Alcohol Mediated Liquefaction of Lignocellulosic Materials: A Mini Review
- 1. Department of Chemical and Biomolecular Engineering, National University of Singapore, Singapore
ABSTRACT
Acid catalysed liquefaction of lignocellulosic materials in the presence of various alcohols is an efficient way to prepare alkoxy-glycosides and levulinic esters. In this mini review, the mechanism of the liquefaction process and the applications of liquefied products, such as upgrading into polyurethane and phenolic resol resin, are highlighted.
CITATION
Pierson Y, Bobbink F, Yan N (2013) Alcohol Mediated Liquefaction of Lignocellulosic Materials: A Mini Review. Chem Eng Process Tech 1(2): 1014.
INTRODUCTION
The coming decades will be marked with the gradual depletion of fossil fuel resources [1], necessitating sustainable technologies to emerge and to replace those in current use. Lignocellulosic biomass, the most accessible and abundant biomass, receives a remarkably increased interest from the scientific community [2,3]. Nevertheless, significant fundamental research are still required [4] to enable the manufacturing of second generation biofuels and biomaterials from woody biomass industrially viable.
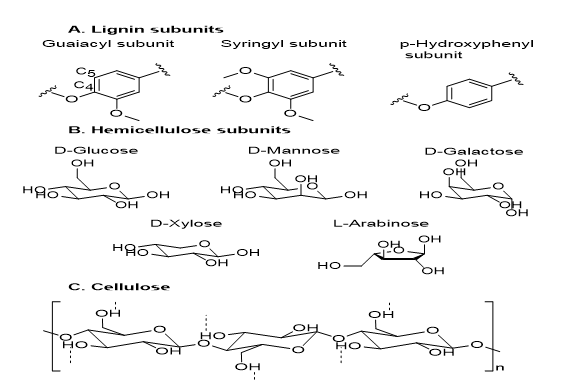
Woody biomass has three main components, namely lignin, hemicellulose and cellulose [5]. Lignin, a branched polymer composed mainly of phenyl derived alcohols, confers to cell walls structural reinforcement and water proofing. Three main subunits, including guaiacyl, syringyl and p-hydroxyphenyl are found in lignin. These moieties are further crosslinked either by C-O or C-C bonds. Hemicellulose, a carbohydrate polymer mainly composed of hexose and pentose sugars, is composed of five subunits, including D-glucose, D-Mannose, D-Galactose, D-Xylose and L-arabinose [6] (Figure 1).
Figure 1: Lignocellulosic components. Lignin (A) is a branched polymer, mainly composed of phenolic subunits. Hemicellulose (B) is formed by different hexoses and fructoses. Cellulose (C) is a linear polysaccharide formed by solely D-glucose units.
Different moieties such as methyl and acetyl groups can be incorporated on the sugar monomers [7]. The hemicellulose polymer chains can be branched and interestingly, can be crosslinked with lignin [8,9]. While being non-covalently bound to cellulose, this copolymeric structure creates an efficient sheath to protect the cellulose fibres and strengthens lignocellulosic cell walls. Finally, cellulose is a linear polysaccharide composed solely of D-glucose units (Figure 1, C) linked together by β-1-4 glycosidic bonds. It is the most abundant organic polymer on earth [10]. Proportion of cellulose in hardwoods usually ranges from 40 to 55 wt%. Due to its organized structure and the strong stabilizing effect of intra and intermolecular hydrogen bonding, [11,12] cellulose is relatively resistant to physical and chemical treatments. Chemical liquefaction of woody biomass has been studied for decades. By liquefaction process, the biomass components are depolymerized to liquid products, which are potential intermediates to produce various value-added polymers or chemicals. It is an economically feasible method for the transformation of biomass because it can convert biomass into materials that could be easily utilized or further upgraded under relatively low temperatures with a short reaction time. Among other methods, alcohol mediated liquefaction of lignocellulosic materials is particularly interesting and have been extensively carried out. A wide array of alcohols such as ethylene glycol (EG) [13,14], propylene glycol (PG) [15], polyethylene glycol (PEG), glycerol (Gly) [16] and phenol [17,18] were screened and they exhibited remarkable abilities to liquefy cellulose. In addition, two cyclic carbonates—ethyl carbonate (EC) and propyl carbonate (PC) [19] — appeared to be suitable candidates as liquefaction reagents. This mini review will highlight most representative liquefaction process in the presence of these reagents. The reaction mechanism, the product characteristics, the effect of different acids, and the influence of acid-alcohol ratios [20] on the liquefaction are discussed.
The purpose of using polyhydoxyl alcohols or phenols to liquefy woody substances is not for the production of biofuels as more efficient methods already exist. Polyhdroxyl compounds are often too expensive to produce cost efficient biofuels. Instead, glycosides and levulinic esters are obtained after woody biomass liquefaction in the presence of polyols and phenols. Alkyl glycosides can be used as detergent, surfactant, emulsifier as well as solubilizer, some widespread cosmetic chemicals. In addition, the diverse glycosides being produced through this method might be employed to a wide array of applications which are partially reviewed in the last section of the article. For example, glycosides can be further utilized to manufacture biodegradable polyurethane (PU) resins and phenolic resin foams [21].
Levulinic esters are important platform molecules and different derivatives can be used for multiple purposes. Methyl, ethyl and butyl levulinates are useful components in the fuel industry. They can be used as oxygenated fuel additive in regular diesel engines [22]. Ethyl levulinate is also used nowadays as a food additive.
The general liquefaction methodology is straightforward. The process is simply achieved by adding an excess of solvent with a catalytic amount of acid with dried cellulose. The solution is stirred at a set temperature for an appropriate time. Afterwards the reaction is generally quenched by adding either 1,4-dioxane or distilled water and neutralised with a base (KOH or NaOH are most common). The reaction parameters in various reports are compiled in (Table 1.)
Table 1: Summary of the solvents, lignocellulosic sources and the relative quantities (%w/w) of reactants with respect to the solvent mass. The time in the table is the duration required to achieve > 90% yield.
|
Cellulose source |
% Dry cellulose |
Acid |
% Acid |
Temperature [°C] |
Time [min] |
|
Whatman filter paper, cotton linters |
20 |
SA, PTSA |
3% |
150°C |
240 |
|
Saw dust |
17 |
SA |
0.24% |
158°C |
240 |
|
Milled wood, commercial cellulose |
20 |
SA |
3% |
140°C |
10-20 |
|
Milled wood, commercial cellulose |
20 |
SA |
3% |
150°C |
30-40 |
|
Milled wood, commercial cellulose |
20 |
SA |
3% |
150°C |
60 |
|
Milled wood, commercial cellulose |
20 |
SA |
3% |
150°C |
100 |
|
Milled wood, commercial cellulose |
20 |
SA |
3% |
150°C |
60 |
|
Milled wood, commercial cellulose |
20 |
SA |
3% |
140°C |
30 |
|
Whatman filter paper |
9 |
PTSA |
9.1% |
130-150 |
40-180 |
Note that this table does not intend to be exhaustive but to provide representative examples. Other experimental conditions might be found in the literature. The time in the table is the duration required to achieve > 90% yield, which ranges from 10 min to 4 hours. The main methods for product analysis are HPLC, 1 H-NMR, 13C-NMR, FT-IR, and size exclusion chromatography.
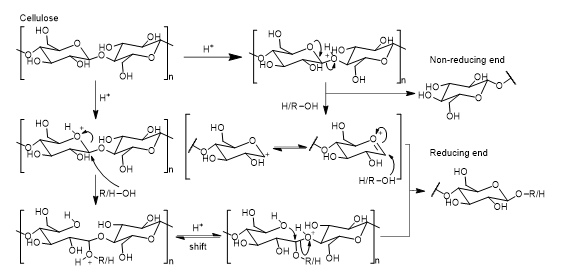
To thoroughly understand the general mechanism of cellulose depolymerisation, cellobiose, a D-glucose disaccharide, has been used as a model compound. The study of its glycosidic bond hydrolysis mechanism provides an insight on the cellulose hydrolysis mechanism. It was confirmed later that cellulose and cellobiose follow the same liquefaction pathway [23,24]. In addition, linear alcohols such as PG, EG and PEG do not affect the reaction mechanism. As such, a general mechanism for cellulose liquefaction can be proposed, as shown in (Figure 2.)
Figure 2: Two possible pathway for cellulose depolymerisations.
Cellulose hydrolysis follows two possible mechanisms. One is the closed chain path and other is the opened chain path. In the closed chain path the oxonium formed expresses a strong electrophilic character. It is believed that the alcohol hydroxy group acts as a nucleophile consequently leading to the formation of glycosides. In the opened chain path, the protonation of the ring oxygen leads to an increased electrophilicity of the C-1. This is consequently followed by a nucleophilic attack from the hydroxy group on C-1. These two mechanisms have been studied through the stability of methyl glycosides and evidence has pointed to the higher stability of the oxonium intermediate [25].
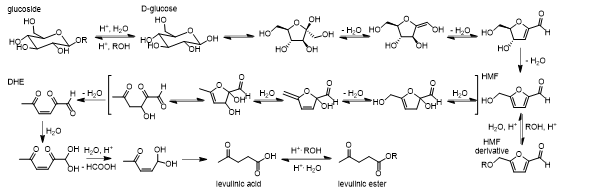
The presence of alkoxy glycosides in such conditions was reported [18,26] but as water is part of the reaction mixture it is impossible to exclude the possibility that cellulose hydrolysis is faster or that water acts as a co-catalyst. A reasonable mechanical pathway describing the transformation of glycoside to levulinate esters would logically ensue from the previous assumption. Indeed, transformation from a glycoside to a 5-(alkoxymethyl) furfural without hydrolysis seems unlikely. Mechanistically, in order to isomerise to fructose the opening of the D-glucose ring proceeds through the formation of an aldehyde. In the case of a glycoside this formation seems unlikely as the aldehyde formation would be hindered by the alkoxy group.
Glycoside hydrolysis to D-glucose was reported to be an equilibrium process through the formation of an oxonium [27], providing a strong argument that cellulose depolymerisation proceeds through the closed chain pathway. In the case of a solvolysis, the high alcohol concentration and acidic conditions would shift the equilibrium towards the glucose alkoxylation. In order to fully convert the glycosides, the alkoxy group needs to follow hydrolysis under acidic condition releasing a D-glucose unit. This unit can, in the presence of water and acid, isomerise to fructose, which would be easily converted to 5-(hydroxymethyl) furfural (HMF) by dehydration [28]. Two pathways are leading to the transformation of glucose to HMF: the first one keeps the fructofuranose ring intact while the second one proceeds through open intermediates. However, the closed chain pathway has been shown to be dominant under acidic conditions [28]. Transformation of HMF to its derivatives in the presence of acid and alcohol were reported [29]. The proposed mechanism for the transformation of HMF to 2-5-dioxohex-3-enal (DHE) is widely accepted although no intermediates were identified [30]. Through the formation of levulinic acid, formic acid is released. Another possible transformation of HMF (not shown in Figure 3)
Figure 3: Decomposition mechanism of glycoside to HMF, HMF derivative, levulinic acid and levulinic ester.
yields humic acids [30]. Humic substances are dark brown solid polymers insoluble in most solvents [31]. They are generally undesired products and therefore reaction conditions have to be optimized to totally liquefy lignocellulosic substances and block this reaction pathway [32]. Due to the relatively high concentration of alcohols, levulinic acid undergoes esterification to yield levulinates. The sulfuric acid catalysed reaction of levulinic acid towards butyl levulinate in aqueous solution has been proven to be thermodynamically favourable (Kcalc ≈ 893 at 150°C) [33]. This scenario is expected to be similar in the presence of different alcohols as long as the alcohols are in large excess.
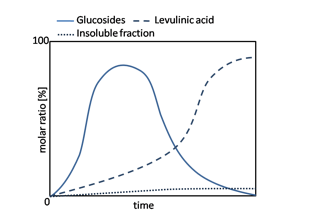
The reaction kinetics is similar for all the different solvolysis reagents and the molar ratio profile [16] is similar to the one shown on (Figure 4.)
Figure 4: Typical molar ratio profile of the cellulose solvolysis reaction.
The reaction sequence starts by the formation of glycosides, which are then steadily and completely transformed to levulinates. Humic acids are formed in a variable extend depending on the reaction conditions. This general representation is adapted from the different studies of cellulose solvolysis presented previously.
Cellulose crystallinity and polymerization degree (DP) play important roles in the solvolysis [34] reaction rate and yield. Under the same conditions, the depolymerisation yield fluctuates heavily with respect to the cellulose average molar mass. The acid strength also plays an important role in the liquefaction process. A study [13] shows that cellulose with an average molar mass of 76,000, 699,000 and 1,910,000 g/mol is converted in EG with SA up to 98.9, 95 and 85% for the three samples. However, by using PTSA the yield was decreased to 98.7, 85 and 72%, respectively.
Cellulose degradation was reported to follow two different modes, the ‘quantum mode’ [35] for microcrystalline cellulose or the random mode for low organization cellulose fibres. Both of them are following first-order kinetics. Different materials with DP ranging from 470 (microcrystalline cellulose) to 11790 (cotton linters) were submitted to solvolysis mediated by EG and catalysed by PTSA at 150°C. Residue sizes were analysed by X-ray diffraction and size-exclusion chromatography. The reactions were monitored by taking samples at several time intervals. Indeed, amorphous regions, due to their larger surface area, are more susceptible for liquefactions. Compared to crystalline cellulose, the reaction rate constants were 57 times faster for cotton linters (DP = 11790), 21 times faster for Whatman paper (DP = 4320) and 6 times for microcrystalline cellulose (DP = 470).
CARBONATES: A SPECIAL CASE
Interestingly, cyclic compounds such as EC and PC are more efficient and seem to solubilise cellulose six to eight times faster than EG. These compounds possess a higher permittivity value, promoting their relative acidity [36]. Moreover, under acidic conditions EC and PC are degraded to EG and PG, as evidenced by CO2 evolving from the system [37] and further confirmed by studies on the carbonates degradations [37]. Unfortunately, toxic volatile side products such as dioxolanes are formed during PC degradation [38] which makes this reagent unpractical for large scale biomass liquefaction. EC mediated degradation of softwoods shows remains of residues [19]. Those residues are often humic substances or un-derivatized cellulose. This problem is addressed by the employment of a mixture of EG/EC with a ratio of 1:4, enabling the quick and total liquefaction of the starting materials.
The use of glycosides for biomaterial and additives production
PU films were prepared from liquefied wood dust using the PEG400/Gly (9:1) in the presence of sulphuric acid [21]. The liquefied wood was copolymerized with polymeric methylene diphenylene diisocyanate. The film mechanical properties vary with respect to the isocyanate to hydroxy ratio. At higher liquefied wood ratio, the rigidity of the end product is enhanced.
PU foam production, using liquefied waste paper, has also been reported, [39] which show similar properties as liquefied wood foams. Interestingly, these materials exhibit excellent thermal stability, no potent carcinogenic or mutagenic character and are also biodegradable to some extent. The waste paper was liquefied using PEG400/Gly (4:1) and PU foam was prepared using diphenylmathane diisocyanate and silicone surfactants. The liquefaction of waste paper using PEG and glycerol provides the polyols for the PU production.
Products from wood solvolysis in the presence of phenol and sulphuric acid can be further converted to produce phenolic foams and resins. It has been reported that by reacting liquefied wood with formaldehyde under alkaline conditions, resol resin was prepared [40]. Interestingly, milder conditions (lower temperature and reaction time) are required to prepare lignocellulosics derived resins than conventional methods. The foam is prepared by reacting the liquefied wood with poly(ethylene ether) as surfactant, HCl and diisopropyl ether as blowing agent.
These efforts open opportunities to novel, ecologically friendly waste treatment to value added materials. However on large scale application the use of acids presents a major disadvantage. Indeed, the reactive batch has to be neutralized and corrosive resistant reactors have to be used [41]. Moreover, separation of the complex products might involve environmentally unfriendly solvent as well as energy intensive distillation operations.
CONCLUSION
In this article the general method to liquefy lignocellulosic material by acidic catalysis in the presence of alcohols is briefly reviewed. The mechanism and kinetic study of cellulose depolymerisation to levulinic acid shows a complex pathway evolving through multiple intermediates. The relevance between liquefaction yield and the type of alcohols, acids, reaction conditions are discussed. Various applications of alkoxyglycosides derivatives e.g. phenolic resol resins, surfactant, polyurethane foams and fibres provides a positive outlook of this strategy to convert bio-wastes into value added, environmentally friendly materials and chemicals.
REFERENCES
- Shafiee, S.; Topal, E. Energ Policy 2009, 37, 181.
- McKendry, P. Bioresource Technol 2002, 83, 55.
- Corma, A.; Iborra, S.; Velty, A. Chem Rev 2007, 107, 2411.
- Huber, G. W.; Iborra, S.; Corma, A. Chem Rev 2006, 106, 4044.
- Whetten, R.; Sederoff, R. Plant Cell 1995, 7, 1001.
- Timell, T. E. Wood Science and Technology 1967, 1, 26.
- Hansen, C. M.; Bjorkman, A. Holzforschung 1998, 52, 335.
- Myton, K. E.; Fry, S. C. Planta 1994, 193, 326.
- Ralph, J.; Grabber, J. H.; Hatfield, R. D. Carbohyd Res 1995, 275, 167.
- OSullivan, A. C. Cellulose 1997, 4, 173.
- Jorgensen, H.; Kristensen, J. B.; Felby, C. Biofuel Bioprod Bior 2007, 1, 119.
- Nishiyama, Y.; Sugiyama, J.; Chanzy, H.; Langan, P. J Am Chem Soc2003, 125, 14300.
- Jasiukaityte, E.; Kunaver, M.; Strlic, M. Cellulose 2009, 16, 393.
- Zhang, T.; Zhou, Y. J.; Liu, D. H.; Petrus, L. Bioresource Technol 2007,98, 1454.
- Uraki, Y.; Sano, Y. Holzforschung 1999, 53, 411.
- Kubo, S.; Yamada, T.; Hashida, K.; Ono, H. Chem Lett 2007, 36, 502.
- Mun, S. P.; Jang, J. P. J Ind Eng Chem 2009, 15, 743.
- Zhang, Y. C.; Ikeda, A.; Hori, N.; Takemura, A.; Ono, H.; Yamada, T.Bioresource Technol 2006, 97, 313.
- Yamada, T.; Ono, H. Bioresource Technol 1999, 70, 61.
- Kobayashi, M.; Asano, T.; Kajiyama, M.; Tomita, B. J Wood Sci 2004,50, 407.
- Kurimoto, Y.; Takeda, M.; Koizumi, A.; Yamauchi, S.; Doi, S.; Tamura, Y. Bioresource Technol 2000, 74, 151.
- Hayes, D. J. Catal Today 2009, 145, 138.
- Xiang, Q.; Lee, Y. Y.; Pettersson, P. O.; Torget, R. Appl Biochem Biotech2003, 105, 505.24.
- Hall, G. S. Nature 1984, 310, 521.
- Edward, J. T. Chem Ind-London 1955, 1102.
- Yamada, T.; Aratani, M.; Kubo, S.; Ono, H. J Wood Sci 2007, 53, 487.
- Timell, T. E. Can J Chem 1964, 42, 1456.
- Antal, M. J.; Mok, W. S. L.; Richards, G. N. Carbohyd Res 1990, 199, 91.29. R.J.H. Grisel, J. C. v. d. W., E. de Jong, W.J.J. Huijgen Catal Today 2013.
- Horvat, J.; Klaic, B.; Metelko, B.; Sunjic, V. Tetrahedron Lett 1985, 26, 2111.
- Ikan, R.; Ioselis, P.; Rubinsztain, Y.; Aizenshtat, Z.; Miloslavsky, I.; Yariv, S.; Pugmire, R.; Anderson, L. L.; Woolfenden, W. R.; Kaplan, I. R.; Dorsey, T.; Peters, K. E.; Boon, J. J.; Deleeuw, J. W.; Ishiwatari, R.; Morinaga, S.;
- Yamamoto, S.; Macihara, T.; Mullervonmoos, M.; Rub, A. Sci Total Environ 1992, 118, 1.
- Hu, X.; Li, C. Z. Green Chem 2011, 13, 1676.
- Bart, H. J.; Reidetschlager, J.; Schatka, K.; Lehmann, A. Ind Eng Chem Res 1994, 33, 21.
- Jasiukaityte-Grojzdek, E.; Kunaver, M.; Poljansek, I. Bioresources 2012,7, 3008.
- Calvini, P. Cellulose 2005, 12, 445.
- Riddick, J. A.; Bunger, W. B.; Sakano, T. K. 1986.
- Peppel, W. J. Ind Eng Chem 1958, 50, 767.
- Chernysh.Da; Polyansk.Ng Zh Org Khim+ 1971, 7, 212.
- Lee, S. H.; Teramoto, Y.; Shiraishi, N. J Appl Polym Sci 2002, 83, 1482.
- Lee, S. H.; Teramoto, Y.; Shiraishi, N. J Appl Polym Sci 2002, 84, 468.
- Rinaldi, R.; Schuth, F. Chemsuschem 2009, 2, 1096.