone or as part of a heterogeneous group of syndromes (Adams-Oliver syndrome, Bart’s syndrome, and Johanson-Bilzzard) [31,59]. Scalp lesions may be associated with complications such as infection, hemorrhage, thrombosis and seizures [31]. According to Çaksen & Kurto?lu [59], this disease may be isolated or associated with anomalies of the skin, eyes, ear, nose, neck, limbs and developmental defects in different systems (cardiovascular, gastrointestinal, genitourinary and central nervous). Its etiology is probably related to genetic factors, teratogenic factors, infections and vascular accidents [12,32].
Despite the fact that the amniotic band syndrome (ABS) is classified as a differential diagnosis of acrania, especially when the constriction band is located in the skull area [17], there are studies in the literature with the association of both [1,6,17,60], as in our case report. This anomaly also known as congenital constriction band presents as an uncommon pathology, with occasional occurrence in nature and without genetic predisposition [25]. Its etiology is uncertain, but the most accepted is that there is an early amniotic rupture causing the amniotic sac to separate from the chorion (membrane involving not only the embryo but all other embryonic attachments such as amnion, yolk sac and alantoide), allowing the embryo or fetus to enter the chorionic cavity. From the chorionic surface of the amnion emanate fibrous bands, which capture the fetal body components [35,36]. In this phase, transient oligodramnia occurs [17]. The morphology of the various clinical manifestations of this syndrome further supports the concept of local compression etiology [37]. Asymmetrical limb involvement is a rule [34].
The ABS has several types of clinical presentation, as ring of constriction in the fingers and toes, syndactyly, acrosydactyly, congenital club foot and intrauterine amputation [37,54]; associated facial, head and upper body formation such as cleft lip, anencephaly, encephalocele, microphthalmia, thoracochisis, extrathoracic heart and gastroeschisis [34]. Constriction in the fetal head can cause failure of the membrane migration of the neurocranium leading to acalvaria [17].
The prevalence of ABS varies from 1:1200 to 1:15000 live births, being slightly more common in female newborns and in afro-caribbean population [10]. Neonatal diagnosis is accurately recognized in only 29% to 50% of cases and is considered an etiological factor in acalvaria [17]. Although reports in medical literature indicate that the incidence is equal in both sexes [55], and there is no preference for ethnic groups [56], Pardini Jr. et al. [34], found a higher occurrence of the syndrome in male and white children.
Cincore et al. [6], described a case of association of acrania and ABS made in prenatal diagnosis by means of transvaginal ultrasound examination in the third trimester. Still according to these authors, the literature reports an association of this syndrome and postnatal acrania, but not diagnosed during prenatal ultrasound. In our study, the diagnosis, also performed during the prenatal period, showed in the obstetric ultrasound with gestational age of 14 weeks and 6 days fetal acrania, and at 21 weeks, still in the second trimester of gestation, tortuosity of the right radium and ulna.
After being informed of her child’s diagnosis and being counseling, the patient decided to carry out full term pregnancy with a delivered baby weighing 2,510gr, after a spontaneous vaginal delivery. The NB presented at birth a weight compatible with gestational age, although in the literature a prevalence of low birth weight in children with congenital anomalies is described [38]. According to studies, this prevalence could be a result of the effect of the fetal growth abnormalities themselves [61] or the increased risk of malformations in the group of newborns of a post-bariatric pregnancy [45,46], even though the real risk is still uncertain and the results in the medical literature are still quite conflicting.
In recent years, with an exponential increase in the number of bariatric procedures, with approximately half of them performed in women of childbearing age, there has been a notable increase in the number of women who become pregnant after having undergone bariatric surgery [44,62]. These procedures involve the risk of nutritional deficiencies, and nutrition is a crucial aspect during pregnancy. Therefore, knowledge and awareness of the consequences of this surgery on maternal and fetal outcomes is essential.
According to Gonzáles research group [62], RYGB is considered the gold standard as a procedure in bariatric surgery since it combines restrictive and malabsorption effects, allowing an optimal efficacy of combined weight loss without serious nutritional and vitamin deficiencies, which can develop after pure malabsorption techniques. There is no consensus in the literature regarding the risks of gestations after malabsorption procedures [63].
The mother’s baby after bariatric surgery made use of oral contraceptive which did not prevent her from getting pregnant. Kominiarek [64], research reports that studies suggest that oral contraception may not be as effective after bariatric surgery as a result of decreased absorption. Johansson et al. [65], in a prospective study, stated that despite the known adverse effects of gastric bypass surgery on iron, vitamin B12 and folate metabolism, they did not find in their research a significant effect of bariatric surgery on the general risk of congenital malformations, when compared to the control group of pregnant women. Unfortunately, we did not find research data on this topic in Brazil.
In our uncommon case report, all the macroscopic findings were as described in scientific literature, confirming the diagnosis of acrania-exencephaly associated with amniotic band syndrome, congenital heart disease and peridiscal atrophy optical. However, we could not explain how the baby survived for 9 days.
It is not possible to state, in this case, that the fetal malformation is directly related to maternal nutritional deficiency due to the malabsorption caused by bariatric surgery. However, literature states that it is essential that patients undergoing this procedure have a careful multiprofessional follow-up [44], since nutritional deficiencies can as a consequence affect the intrauterine environment and the developing fetus. In this sense, the concept may present serious short- and long-term complications such as neural tube abnormalities and growth restriction [63]. Pregnancy after bariatric surgery, with careful medical and nutritional monitoring, appears to be safe. Currently, with the increase in the number of cases of women of childbearing age who undergo this surgery, more research on this topic becomes relevant.
ACKNOWLEDGEMENTS
This manuscript is an original work. The authors have read and approved the paper and accept responsibility for its contents. This manuscript has not been previously published whole or in part, and is not under consideration for publication by any other peer-reviewed journal. All authors have contributed to this manuscript, reviewed and approved the current form of the manuscript to be submitted.
REFERENCES
16. Kwon TH, King J, Jeanty P. Acrania: review of 13 cases. 1991-01-01-12 The Fetus.net.
22. Ribeiro EM. Anencephaly: updated, guidelines and ethical features. Femina. 2004; 32: 447-454.
23.Oliveira ALB. Epidemiology of neural tube defects at the hospital de clinicas de Porto Alegre [dissertation]. Postgraduate Program in Medicine: Medical Sciences. Federal University of Rio Grande do Sul, 2008. 24.Singh AK, Upadhyaya DN. Sincipital encephaloceles. J Craniofac Surg. 2009; 2: 1851-1855. 25.Beltrán RP, Montaño JN, Muñoz FR, Landa FM., Díaz NR. Occipital encefalocele. Rev la Soc Boliv Pediatría. 2002; 41: 71-73. 26.Alam A, Adhi M, Bano R, Zubair A, Mushtaq A. Meckel Gruber Syndrome: Second trimester diagnosis of a case in a non-consanguineous marriage. Pak J Med Sci. 2013; 29: 234-236. 27.Rauch F, Glorieux FH. Osteogenesis imperfecta. Lancet. 2004; 363: 1377-1385. 28.Alharbi SA. A Systematic Overview of Osteogenesis Imperfecta. Mol Biol. 2016; 5:150. 29.Kim CA. Osteogenese imperfecta – Review. Pediatria (São Paulo).1993; 15: 8-21. 30.Henriques JG, Pianetti Filho G, Giannetti AV, Henriques KS. [Large scalp and skull defect in patient with aplasia cutis congenita]. Arq Neuropsiquiatr. 2004; 62: 1108-1111. 31.Brzezinski P, Pinteala T, Chiriac AE, Foia L, Chiriac A. Aplasia cutis congenita of the scalp--what are the steps to be followed? Case report and review of the literature. An Bras Dermatol. 2015; 90: 100-103. 32.Dutra LB, Pereira MD, Kreniski TM, Zanon N, Cavalheiro S, Ferreira LM. Aplasia cutis congenita: management of a large skull defect with acrania. J Craniofac Surg. 2009; 20: 1288-1292. 33.Mézel A, Manouvrier S. Síndrome de bridas amnióticas. EMC - Aparato Locomotor. 2011; 44: 1-10. 34.Pardini Jr AG, Santos MA, Freitas AD. Congenital constriction bands. Acta ortop bras. 2001; 9: 5-12. 35.Kino Y. Clinical and experimental studies of the congenital constriction band syndrome, with an emphasis on its etiology. J Bone Joint Surg Am. 1975; 57: 636-643. 36.Nogueira FCS, Cruz RB, Machado LP, Ramos BLF, Madureira Jr JL, Pinto RZA. Amniotic band syndrome: case report. Rev bras ortop. 2011; 46: 56-62. 37.Light TR, Ogden JA. Congenital constriction band syndrome. Pathophysiology and treatment. Yale J Biol Med. 1993; 66: 143-155. 38.Aguiar MJ, Campos AS, Aguiar RA, Lana AM, Magalhães RL, Babeto LT. [Neural tube defects and associated factors in liveborn and stillborn infants]. J Pediatr (Rio J). 2003; 129-134. 39.Peralta CF, Barini R. [Obstetric ultrasound between the 11th and 14th weeks: beyond the screening for chromosomal abnormalities]. Rev Bras Ginecol Obstet. 2011; 33: 49-57. 40.Tonni G, Centini G, Bonasoni MP, Ventura A, Pattacini P, Cavalli P. Acrania-anencephaly associated with hypospadias. Prenatal ultrasound and MRI diagnosis and molecular folate metabolism pathway analysis. Fetal Pediatr Pathol. 2012; 31: 379-387. 41.Nogueira FCS, Da Cruz RB, Machado LP, Ramos BLF, Junior JLM, De Almeida Pinto RZ. Amniotic band syndrome: case report. Rev Bras Ortop. 2011; 46: 56-62. 42.Hamisa M, Dabeesa N, Ataallab WM, Ziada DH. Magnetic resonance imaging versus Ultrasound examination in detection of prenatal fetal brain anomalies. Egypt J Radiol Nuclear Medi. 2013; 44: 665-667. 43.Ilias EJ. Considerações sobre gravidez após cirurgia bariátrica: evidências atuais e recomendações [in Portuguese]. Rev Assoc Med Bras. 2008; 54: 475. 44.Andreassen MS, Ferraz LF, Jesus SNR, Piano A, Azevedo CH, Perez AIC. Evaluation of the fetal maternal binomial after bariatric surgery. BEPA, Bol. epidemiol. paul. 2012; 9: 21-29. 45. Cools M, Duval ELIM, Jespers A. Adverse neonatal outcome after maternal biliopancreatic diversion operation: report of nine cases. Eur J Pediatr. 2006; 165: 199-202. 46. Salinas PH, Naranjo DB, Rojas CJ, Retamales MB, Vera NF, Sobrón BM. Cirurgia Bariátrica y Embarazo. Rev Chil Obstet Ginecol. 2006; 71: 357-363. 47. Fonseca EB, Sá RAM, Francisco RPV, Raskin S, Cabral ACV, Zugaib M. Recomendação sobre Suplementação Periconcepcional de Ácido Fólico na Prevenção de Defeitos de Fechamento do Tubo Neural (Anencefalia e outros defeitos abertos do tubo neural). Guia Prático de Condutas [in Portuguese]. FEBRASCO. Federação Brasileira dasssociações de Ginecologia Obstetrícia. 2012. 48.Medical Task Force on Anencephaly. The infant with anencephaly. N Engl J Med. 1990; 322: 669-674. 49.CDC. Birth Defects. Facts about Anencephaly. Division of Birth Defects and Developmental Disabilities, NCBDDD, Centers for Disease Control and Prevention. 2015. 50.Liao SL, Tsai PY, Cheng YC, Chang CH, Ko HC, Chang FM. Prenatal Diagnosis of Fetal Encephalocele Using Three-dimensional Ultrasound. 2012; 20: 150-154. 51.Dhirawani RB, Gupta R, Pathak S, Lalwani G. Frontoethmoidal encephalocele: Case report and review on management. Ann Maxillofac Surg. 2014; 4: 195-197. 52.CDC. Birth Defects. Facts about Encephalocele. Division of Birth Defects and Developmental Disabilities, NCBDDD, Centers for Disease Control and Prevention. 2016. 53.Wang Y, Liu G, Canfield MA, Mai CT, Gilboa SM, Meyer RE, et al. Racial/ ethnic differences in survival of United States children with birth defects: a population-based study. J Pediatr. 2015; 166: 819-826. 54.Askins G, Ger E. Congenital constriction band syndrome. J Pediatr Orthop. 1988; 8: 461-466. 55.Wiedrich TA. Congenital constriction band syndrome. Hand Clin. 1998; 14: 29-38. 56.Shetty P, Menezes LT, Tauro LF, Diddigi KA. Amniotic band syndrome. Indian J Surg. 2013; 75: 401-402. 57.Kosnik-Infinger L, Gendron C, Gordon CB, Pan BS, van Aalst JA, Vogel TW. Enzyme replacement therapy for congenital hypophosphatasia allows for surgical treatment of related complex craniosynostosis: a case series. Neurosurg Focus. 2015; 38: 10.
58. Vergani P, Ghidini A, Sirtori M, Roncaglia N. Antenatal diagnosis of fetal acrania. J Ultrasound Med. 1987; 6: 715-717.
59. Caksen H, Kurtoglu S. Our experience with aplasia cutis congenita. J Dermatol. 2002; 29: 376-379.
60. He P, Yang Y, Li DZ. Acrania associated with amniotic bands in a fetus. J Obstet Gynaecol. 2012; 32: 397-398.
61. Hunter GWA. Brain and Spinal Cord. In: Stevenson ER, Hall JG, Goodman RM, editors. Human malformations and related anomalies. Vol.1. Oxford: Oxford University Press; 1993; 109-131.
62. González I, Lecube A, Rubio MÁ, García-Luna PP. Pregnancy after bariatric surgery: improving outcomes for mother and child. Int J Womens Health. 2016; 8: 721-729.