Heavy Metal Ions in Wastewater: A Review on Detection and Toxicity
- 1. Department of Chemical Engineering, Malaviya National Institute of Technology, India
Abstract
The existence of life on Earth is primarily dependent on water. Rapid industrialization, population increase, unplanned urbanization, poor use of natural water resources, and human activity have all negatively impacted water quality. Environmental pollution is becoming more problematic in human life and the environment. Water pollution is mainly increasing quickly as a result of industrial wastewater discharge. The release of numerous organic/inorganic pollutants has increased steadily along with the development of anthropogenic activity. Heavy metals have been identified as one of the priority groups among the several kinds of pollutants because of their detrimental effects on the environment and animals. It is a class of metals and metalloids with an atomic density of more than 4000 kg/m3. Because of their toxicity, heavy metals such as Cu, Pb, Ni, Hg, and many more have been associated with several environmental issues. Even at very low concentrations, the accumulation of ions from these hazardous metals in humans can lead to significant health problems for individuals and the environment. Since they can concentrate and accumulate in a variety of stable forms, heavy metals introduced into the environment are a major source of concern. Heavy metal contamination is one of the worst environmental issues facing the world today. Consequently, it is crucial to monitor and detect heavy metals in wastewater continuously. Various conventional techniques can be applied to measure trace elements in wastewater. This article details heavy metal ions, their impact on human health, the detection technologies, and their working principles.
Keywords
• Detection techniques
• Heavy metal
• Pollutants
• Toxicity
• Wastewater
CITATION
Rajoria S, Vashishtha M, Sangal VK (2023) Heavy Metal Ions in Wastewater: A Review on Detection and Toxicity. Chem Eng Process Tech 8(3): 1082.
INTRODUCTION
"Heavy metal ions" was the general term used to characterize the discharge of contaminants into soil, water, or air. Heavy metal wastewater is being released directly or indirectly into the environment, especially in emerging countries where sectors like metal plating, mining, fertilizers, leather factories, batteries, and pharmaceuticals are developing rapidly. Despite being used extensively for a decade, the term "heavy metal ions" still has inconsistent definitions in the literature. One definition is used to classify metals and metalloids with comparatively high densities (3–7 g/cm). Heavy metals are naturally occurring elements with atomic weights between 63.5 and 200.6 g/mol and densities or specific gravities of more than 5 g/cm [1,2]. This category includes a wide range of elements, but only those indicated in Table 1 are relevant in the environmental context. Industries that contain heavy metals, such as iron (Fe), lead (Pb), copper (Cu), arsenic (As), cadmium (Cd), chromium (Cr) [3], nickel (Ni), mercury (Hg), and Zinc (Zn), are the most hazardous of all chemical-intensive industries because they release a lot of wastewater contaminated with metals [4,5]. Some metals,such as manganese (Mn), magnesium (Mg), Cu, Cr, Fe, Ni, and Zn, are frequently referred to as trace elements since they are only micronutrients that are necessary for the physiological and metabolic processes of living things. Documentation from the Agency of Toxic Substances and Diseases Registry (ATSDR) indicates that four primary heavy metals are classified as extremely harmful to humans, animals, and plants [6]. These are Pb, Hg, Cd, and As. In contrast to organic pollutants, heavy metals are known to be poisonous or carcinogenic in large quantities, and they have a tendency to accumulate in living things. Due to their great solubility in aquatic environments, living things can absorb heavy metals. Heavy metal concentrations may accumulate in the human body once they reach the food chain. Long-term exposure to heavy metals can result in nephrotoxicity, encephalopathy, cancer, growth and developmental anomalies, chronic poisoning, and cardiovascular and neurological disorders [7]. Consequently, the World Health Organization (WHO) and the United States Environmental Protection Agency (USEPA) [8] have established maximum contamination limits standards (MCLS) regarding these hazardous heavy metals and the health effects they can have on people when those levels are exceeded in drinking water [9,10]. Some of these limits are summarized in Table 1.
Table 1: Sources of some heavy metals, their toxicity, and MCLS in drinking water according to the WHO and USEPA [8, 11, 12].
|
Heavy metals |
Main sources |
Toxicities |
Maximum concentration limits by WHO in drinking water (mg/L) |
Maximum concentration limits by USEPA in drinking water (mg/L) |
|
Arsenic (As) |
Burning of fossil fuel, mining, and pesticides |
Vascular disease, visceral cancers |
< 0.01 |
0.05 |
|
Cadmium (Cd) |
Mining, welding, refining pesticide, fertilizer, smelting, and plastic |
Kidney and lung damage, renal disorder |
0.005 |
0.005-0.01 |
|
Chromium (Cr) |
Textile industry, steel, electroplating, and dyes |
Diarrhea, nasal, and sinus cancer |
< 0.05 |
0.05-0.1 |
|
Nickel (Ni) |
Storage batteries, electroplating, porcelain enameling, and paint |
Dermatitis, nausea, chronic asthma, coughing, human carcinogen |
0.20 |
0.134-0.2 |
|
Copper (Cu) |
Electroplating, mining, pesticides, batteries, and copper cooking pots |
Liver damage, insomnia |
< 0.5 |
1.3 |
|
Lead (Pb) |
Batteries, mining, paint, pigments, explosives, and electroplating |
Kidney and lung diseases, damage the nervous system |
< 0.01 |
0.005-0.006 |
|
Mercury (Hg) |
Batteries, mining, paper and paint industries |
Rheumatoid arthritis, damages nervous system |
0.00003 |
0.0003 |
|
Zinc (Zn) |
Mining, refineries, brass manufacturing, and plumping |
Increased thirst, depression |
< 2 |
5 |
|
Manganese (Mn) |
* |
Neurological effects, psychological symptoms |
< 0.12 |
* |
WHO: World Health Organization; USEPA: United States Environmental Protection Agency; *: Data not available.
Abbreviations: ATSDR: Agency of Toxic Substances and Diseases Registry; WHO: World Health Organization; USEPA: United States Environmental Protection Agency; MCLS: Maximum contamination limits standards; AAS: Atomic Absorption Spectroscopy; ICP-MS: Inductively Coupled Plasma-Mass Spectrometry; HR-ICP-MS: High-resolution inductively coupled plasma mass spectrometry; MC-ICP-MS: Multi-collector inductively coupled; ICP-OES: Inductively Coupled Plasma-Optical Emission Spectrometry; RF: Radio frequency; PMT: Photomultiplier; CCD: Charge capacitive discharged; XRF: X-ray fluorescence spectrometer; AFM: Atomic Force Microscopy; SPM: Scanning probe microscopy (SPM).
We need a way to identify the presence of these harmful metal ions in water samples before we can remove them. This will enable us to quantify the pollution level quantitatively and select an appropriate removal strategy. For this reason, an economical, time-efficient, and ecologically friendly heavy metal detection technology must be created. Conventional techniques that have been used so far for the detection of heavy metals like atomic absorption spectroscopy (AAS), inductively coupled plasma- optical emission spectrometry (ICP-OES), ICP-MS spectrometry (ICP-MS), atomic force microscopy (AFM), and x-ray fluorescence spectrometer (XRF). A detection method must also have sufficient sensitivity to accurately identify tiny quantities of metal ions. Depending on the number of components to be analyzed, the anticipated range of analyte concentrations, and the volume of samples to be tested, the best technology can be selected to meet business needs. These methods are quite selective and sensitive, but there are still a few issues with detecting heavy metals. These include the complexity of the operating procedures and the challenge of detecting in real environments. Thus, focusing on heavy metal detection techniques, the article examines the broader area of heavy metal pollution in wastewater. This review also presents details on different types of heavy metals and their toxic effect on human health.
TYPES OF HEAVY METALS AND THEIR EFFECTS
Zinc (Zn): Zn is rarely found in nature; however, since it can be easily extracted from ores and is available in limited amounts, it has been used for a long time. Several minerals include zinc,such as ZnO, ZnS, ZnCO3, Zn2SiO4, etc. It is a trace element that is necessary for maintaining human health [2]. It controls various metabolic activities and is essential for the physiological functions of living tissue. On the other hand, an excess of zinc can lead to serious health issues like anemia, vomiting, nausea, skin irritations, and cramping in the stomach.
Cadmium (Cd): Cd appears as a natural deposit, which includes other elements. In industrial wastewater, it is also the heavy metal that is most harmful. It is vital for businesses such as alloys, phosphate fertilizers, stabilizers, cadmium-nickel batteries, and plating [11-13]. The Cd compounds are highly toxic and concentrated in the ecosystem, even in low quantities. They also reveal side effects such as lung cancer, liver toxicity, and other illnesses.
Lead (Pb): Pb is frequently found in inorganic compounds in the 2+ oxidation state. Tetraethyl and tetramethyl lead are the most commonly used fuel additives due to their general characteristic of low volatility in the gasoline evaporation process. Alkyl lead, which is added to motor gasoline, is the primary source of lead emissions, accounting for around 80% of all emissions released into the environment. The extraction and smelting of lead ores, as well as compound production and refinement, are other notable sources of Pb. It can harm the reproductive system, central nervous system, basic cellular processes, brain functioning, and kidney damage. The hazardous symptoms are headaches, sleeplessness, dizziness, irritability, hallucinations, muscular weakness, and kidney impairment [7].
Chromium (Cr): Cr is the most easily accessible seventh element on Earth. Ores containing crocoite (PbCrO4), ferric chromite (FeCr2O4), and chrome ocher (Cr2O3) are the forms in which Cr is found. The tanning, textile, leather, and electroplating industries are the main industrial sources of Cr. These sectors produce waste products containing trivalent and hexavalent forms of Cr (III) [14,15]. For plants, animals, and other living things, Cr (VI) is typically more poisonous than Cr (III). The industry that produces chromate salts is the main source of Cr (VI). Cr(III) is essential for the metabolism of fats and sugars. Cr(VI) can cause a range of major health problems, from minor skin irritation to lung cancer. It also affects human physiology and accumulates in the food chain [16].
Mercury (Hg): Hg is a neurotoxin that can potentially harm the central nervous system. High concentrations of Hg cause chest pain, dyspnoea, and kidney and lung function damage. Minamata Bay is a well-known instance of mercury poisoning [2].
DETECTION TECHNIQUES OF HEAVY METALS
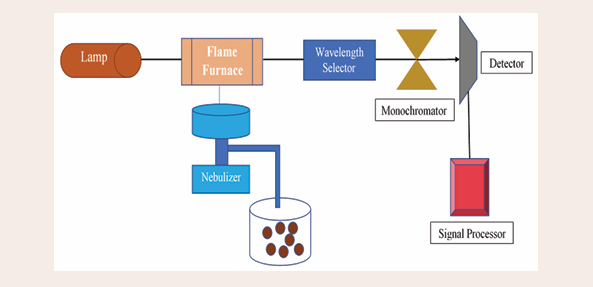
Atomic Absorption Spectrometry (AAS): The origins of AAS can be traced to the 1950s when Alan Walsh and his colleagues developed a method to detect specific metal concentrations in test samples. The analytical technique known as AAS is used to quantify more than 70 distinct elements either directly in solid samples or solutions. The basic idea of AAS is that light excitation causes electrons of atoms to transition to higher energy levels, and subsequent de-excitation causes the atoms to emit light at frequencies specific to that particular atom. Figure 1 shows
Figure 1: Block diagram of Single-beam AAS instrument.
a block diagram of a typical AAS. It consists of a main light source, a monochromator, a detector, an electronic "readout" device, and an atomizer to create gas-phase ions for detection. The technique is sensitive because the width of the transition line is extremely narrow; as a result, it can be considered an "elemental" sensitivity. It must be atomized before the sample can be examined at the atomic level. Although other atomization processes exist, flame and electro-thermal atomization are used to achieve this. After the sample atomization, radiation is applied, and the radiation flux is recorded. Before this, a measurement of the radiation flux is carried out without the sample. Thus, the Lambert-Beer law can compute the absorbance, or the ratio of the fluxes with and without the sample, and translate it into analyte concentration. The concentration is determined from a calibration curve produced by standards with known concentrations. The device includes flame and furnace spectroscopy for low detection limit and trace metal analysis. Even though recent scientific developments in ICP-OES and ICP-MS and other analytical procedures have overtaken AAS, its greater specificity makes it a well-respected, superior, dependable, and extensively utilized chemical technique for testing any type of material. With a focus on heavy metal analysis, it has been extensively studied and verified that numerous workers from various parts of the world have employed this technique.
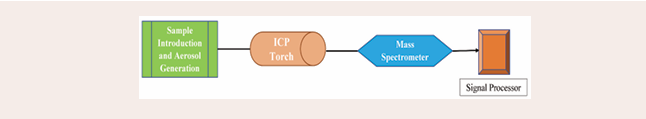
Inductively Coupled Plasma Mass Spectrometry (ICP- MS): ICP-MS is a multi-element method that atomizes the sample with a plasma source so that the mass spectrometer can identify the ions. Ions are separated using mass spectrometers based on their mass-to-charge ratio. This method has excellent detection limits in the ppt (parts per thousand) range. In recent years, speciation analysis with ICP-MS has become a standard procedure for ultrasensitive detection of metalloids and compounds containing trace metals. Industrial analysis of metals, chemicals, advanced synthetic materials, environmental analysis, clinical and biological materials, food, and beverages were among the many uses of this instrument that emerged from the literature review. Other speciation methods, such as optical spectrometry, inductively coupled plasma atomic emission spectroscopy, and atomic absorption spectroscopy, have been replaced by ICP-MS because they are less sensitive and cannot provide simultaneous multi-elemental detection [17]. Even in situations when a particular trace element is dispersed among multiple species, ICP-MS can identify the species that contain the trace element. It is possible to analyze every component at once in a minute. Unlike other approaches, method development requires highly competent operators. ICP-MS instruments are classified into MC-ICP-MS (multi-collector inductively coupled plasma mass spectrometry) and HR-ICP-MS (high-resolution inductively coupled plasma mass spectrometry). In Figure 2, the ICP-MS block diagram is displayed.
Figure 2: Block diagram of ICP-MS instrument
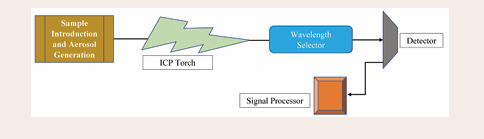
Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES): To determine trace metals, an analytical method called ICP-OES is employed. The atoms in the samples are excited by a plasma source in this multi-element method. A detector computes the intensity of the light released by these excited atoms, which is correlated with the concentration. The light has a specific wavelength. A critical benefit of the ICP approach is that samples are successfully atomized through heating to 10,000 ºC. The primary advantage of this method is that it can meet the increasing need for simultaneous multi-element determination at the nanogram/milliliter level for multiple trace elements, especially for limited amounts of biological samples. Even the most widely used methods, such as AAS, reportedly fall short of these requirements. It is possible to make determinations across a large linear range and at low concentrations for refractory elements (B, P, W, Zr, U). In a single sample test of less than a minute, sixty elements can be analyzed using the ICP approach. Additionally, the device is only specific for a single set of metal analyses. Figure 3 shows
Figure 3: Block Diagram of ICP-OES instrument.
the block diagram for the ICP-OES. The most common types of sources and detectors are radio frequency (RF) powered torches, polychromators for wavelength selection, photomultipliers (PMTs), and charge capacitive discharge arrays (CCDs).
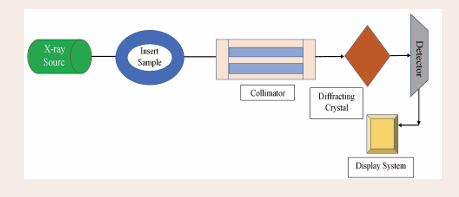
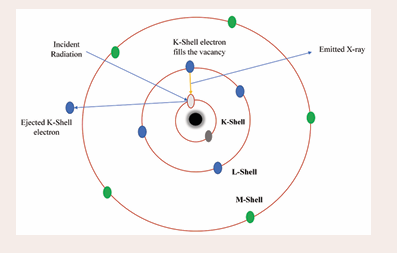
X-ray fluorescence spectrometer (XRF): The fundamental principle of XRF spectroscopy is a straightforward relationship between the basic physics of atom-radiation interaction. It is a very sensitive analytical tool used for analyzing heavy metals. As illustrated in Figure 4,
Figure 4: A block diagram of the XRF instrument.
the X-ray fluorescence spectrometer's block diagram comprises an X-ray source, sample chamber, collimator, fluorescence detector, data processing, detector, and display system. It is well known that XRF offers advantages over other multi-elemental methods like ICP-MS/ICP-OES. The primary benefits of XRF instrument analysis include the minimal preparation needed for solid samples, faster overall, less hazardous waste generated, cheap operating costs, and portability [18]. For the detection limits, accuracy, and precision of various trace elements and heavy metals in various environmental samples, such as biological samples, contaminated soils and sediments, standard reference soil samples, and estuarine sediments, a small number of comparative studies between the XRF technique and conventional ICP-OES have been conducted. This is due to the complexity of environmental samples and multi-element measurement. Ionization of a substance occurs when it is exposed to X-rays or gamma-ray radiation. As seen in Figure 5,
Figure 5: A block diagram of K-capture included in XRF instrument.
such a high-energy radiation bombardment of the material can even evict electrons from the inner orbitals of the K or L shell. Electrons from higher energy shells occupy these vacancies, causing photon or fluorescence emission known as XRF, which can be monitored [19]. The fluorescence spectrum of each element has a different set of energies associated with multiple peaks of different intensities because each element has a different set of energy levels. Thus, XRF is a helpful method for easily determining a sample's elemental composition.
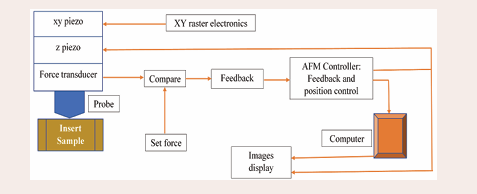
Atomic Force Microscopy (AFM): AFM is a method that provides previously unprecedented resolution and accuracy when measuring surface structure. This technique is included under scanning probe microscopy (SPM), which was first used in 1981. The three primary functions of AFM are imaging manipulation and force measurement. In the process of measuring forces, the forces between the probe and the sample are calculated using AFM as a function of their mutual separation. To keep the distance between the probe and the sample constant, this provides a signal to the feedback control. The sensor's voltage output increases as it comes in contact with the sample. The standard parts of a force sensor include an optical lever system and a cantilever with an integrated tip. Small movements are measured by this optical sensor, which has a laser-focused behind a reflective cantilever probe. A high-resolution image of the three-dimensional shape of a sample surface can be created through scanning by using the probe's response to forces applied by the sample. The forces between the sample and the tip can also be used in manipulation to modify the characteristics of the sample in a controlled manner. It should be quite obvious from the discussion above that the AFM is a highly complex device. In addition, the AFM is incredibly flexible because it may be used in many ways based on the researcher's goal. With all these features, the AFM is a vital tool for microscopy. The block diagram of ICP- MS is shown in Figure 6.
Figure 6: A block diagram of the AFM instrument.
CONCLUSIONS AND FUTURE PERSPECTIVE
One of the major environmental issues is water pollution caused by heavy metal ions in wastewater. Researchers have developed several techniques for detecting heavy metal ions from aqueous systems to make water safe for consumption. This is because heavy metal ion toxicity has been shown to cause numerous health difficulties for living things. Several different kinds of health issues in humans, animals, and plants have been linked to their presence in the environment. To protect people's health and the environment, wastewater must be analyzed for heavy metal contamination. The main issue for the present and future is the harm caused by consuming various items containing metal ions. As a result, it could be necessary to evaluate wastewater for a range of metals in various wastewater matrices at various concentrations. For the purpose of quantifying heavy metals in wastewater, numerous well-established analytical techniques exist. The elements that need to be identified and the number of samples that need to be run will determine which technique best meets the needs of the business. Before application, one should consider the environmental impact, overall detection performance, and economic factors like capital investment and operating cost. This review provides a resource for future research on heavy metal ion detection in wastewater.
REFERENCES
- Rajoria S, Vashishtha M, Sangal V.K. Treatment of electroplating industry wastewater: a review on the various techniques. Environ Sci Pollut Res. 2022; 29: 72196–72246.
- Fu F, Wang Q. Removal of heavy metal ions from wastewaters: A review. J Environ Manage. 2011; 92: 407–418.
- Bajpai S, Dey A, Jha MK, Gupta SK, Gupta A. Removal of hazardous hexavalent chromium from aqueous solution using divinylbenzene copolymer resin. Int J Environ Sci Technol. 2012; 9: 683–690.
- Sasidharan R, Kumar A. Magnetic adsorbent developed with alkali- thermal pretreated biogas slurry solids for the removal of heavy metals: optimization, kinetic, and equilibrium study. Environ Sci Pollut Res. 2022; 29: 30217–30232.
- Barakat MA. New trends in removing heavy metals from industrialwastewater. Arab J Chem. 2011; 4: 361–377.
- Michael A. Heavy Metals in Soil: A Review. Chem Eng Process Technol. 2023; 8: 1076.
- Azimi A, Azari A, Rezakazemi M, Ansarpour M. Removal of Heavy Metals from Industrial Wastewaters: A Review. Chem Bio Eng Rev. 2017; 4: 37–59.
- Al-Qodah Z, Al-Shannag M. Heavy metal ions removal from wastewater using electrocoagulation processes: A comprehensive review. Sep Sci Technol. 2017; 52: 2649–2676.
- Rajoria S, Vashishtha M, Sangal VK. Review on the treatment of electroplating industry wastewater by electrochemical methods. Mater Today Proc. 2021; 47: 1472–1479.
- Shrestha R, Sijan Devkota, Sudip Sharma, Rajendra Joshi, Arjun Prasad Tiwari, Hak Yong Kim, et al. Technological trends in heavy metals removal from industrial wastewater: A review. J Environ Chem Eng. 2021; 9: 105688.
- Vidu R, Ecaterina Matei, Andra Mihaela Predescu, Badriyah Alhalaili, Cristian Pantilimon, Claudia Tarcea, et al. Removal of heavy metals from wastewaters: A challenge from current treatment methods to nanotechnology applications. Toxics. 2020; 8: 1–37.
- Sawan S, Maalouf R, Errachid A, Jaffrezic-Renault N. Metal and metal oxide nanoparticles in the voltammetric detection of heavy metals: A review. TrAC - Trends Anal Chem. 2020; 131: 116014.
- Carolin CF, Kumar PS, Saravanan A, Joshiba GJ, Naushad M. Efficient techniques for the removal of toxic heavy metals from aquatic environment: A review. J Environ Chem Eng. 2017; 5: 2782–2799.
- Sadyrbaeva TZ. Removal of chromium (VI) from aqueous solutions using a novel hybrid liquid membrane - electrodialysis process. Chem Eng Process Process Intensif. 2016; 99: 183–191.
- Malaviya P, Asha Singh. Physicochemical Technologies for Remediation of Chromium-Containing Waters and Wastewaters. Crit Rev Environ Sci Technol. 2011; 41: 1111–1172.
- Vardhan KH, Kumar PS, Panda RC. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J Mol Liq. 2019; 290: 111197.
- Soodan RK, Pakade YB, Nagpal A, Katnoria JK. Analytical techniques for estimation of heavy metals in soil ecosystem: A tabulated review. Talanta. 2014; 125: 405–410.
- McComb JQ, Rogers C, Han FX, Tchounwou PB. Rapid screening of heavy metals and trace elements in environmental samples using portable X-ray fluorescence spectrometer, A comparative study. Water Air Soil Pollut. 2014; 225: 2169.
- Malik LA, Bashir A, Qureashi A, Pandith AH. Detection and removal of heavy metal ions: a review. Environ Chem Lett. 2019; 17: 1495–1521.