Chemical Durability and Structure Analysis of Simulated Radioactive Waste Glasses
- 1. Reactors Department, Nuclear Research Center, Atomic Energy Authority, Egypt
- 2. Radiation Chemistry Department, National Center for Radiation Research and Technology, Egypt
ABSTRACT
Borosilicate glasses are used to accommodate wastes arising from day to day operations and the decommissioning of nuclear installations. Investigating the immobilization systems, chemical durability and structure under normal conditions have gradually increased and over the next two decades will become the main focus of research. In this study several models include comprehensively the relevant aspects which are known to lead to glass formation were investigated. Chemical durability was evaluated for three borosilicate glasses by immersed in hydrogen peroxide. Physical properties such as Dietzel field strength are evaluated for three borosilicate glasses in the perspective of being capable to apply to the waste forms. The Dietzel field strength values span within approximately wide range of 0.2 to 1.6, which are signifying the extent of the electrostatic attraction force between oxygen and elements. The arrangement ordered of oxides is approximately convincing in the range of Dietzel field strength (A) 1. Results indicate that oxides construct the glass network have A 0.35, as these oxides, which are alkali oxide are known to reshape network. Intermediate oxides may show both behaviors in consistent with glass composition. Dietzel approach showed that both high charge and limited size of the cations favor the creation of glassy oxides. Chemical activities were calculated using overall dissolution rate, which indicated solid surface layer hydrolysis reaction.
KEYWORDS
• Radioactive waste
• Immobilization
• Borosilicate glass
• Leaching
• Dietzel field strength
CITATION
Farid O, Abdelaziz Y, Waly S (2022) Chemical Durability and Structure Analysis of Simulated Radioactive Waste Glasses. Chem Eng Process Tech 7(1): 1063.
INTRODUCTION
The main challenging task for nuclear industry is the safe management of nuclear waste, as approval of the public to build novel nuclear facilities by discovering a safe way to take care of these wastes. Solid, liquid or gaseous wastes originate from every stage of the nuclear fuel cycle and are treated to ensure they comply relatively with strict environmental and regulatory standards before final disposal. Immobilization of nuclear wastes is the first step towards the safe management of these wastes; the immobilization system should be durable [1]. Different immobilization matrices were proposed, these includes cement, polymer, glasses, ceramic, and Synroc. Vitrification is the transformation of oxides into a glass (a non-crystalline amorphous solid) by melting oxides until they turn into a liquid, then cooling the liquid quickly, as it passes through the glass transition phase to form a glassy solid. As this vitrification is peculiar to amorphous materials or turbulent systems and occurs when the bonding between elementary particles (atoms, molecules and formation masses) is higher than a certain threshold value [2]. This is explained as amorphous materials have a characteristic threshold temperature called the vitreous temperatures (Tg). These amorphous materials at a temperature below Tg are glassy, while at a temperature above Tg they are molten. Vitrification is one of the most proven processes to immobilize nuclear wastes, where the wastes are combined in glass or glass like material [3]. Due to their respectively low processing temperature, borosilicate and phosphate glasses are the most applied glass for vitrification. Some compounds in nuclear wastes have limited solubility in borosilicate glasses i.e. platinoids, refractory oxides and cations of high oxidation state [4].
Safety assessment studies for waste form matrices are focused on glass structure understanding. A glass structural model should detail structure-properties relationships, depict atomic arrangements at short and medium range, and have general idea applicable to a large number of systems [5]. Longterm assessment studies are confronted by the quantification of coordination number; bond type, bond strength, and their roles in strengthen long-term glass degradation. Dietzel [6] classified the oxides according to their ability to form or modify glass lattice in light of their positive cation field. The cation field intensity is defined as charge to distance square ratio according to Jantzen et al. [7]. Dietzal's field strength identifies the structure of glass formation and the mechanical properties changes. It evaluates the different in internal energy due to change in Dietzal's field strength, and identify the chemical affinity for the solid surface layer hydrolysis reaction. The main question when quantifying the rate of glass dissolution is that there are two different distinctive glass surface reactions that can guide dissolution rate deviation with solution composition:
(a) The reverse reaction, as at equilibrium ions are leaning to reprecipitate.
(b) The forming reaction, as the dissolution rate control or precursor of surface.
These effects are often determined in premise of the chemical affinity for the dissolving phase leading to the equivalence of the form. The chemical affinity of the dissolving phase is often quantified in terms of inverse reaction and forming reaction. The overall dissolution rate of metals or glass may be affected by several factors. Firstly, aqueous carry away of chemical species from its surface, secondly the effect of the reverse reaction in conditions close to equilibrium, and thirdly, the rate of equilibrium dissolution away from equilibrium [8].
The aim is to identify the containment performance and degradation resistance and structure-properties relationships that control leaching mechanism for each metal group. In this study, the short-term temporal evolution of waste glass matrices will be examined. Initial glass leaching properties of all matrix components in three borosilicate waste glasses will be evaluated. The performance of the immobilization process was assessed by using MCC-2 leaching test. This test is classified as a static standard that was performed at 90 °C. In this context, MCC-2 leaching test was performed and identified Dietzel field strength which explains structure-properties relationships.
EXPERIMENTAL
Material
Three borosilicate-based glasses are under investigation in this project. The nominal compositions of the glasses are shown in 1 which is based on the weight percentage. The compositions are batch compositions based on starting powders giving indication of glass composition at best [9] Table 1.
Table 1: The composition of the waste glasses (wt%).
|
Sample Compound |
MW Baseline |
CaZn + Magnox |
Blend |
|
SiO2 |
61.7 |
44.26 |
46.28 |
|
B2O3 |
21.9 |
17.95 |
16.43 |
|
Na2O |
11.1 |
9.01 |
8.33 |
|
Li2O |
5.3 |
2.11 |
3.98 |
|
CaO |
-- |
1.39 |
-- |
|
Al2O3 |
-- |
4.11 |
1.91 |
|
BaO |
-- |
0.40 |
0.47 |
|
CeO2 |
-- |
0.96 |
1.45 |
|
Cr2O3 |
-- |
0.63 |
0.51 |
|
Cs2O |
-- |
0.89 |
1.59 |
|
Fe2O3 |
-- |
2.79 |
2.06 |
|
Gd2O3 |
-- |
0.06 |
4.16 |
|
La2O3 |
-- |
0.52 |
0.73 |
|
MgO |
-- |
4.10 |
1.61 |
|
MoO3 |
-- |
1.32 |
2.49 |
|
Nd2O3 |
-- |
1.53 |
2.17 |
|
NiO |
-- |
0.39 |
0.34 |
|
Pr2O3 |
-- |
0.52 |
0.66 |
|
RuO2 |
-- |
0.52 |
0.55 |
|
Sm2O3 |
-- |
0.32 |
0.49 |
|
SrO |
-- |
0.24 |
0.41 |
|
TeO2 |
-- |
0.15 |
0.28 |
|
Y2O3 |
-- |
0.16 |
0.31 |
|
ZnO |
-- |
4.43 |
-- |
|
ZrO2 |
-- |
1.24 |
2.82 |
|
Total |
100 |
100 |
100 |
MW Baseline is a base glass composition that is used for mixing with the nuclear waste. The prepared glasses samples were processed using a melt quenching technique, where reagent grade powders were combined in appropriate ratio, and then milled to homogenize mixers. After that, the batches were melted in a platinum crucible at 13000 °C for 1 h then it stirred for 3 h, before casting into small blocks by a preheated stainless steel mould. The batches were cooled down before being disposed into an annealing furnace at 400°C for 1 h. These glass samples were allowed to cool to room temperature at a rate of 3°C/min. CaZn+Magnox represents calcined Magnox which has been mixed with CaO and ZnO2 as a base glass. Blend is another glass which represents waste. B2 O3 and SiO2 act as network formers. Ca, Al, Cr, Fe, Mg, Mo, Zn, Zr may act as modifiers or intermediates. Other elements are simulant fission products which resembles nuclear waste. These batches were chosen as likely durable compositions.
Leaching test
The performance of the immobilization process was examined by conducted MCC-2 (Material Characterization Center-2) method. This test is classified as a static standard that conducted at 90 °C using surface area to volume ratio (SA/V) of the samples must be approximately 10 m-1.The samples are sectioned to about 1x1x0.5 cm and polished before being immersed in Hydrogen peroxide. Vessels of Perfluoroalkoxy (PFA) are used for this test as there is no interaction with water and it can resist high temperatures up to 200 °C. To make sure that the samples are not contaminated with the environment, leach testing is performed in a vacuum oven and leached samples are freshly made for each characterization test.
Any noticeable changes in the pH of the leachant after leaching tests were detected for both studied samples. For reference sample, the pH changed from 6.4 to 9, where for modified glass, the pH changed from 6.4 to 8.5. The induced alkalinity in the leachant was observed in previous literatures and was attributed to alkali ions leaching [10].
For this project, a Perkin Elmer ICP-OES instrument was used to measure the concentration of leachates. 5ml leachates from each sample were taken for each measurement. Beforehand, standard solutions for the elements were prepared for required concentrations by dilution technique. In order to get a precise and an accurate result, measurements were repeated at least three times, calculating mean values and standard deviations.
RESULTS AND DISCUSSION
Surface investigation
Preliminary surface investigation was conducted by applying glass samples to SEM. Results are reported in Figure 1,
Figure 1: SEM of borosilicate glass where microcracking appears after leaching for 7 days at 90°C and SA/V~10 m-1.
which shows that three borosilicate-based glasses which are reference glass A, CaZn+Magnox B, and blend glass C.
The surface of the reference glass was observed and found to be homogeneous except for some fine oval inclusions, ie, region 1 and 2, with dimensions of 16_24 and 32_22 mm. CaZn+Magnox has been observed and the glass has been reported to contain foreign impurities or immiscible components. The blend glass was observed and found to contain impurities due to the presence of bubbles and/or foreign impurities and/or immiscible components. The interpretation of this is attributed to the assumption of phase separation and Greaves model of the modified continuous random network for borosilicate glass, where the random network is subjected to the separation of immiscible liquid phases and to the emergence of a water-soluble alkaline borate phase and a silica-rich phase.
Leaching analysis
The overall ion release rate from glass was evaluated using the MCC-2. This technique is widely used to estimate the rate of glass dissolution. Normal solubility rate is estimated by (DR) of the glass-forming elements during the hydrogen peroxide immersion. DR is evaluated by the equation (1).
(1)
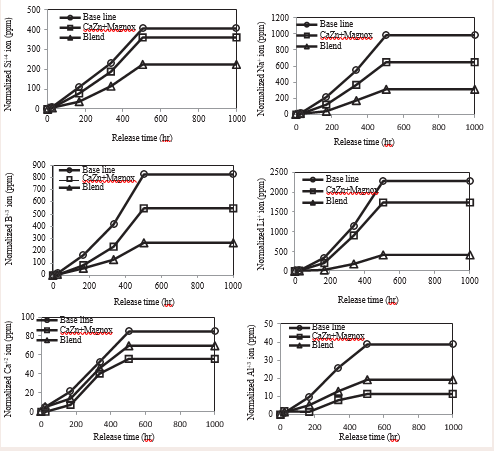
where W is the initial weight of the sample [g], S is surface area of the sample At is the leached mass of element from glass sample [g], A0 is the initial content of the element [g],and d is the immersion time. The remaining concentrations of the leached elements were analyzed with ICP-MS (Inductively coupled plasma mass spectrometry). The normalized dissolution rates of the glass constituent elements such as Silicon (Si+4), boron (B+3), sodium (Na+ ), lithium (Li+ ), calcium (Ca2+) and aluminum (Al3+) are depicted in Figure 2.
Figure 2: Normalized ion release for Silicon (Si+4), boron (B+3), sodium (Na+ ), lithium (Li+ ), calcium (Ca2+) and aluminum (Al3+) from base line, Ca+ZnMagnox, and blend glass.
Figure 2 displays ion release profiles for base line, CaZn+Magnox, and blend glass composites. The results show that normalized ion release of silicon (Si+4), boron (B+3), sodium (Na+ ), lithium (Li+ ), have the same pattern, however calcium (Ca2+) and aluminum (Al3+) have different behavior. Silicon (Si+4),boron (B+3), sodium (Na+ ),lithium (Li+ ), calcium (Ca2+) and aluminum (Al3+) reached38,84,115,985,826,405 ppm (respectively) for base line. Silicon (Si+4), boron (B+3), sodium (Na+ ), lithium (Li+ ), calcium (Ca2+) and aluminum (Al3+) reached 11,55,1740,650,548,406 ppm(respectively) for Ca+ Magnox. Silicon (Si+4), boron (B+3), sodium (Na+ ), lithium (Li+ ), calcium (Ca2+) and aluminum (Al3+) reached 19,69,410,314,266,224 ppm (respectively) for blend.
Ion releases for all elements in base line are higher than CaZ+ Magnox and blend glass. Ion release profiles could be divided into two stages; first 504 hr, release of all elements followed almost linear relationship with time, later it comes to stable point till 1000 hr. The ion release profiles for all glass investigated showed similar trends. The CaZn + Magnox and blended glasses showed lower ion release rates compared to base glass. This is explained by their high chemical toughness. Its durability is attributed to the presence of Si content and presence of Ca2 + in CaZn + Magnox in the tempered glass, which can help cross-linking the glass structures. Based on the addition of Ca2+ ions as in CaZn+Magnox glass, the oxides (particularly alkaline) play an important function in the chemical durability of the glass structure. This was noted by Farid et al. [11], who noted that the rate of determination of ion release and degradation of borosilicate glass decreased with increasing calcium intake. In addition, calcium ions can construct a chelating structure via ionic and interfering bonds. Kim et al. [12], found that the effect of adding metals (particularly alkaline) on the rate of glass degradation, follow the order Fe > Mg > Ca.
Glass structure
In order to understand new glass structures, the simplified metal oxides classifications from Dietzel based on field strength (A) has produced countless guidelines for glass scientists. Field strength is associated with ionicity of metal-oxide bonds, since bonds comprising components with high field strength possess large amounts of ionic character, and high field strength bonds have more valence or directionality for their bonding environment. The field strength (A) is defined by the ability of cations to enter the glass structure by their field strength.
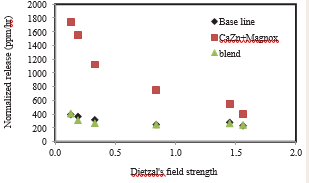
where Z is cation valence, rc and ro are cation and oxgen radii respectively. In our recent study of silicon (Si+4), boron (B+3), sodium (Na+ ), lithium (Li+ ), calcium (Ca2+) and aluminum (Al3+) component within three different glass composite, were evaluated as shown in Figure 3.
Figure 3: Normalized rate of ion released of silicon (Si+4), boron (B+3), sodium (Na+ ), lithium (Li+ ), calcium (Ca2+) and aluminum (Al3+) versus Dietzal's field strength.
Molar normalization was calculated by dividing released cations rate by the molar concentration of the cation within glass composition (0.16, 0.16 and 0.24 for Na, Ca and Mg respectively). The Dietzel field breadth spans within a relatively broad range of 0 to 2 and is indicative of the breadth of the Dietzel field to the size and strength of electrostatic attraction between the element and oxygen inside the glass lattice. It appears from the results that the ordered sequence of the oxides is relatively convincing, as the results indicate that the oxides form the vitreous network. For A ?0.35, oxides (particularly alkaline) as in CaZn+Magnox glass are known to modify the lattice. The modulators of this lattice or this polyhedral arrangement lead to a strong glass structure. These represented two extreme behaviors, intermediate oxides may exhibit both behaviors depending on the structure of the glass. From this, Dietzel's approach shows that both the small size of the cation and its high charge favor the formation of glass oxides with a strong glass structure. This study conclude alkaline silicates easily form glasses with a strong glass structure and cations such as Si that readily form with oxygen are called polyhedra and triangles, lattice-formers, and such coordination polyhedra leads to a glassy structure.
Chemical activity
The aim of this current study is to study the chemical affinity, which in turn determines the amount of glass dissolution and leads to the release of ions away from equilibrium, which shows a control of the surface reaction. The release of ions is often specified in terms of the chemical affinity of the melting phase leading to the equation 3.
(3)
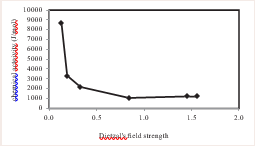
where r designate the overall and forward dissolution rate, respectively, AF represents the chemical affinity for the solid surface layer hydrolysis reaction, which may differ from that of the bulk solid. E refers to Temkin’s average stochiometric number, which equal to the ratio of the rate of destruction of the activated or precursor complex relative to the overall rate, R stands for the gas constant, and T represents absolute temperature in Kelvien. Chemical activity versus Dietzal's field strength is shown in Figure 4
Figure 4: Chemical activity versus Dietzal's field strength.
the dissolution of the glass close to the surface layer of the solid consists of a layer rich in amorphous silicon. They indicated that the chemical affinity of the released ions can be assumed to be equal to the chemical affinity of amorphous silica. In contrast, Rose and Mukunoki [5,13], suggested that the chemical affinity for amorphous silica can be assumed to be equal to the chemical affinity for amorphous gel containing SiO2-, Al(OH)3-, Fe(OH)3 , Ca(OH)2 and Mg(OH)2 . Each of these studies accurately described various glass dissolution data sets as a function of solution composition at near to equilibrium conditions. This study concluded that the release of ions can be predicted and controlled based on their composition. This study describes glass dissolution datasets and ion release accurately as a function of solution composition under conditions close to equilibrium.
CONCLUSIONS
The current study focuses on the dissolution rates of borosilicate glass in conditions far from equilibrium. The dissolution rates allow studying the chemical affinity of the melt phase and can determine the effect on the rates of the active formation reaction or precursors independently of those resulting from the reverse reaction. This study concluded that the quantity and type of ions releasing are case-sensitive to the glass composition as well as the chemical durability. Therefore, it could be concluded that ion release profiles could be predictable and controllable from waste glasses based on their composition. The ion release features are attributed to the Ditzel field strength. It also note that low trends of Na+ compared to Ca2+ in CaZn+Magnox and blend glasses. This leads to weak glassy structure and collapse of the longer glass chain, which is attributed to the lower Dietzal field strength of Na compared to Mg2+ and Ca2+ and the collapse of the glass chain. Thus, two values for the single bond strength were computed corresponding to Dietzal's field strength criterion. It is clear that Dietzel's approach confirms that both the small size of the cation and its high charge lead to the formation of strong-structured glass oxides. Some of these cations, such as Si, in the presence of oxygen, can easily form polyhedrons and triangles with oxygen. Also, forming this lattice or this polyhedral arrangement leads to a strong glass structure. The chemical affinity of the leading melt phase corresponds to controlled glass dissolution rates and it appears far from stable equilibrium at surface. These results confirm the ability of borosilicate glass to contain different waste in its cores in harsh conditions.
ACKNOWLEDGEMENT
The authors thankfully acknowledge Wesam Gomaa, Radiation Chemistry Department, National Center for Radiation Research and Technology, Atomic Energy Authority for providing SEM samples.
REFERENCES
- Abou hussein EM. Vitrified Municipal Waste for the Immobilization of Radioactive Waste: Preparation and Characterization of Borosilicate Glasses Modified with Metal Oxides. Silicon. 2019; 11: 2675-2688.
- Calahoo C, Wondraczek L. Ionic glasses: Structure, properties and classification. Journal of Non-Crystalline Solids. 2020; 8: 100054.
- Nathan J. Cassingham, Martin C. Stennett, Paul A. Bingham, Neil C.Hyatt, Giuliana Aquilanti. The Structural Role of Zn in Nuclear WasteGlasses. International Journal of Applied Glass Science. 2011; 2: 343-353.
- Farid OM, Abdel rahman RO. Preliminary assessment of modified borosilicate glasses for chromium and ruthenium immobilization. Materials Chemistry and Physics. 2017; 186: 462-469.
- Osama M Farid, Michael I Ojovan, A Massoud, RO Abdel Rahman. An Assessment of Initial Leaching Characteristics of Alkali-Borosilicate Glasses for Nuclear Waste Immobilization. Materials. 2019; 12: 1462.
- Osama M. Farid, MI Ojovan, RO Abdel Rahman. Evolution of cations speciation during the initial leaching stage of alkali-borosilicate- glasses. MRS Advances. 2020; 5: 185-193.
- IAEA Radioactive Waste Management Glossary. Vienna, International Atomic Energy Agency. 2003.
- 8.Jantzen CM, Plodinec MJ. Thermodynamic model of natural, medieval and nuclear waste glass durability. Journal of Non-Crystalline Solids. 1984; 67: 207-223
- Kim M, Corkhill CL, Hyatt NC, Heo J. Development, characterization and dissolution behavior of calcium-aluminoborate glass wasteforms to immobilize rare-earth oxides. Scientific Reports. 2018; 8: 5320.
- Lee WE, Ojovan MI, Stennett MC, Hyatt NC. Immobilisation of radioactive waste in glasses, glass composite materials and ceramics. Advances in Applied Ceramics. 2006; 105: 3-12.
- Mukunoki A, Kikuchi T, Chiba T, Sakuragi T, Kogure T. Sato T. Dissolution Behavior of Lead Borate Glass under Simulated Geological Disposal Conditions. MRS Advances. 2018; 3: 1139-1145.
- Rose PB, Woodward DI, Ojovan MI, Hyatt NC, Lee EW. Crystallisation of a simulated borosilicate high-level waste glass produced on a full- scale vitrification line. Journal of Non-Crystalline Solids. 2011; 357: 2989-3001.
- Trocellier P, Djanarthany S, Chêne J, Haddi A, Brass A, Poissonnet A, Farges F. Chemical durability of alkali-borosilicate glasses studied by analytical SEM, IBA, isotopic-tracing and SIMS. Nuclear Instruments & Methods in Physics Research Section B-beam Interactions With Materials and Atoms. 2005; 240: 337-344.