Isolation of Substances having Anti-Nematicidal Activity from Streptomyces Sp. An110065
- 1. Microbiome Convergence Research Center, Korea Research Institute of Bioscience and Biotechnology (KRIBB), South Korea
- 2. Synthetic Biology and Bioengineering Research Center, Korea Research Institute of Bioscience and Biotechnology (KRIBB), South Korea
- 3. Department of College of Pharmacy, Chungnam National University, South Korea
- 4. Department of Bio Environmental Chemistry, Chungnam National University, College of Agriculture and Life Science, South Korea
- 5. Department of Microbiology, Chungbuk National University, South Korea
- 0. Authors contributed equally
ABSTRACT
Background: Pine wilt disease (PWD) is a disease caused by the parasitization of Pinus spp. by Bursaphelenchus xylophilus, a pine wood nematode. This nematode is parasitic to insects and is thus transferred to the tree, growing in the tree for a short period of time before blocking nutrients and killing the tree. This is now becoming a serious problem globally. Actinomycetes are known to produce a variety of physiologically active substances. In this study, we search for a substance that kills pine wood nematodes from the secondary metabolites of actinomycetes.
Result: We selected Streptomyces sp. AN110065, which has nematicidal activity against B. xylophilus, from among 5,000 actinomycetes. As a result of a 16S rRNA sequence analysis, 99.78% similarity to Streptomyces netropsis NBRC 3723 was found. In addition, by separating the active compound, pimprinethine was identified through an analysis of the 1H-NMR, 13C-NMR, HMQC, 1H-1H COSY, and HMBC spectra. As a result of evaluating the activity of pimprinethine against B. xylophilus, 100% nematode activity was observed after 24 hours at a concentration of 0.4 mg/ml.
Conclusion: This study was performed to isolate useful secondary metabolites produced by soil actinomycetes for the control of pine wilt nematodes. A compound having nematicidal activity was isolated from the selected strain, and its structure was confirmed as pimprinethine. The nematicidal activity of the isolated active ingredient pimprinethine was confirmed, and as the first report on the effect of pimprinethine on PWN, it is proposed as a potential material for the control of pine wood nematodes.
KEYWORDS
Pine wilt disease; Bursaphelenchus xylophilus; Actinomycetes; Pimprinethine; Nematode
CITATION
Kang MK, Kim JH, Moon KH, Jeong HJ, Lee BM, et al (2023) Isolation of Substances having Anti-Nematicidal Activity from Streptomyces Sp. An110065. Chem Eng Process Tech 8(1): 1075.
INTRODUCTION
The pine wood nematode (PWN, Bursaphelenchus xylophilus) causes catastrophic damage to pine trees and is mainly transmitted via vector insects. Pine wilt disease (PWD) first occurred in Japan in the early 1900s, but it was not confirmed until 1971 that the causative agent was a pine tree nematode [1-3]. The PWN is native to North America, i.e., the United States, Canada, and Mexico. In North America, pine trees are not affected because they are resistant. However, the pines of the genus Pinus, including trees in Korea, Japan, Taiwan, China, and Portugal, are susceptible and sustain fatal damage [4-6].
The PWN does not have the ability to move or disperse between trees on its own, and these nematodes live in parasitic worms such as brush-bearing and northern-bearded worms, and infect host plants through wounds that occur when they eat healthy pine branches. After the PWN has infected the host plant, it grows by ingesting nutrients and Botrytis cinerea in the tree [7-10]. Regarding the mechanisms of the death of the pine tree by PWNs, they include flow cell destruction by PWNs, the blockage of water movement by the clogging of the trachea, cell suicide through a hypersensitivity reaction, and stress caused by the cellulase of bacteria possessed by PWNs [11,12].
Currently, there are two methods of controlling PWNs: controlling the vector insects and directly controlling the PWNs. In addition, in order to control PWD, healthy pine trees are protected from bestial injections of nematodes or soil drenching [13,14]. Methods include tree injections for nematodes with chemicals such as abamectin or emamectin benzoate, or ground spraying with fosthiazate. Other methods include fumigation, crushing, and incineration to remove larvae from damaged trees [15,16]. Control methods targeting pine worms have stronger effects than control methods targeting vector insects, and a precautionary effect can be expected. However, these approaches are difficult to apply to large-scale forests given the requirement of much labor, the high cost, and the difficulty of working in forests [17,18].
There is an increasing level of demand for alternative control agents with low or no environmental risk available at a low cost as well. Therefore, in order to compensate for the shortcomings of the aforementioned methods, studies of nematode substances that may replace chemical pesticides have been actively conducted in recent years, and the use of microorganisms that produce nematode-active substances is one of the main branches of this work [19,20]. Research is currently active in the area of alternative control materials because substances with high activity among plant-derived substances or microorganisms can be used as biochemical pesticides or for chemical pesticide development [21]. Among them, natural substances derived from microbial metabolites are highly valued for direct use of active ingredients as well as leading substances for the development of new pesticides and research on new points of action [22,23]. Therefore, in this study, nematode activity was assessed after the use of actinomycetes isolated from forest soil to search for an environmentally friendly alternative material that can be used for the control of PWD.
MATERIALS AND METHODS
Screening of Nematicidal Activity and Active Strain Identification
The actinomycetes used in the experiment were from the soil actinomycetes library of the Korea Research Institute of Bioscience and Biotechnology (MRDB, www.mrdb.or.kr). A total of 5,000 actinomycetes were incubated at 28? for seven days in Bennett's (10 g glucose, 1 g yeast extract, 1 g beef extract, 2g peptone and 1 L DW) agar medium, followed by seven days in a GSS broth25 (20 g glucose, 10 g soluble starch, 25 g soybean meal, 2 g NaCl, 0.25 g K2 HPO4 , 2 g CaCO3 , 1 g beef extract, 4 g yeast extract and 1 L DW) medium. Next, 500 δL of each culture broth was extracted with 500 δL of acetone and centrifuged, after which 500 δL of the supernatant was collected and dried. The dried sample was diluted with DMSO at concentrations of 40, 20, and 10 mg/mL. The sample at an amount of 5 δL and the nematode suspension 95 δL were added to a 96-well plate containing 50 nematodes per 100 δL. The final concentrations of the cultures in the wells were 2, 1, and 0.5 mg/mL.
A molecular phylogenetic analysis was performed based on a 16S rRNA gene sequencing analysis of the active strain. Genomic DNA was extracted using acetyltrimethylammonium bromide from mycelium obtained by centrifuging the culture at 4? for 20 min. 16S rRNA was amplified using the PCR method with Ex Tag polymerase (Takara, Ohtsu, Japan) and a universal primer set (27F, 5'-AGAGTTTGATCMTGG CTCAG-3'; 1492R, 5'-TACGGYTACCTTGTTACGACTT-3')28. The conditions for thermal cycling were once at 94? for 5 min and 34 times at 94? for 30 sec, 58? for 30 sec, 72? for 30 sec, and one time at 72? for 7 min. The PCR products were purified using a GeneAll ExpinTM PCR purification kit (GeneAll, Seoul, Korea) and were sequenced by Macrogen (Daejeon, Korea). The obtained sequences were blasted on EZBioCloud (https://www.ezbiocloud.net/identify) and aligned with CLUSTAL W software. The phylogenetic tree was constructed via the neighbor-joining method with 1,000 iterations of bootstrapping using the MEGA program package.
Nematode and Fungi Culturing
B. xylophilus, a causative agent of pine wilt disease, was purchased from the National Forestry Academy and was used in the experiments. A nematode was isolated using a funnel method. After separation, it was used for culturing at 25?C using a feed of Botrytis cinerea for nematodes. B. cinerea was cultured in PDA (DifcoTM, Potato Dextrose Agar, and BD, USA) medium at 25?C for seven days.
Screening for Nematicidal Activity
The actinomyces culture broth was centrifuged and the supernatant was concentrated. Here, 5,000 samples were prepared by dissolving the residue in 50 μL of DMSO and were used in the experiments. In addition, 5 μL of the actinomycetes culture extract and 95 μL of the nematode solution (containing 30-40 nematodes) were tested in 96-well Microtest™ tissue culture plates. For the nematode activity, the 96-well plates were incubated at 25?C for 24 hours. Straight, rigid and immobile nematodes were considered to be dead nematodes. Flexible and motile nematodes were regarded as acid nematodes, and the live nematodes were examined.
All tests were performed three times with treatments replicated three times. Mortality was calculated using the following equation:
General Experimental Procedures
The 1 H NMR (600 MHz), 13C NMR (150 MHz), and 2D NMR spectra were obtained on a JEOL JNM-ECA600 spectrometer with CD3 OD as a solvent. HRESI-MS was conducted using a Shimadzu LCMS-IT-TOF mass spectrometer. The HPLC system consisted of a Hitachi L-2130 pump, a Hitachi UV detector L-2400, and a reversed-phase column (C18, 5 µm, φ10 × 150 mm, Cosmosil). Reversed-phase column chromatography was conducted using RP-C18 silica gel (YMC*GEL ODS-A, 12 nm S-150 µm, YMC Co. Ltd.), and silica gel column chromatography was conducted using Kieselgel 60 (70−230 and 200−400 mesh, Merck).
Anti-Nematicidal Activity Tests
B. xylophilus were reared on the PDA medium covered with B. cinerea cultured in the dark at 25°C for seven days, after which they were washed with water. The nematode suspension underwent counting and was used in the experiment. In order to find the concentration at which the compound exhibits 100% nematicidal activity, nematicidal activity assays were performed at various concentrations. Positive control abamectin and test samples of pimprinethine were treated at concentrations from 0.1 to 1 mg/ml in each case. Each sample was treated with 5 υL in a 96-well plate so that the final concentration ranged from 0.1 to 1 mg ml-1. Thereafter, the nematode suspension was treated with 95 υL per well so that the final volume was 100 δL per well. After incubation for seven days in a 25°C incubator, dead nematodes were counted under a microscope. Mortality was determined as the minimum concentration at which 100% of the nematodes died.
All tests were performed three times with treatments replicated three times. Mortality was calculated using the following equation:
RESULTS
Screening and Identification of Nematicidal Activity by Actinomycetes
We screened for insecticidal activity of 5,000 strain extracts at concentrations of 0.5, 1, and 2 mg/ml. Among them, a strain having 100% activity at the lowest concentration of 0.5 mg/ ml was selected. The activity was compared with the same Streptomyces sp. The isolate strain AN110065, which had 100% anti-nematode activity at all concentrations, was selected after 24 hours from 0.5, 1, and 2 mg/mL of the extract (Table 1).
Table 1: Activity and identification results of active strain
|
Streptomyces sp. strain |
Dose (mg/mL) |
Mortality (%) |
|
AN150764 |
2 |
82 |
|
1 |
65 |
|
|
0.5 |
32 |
|
|
AN160026 |
2 |
76 |
|
1 |
25 |
|
|
0.5 |
0 |
|
|
AN110065 |
2 |
100 |
|
1 |
100 |
|
|
0.5 |
100 |
|
|
AN150294 |
2 |
71 |
|
1 |
53 |
|
|
0.5 |
26 |
|
|
AN150502 |
2 |
88 |
|
1 |
54 |
|
|
0.5 |
0 |
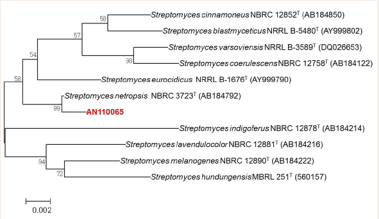
A molecular phylogenetic analysis based on the 16S rRNA gene sequence revealed that AN110065 showed 99.78% similarity with Streptomyces netropsis NBRC 3723 (Figure 1).
Figure 1: Based on 16S rRNA gene sequence, the location of Streptomyces sp. AN110065 was constructed in phylogenetic trees using a neighbor- binding method. The scale bar represents 0.002 substitutions per nucleotide position.
Isolation and Purification of Nematicidal Activity Compound-1
The AN110065 culture broth was separated into a broth layer and a mycelium cake layer by centrifugation (6000 rpm, 20 min). The broth layer was fractionated with various solvents according to polar and non-polar solvent conditions. As a result, the activity of the ethyl acetate layer (516 mg) was confirmed and the sample was characterized by silica-gel TLC. The ethyl acetate layer was purified by silica-gel column chromatography using a linear gradient method with the CHCl3 : MeOH = 50:1 developing solvent. Based on the results, the fraction of CHCl3 : MeOH = 50:1, which shows activity, was separated by size exclusion gel Sephadex LH20 column chromatography. Finally, a high-performance liquid chromatography (HPLC) step was carried out to separate and purify the inhibitory substance.
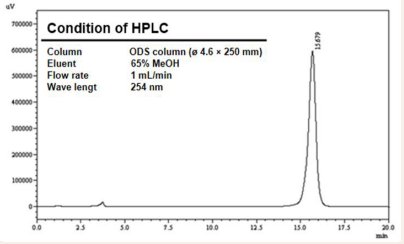
In order to confirm that the separated material was a single substance, it was confirmed by HPLC that the peak of the active substance could be observed in approximately 15.679 minutes when developed with 65% methanol using an ODS column (φ 4.6 × 250 mm) (Figure 2).
Figure 2: HPLC profile of compound-1 isolated from Streptomyces sp. AN110065. HPLC analysis in 65% methanol condition from LH-20 fraction.
1H-NMR and 13C-NMR Spectra of Compound-1
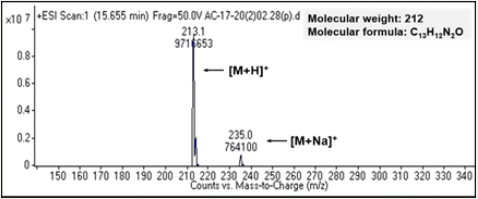
The ESI mass spectrum was measured in positive mode to determine the molecular weight of compound-1. In the positive mode, [M+H]+ was observed at m/z 213.1 and [M+Na]+ at m/z 235.0. The molecular weight was found to be 212, and the molecular formula was determined to be C13H12N2 O (Figure 3).
Figure 3: ESI-Mass spectrum of compound-1.
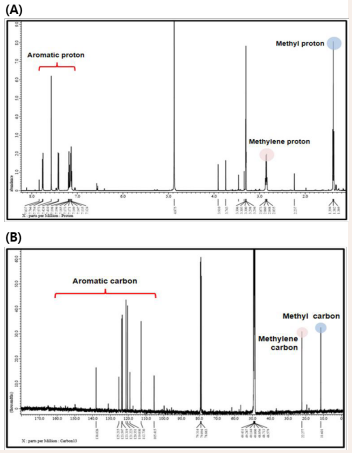
Through the 1 H-NMR and 13C-NMR analyses of compound-1, 13 carbons and 12 protons were found to be present. The 1 H-NMR spectrum showed signals due to six aromatic protons at δH 7.14 to δH 7.76, methyl protons at δH 1.38, and one methylene at δH 2.85. The 13C-NMR spectrum showed that 11 aromatic carbon atoms were present between δC 105.4 and δC 165.0, one methyl carbon at δC 11.6, and methylene carbon at δC 22.3, which were related to 1 H-NMR. The chemical shifts of the protons and carbons were confirmed by 1 H-NMR and 13C-NMR spectroscopy and are summarized in Table 2.
Table 2: 1H and 13C NMR chemical shift of compound-1.
|
Position |
δC (ppm) |
δH (ppm) |
Position |
δC (ppm) |
δH (ppm) |
|
2 |
165.0 |
- |
3′a |
125.2 |
- |
|
4 |
119.0 |
7.15 (s) |
4′ |
120.4 |
7.76 (d) |
|
5 |
149.5 |
- |
5′ |
121.2 |
7.14 (d) |
|
6 |
22.3 |
2.85 (q) |
6′ |
123.3 |
7.19 (t) |
|
7 |
11.6 |
1.38 (t) |
7′ |
112.7 |
7.42 (d) |
|
2′ |
123.6 |
7.57 (s) |
7′a |
138.0 |
- |
|
3′ |
105.4 |
- |
|
|
|
2D NMR Spectrum of Compound-1
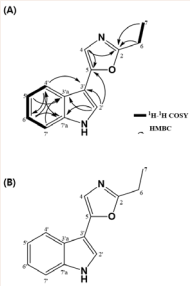
The HMQC spectra of the compound-1 proton-carbon longrange correlations were determined: H-7 (δH 1.38) and C-7 (δC 11.6), H-6 (δH 2.85) and C-6 (δC 22.3), H-4 (δH 7.15) and C-4 (δC 119.0), H-6' (δH 7.19) and C-6' (δC 123.3), H-2'(δH 7.57) and C-2' (δC 123.6), and H-4' (δH 7.76) and C-4' (δC 120.4). A cross-peak between 1 H-1 H through the 1 H-1 H COZY spectrum was confirmed, H-5' (δH 7.14), H-7' (δH 7.42) and H-6' (δH 7.19) linkages at H-6 (δH 2.85), H-4' (δH 7.76), and H-5' (δH 7.14). Finally, the HMBC spectrum was measured from the proton to confirm the position of the carbon at two, three, and four bonds. The results were as follows: long range coupling from H-4 (δH 7.15) to C-2 (δC 165.0), C-5 (δC 149.5) and H-2’ (δH 7.57) to C-3’ (δC 105.4), C-5 (δC 149.5), C-3'a (δC 125.2), C-7’a (δC 138.0) and H-4' (δH 7.76) to C-6' (δC 123.3), C-7’a (δC 138.0), C-3’a (δC 125.2) and H-5’ (δH 7.14) to C-3’a '(δC 125.2), C-7’ (δC 112.7) and H-6’ (δH 7.19) to C-4' (δC 120.4), C-7'a (δC 138.0) and H-7’ (δH 7.42) to C-3’a (δC 125.2), C-5’ (δC 121.2) were observed, confirming the structure of pimprinethine figure 4.
Figure 4: (A) 1H-NMR spectrum and (B) 13C-NMR spectrum of compound-1.
Effect of Active Compound Pimprinethine
The nematicidal activity of pimprinethine, a chemical compound isolated from Streptomyces sp. AN110065 strain, was evaluated to confirm the LD100 value for B. xylophilus. In order to confirm the 100% nematode activity concentration for B. xylophilus, abamectin and pimprinethine were treated at 0.1 to 1 mg/ml, respectively, and observed under a microscope after 24 hours. As a result, it was confirmed that abamectin 0.5 mg/ml and pimprinethine 0.4 mg/ml had a 100% mortality rate figure 5
Figure 5: (A) Correlations in the H1-H1 COSY (bold line) and HMBC spectra (arrow) of pimprinethine. (B) Chemical structure of pimprinethine.
(Table 3).
Table 3: Nematicidal activity of pimprinethine against Bursaphelenchus xylophilus
|
Compound |
Dose (mg/ml) |
Mortality (%) |
|
Abamectin® |
0.5 |
100 |
|
Pimprinethine |
0.4 |
100 |
|
0.2 |
67 |
|
|
0.1 |
23 |
It was found that pimprinethine had the same activity at a slightly lower concentration than abamectin, a positive control. Based on these results, the potential PWD inhibitory activity of pimprinethine can be predicted.
DISCUSSION
PWD is currently the most threatening disease in the forests of Korea, and damaged areas have increased every year since 1988, despite continuous control work [24,25]. Therefore, various methods for controlling PWD are being studied. Among these, studies targeting pine wilt nematodes focus on the selection and use of pesticides with nematicidal activity [26], searches for plants having nematicidal activity against pine wilt nematodes [27,28], the separation of useful substances, and searches for microorganisms with nematicidal activity [29,30]. There are very few practical cases, such as avermectins isolated from Streptomyces avermitilis [31]. Unlike general agricultural ecosystems within forest ecosystems such as pine forests, the food chains of pest groups and natural enemies are stabilized such that when large amounts of chemical control agents are injected to control specific pests, various adverse effects on the forest ecosystem can occur [32]. Therefore, even for research results that cannot be used practically right away, various studies of eco-friendly control methods that can replace the existing chemical control are continuously needed.
In the present study, secondary metabolites produced by strains with activity against pine wilt nematodes from soil actinomycetes were investigated. By screening for nematicidal activity from 5,000 strains from an actinomycetes library, the Streptomyces sp. AN110065 strain, showing activity in an extract at a low concentration, was selected. In order to confirm the taxonomic characteristics of Streptomyces sp. AN110065, 16S rRNA sequencing was performed. As a result, it was confirmed to have 99.78% similarity with Streptomyces netropsis NBRC 3723. A compound having nematicidal activity was isolated from the secondary metabolites produced by this strain. As a result of analyzing the isolated compound through the 1 H-NMR, 13C-NMR, HMQC, 1 H-1 H COSY, and HMBC spectra, it was identified as a pimprinethine compound. The nematicidal activity of a single compound of pimprinethine against B. xylophilus was evaluated, and it was confirmed that it had the same 100% insecticidal rate at the concentrations of 0.5 mg/ml of abamectin and 0.4 mg/ml of pimprinethine.
Actinomycetes are used as a search source for various useful substances in the biological material industry. These actinomycetes are mainly isolated from soil [33], and actinomycetes active against pine wilt nematodes have also been reported [34,35]. It has been reported that the pimprinethine isolated in this study has antiviral and anti-tuberculous effects on EV71 [36]. Recently, nematicidal activity against Caenorhabditis elegans was also reported [37]. Our results suggest for the first time that pimprinethine has nematicidal activity against PWN. This result is reliable because pimprinethine has now known nematicidal activity in C. elegans. Through this study, pimprinethine is expected to be a potential control agent for sufficiently controlling the PWD. Derivatives of this compound are very diverse, and further research requires experiments in in vivo conditions. Therefore, in this paper, we propose interesting results predicted that pimprinethine may have nematicidal activity in various nematodes.
FUNDING
This work was supported Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry (IPET) through (Project No. 321110-4 “Crop Viruses and Pests Response Industry Technology Development”) Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) and was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) & funded by the Korean government (MSIT) (No. 2021M3A9I5023254).
REFERENCES
- Kiyohara T, Tokushige Y. Inoculation experiments of a nematode, Bursaphelenchus sp., onto pine trees. J The Jpn For Soc. 1971; 53:210- 218.
- Futai K. Pine Wilt in Japan: From First Incidence to the Present. In: Zhao BG, Futai K, Sutherland JR, Takeuchi Y, editors. Pine Wilt Disease. Springer, Tokyo. 2008; 5-12.
- Cheng HR, Lin M, Li W, Fang Z. The occurrence of a pine wilting disease caused by a nematode found in Nanjing. Forest Pest and Disease 1983; 4; 1-5.
- Mota MM, Vieira PC. Pine Wilt Disease in Portugal. In: Zhao BG, Futai K, Sutherland JR, Takeuchi Y, editors. Pine Wilt Disease. Springer, Japan, 2008; 33-38.
- Mamiya Y. History of Pine Wilt Disease in Japan. J Nematol. 1988; 20: 219-226.
- Edward OR, Linit MJ. Transmission of Bursaphelenchus xylophilus through oviposition wounds of Monochamus carolinensis (Coleoptera: Cerambycidae). J Nematol. 1992; 24: 133-139.
- Mamiya Y, Enda N. Transmission of Bursaphelenchus lignicolus (Nematoda: Aphelenchoididae) by Monochamus alternatus (Coleoptera: Cerambycidae). Nematologica. 1972; 18: 159-162.
- Morimoto K, Iwasaki A. Role of Monochamus alternatus (Coleoptera: Cerambycidae) as a vector of Bursaphelenchus Lignicolus (Nematoda: Aphelenchoididae). J The Jpn For Soc. 1972; 54: 177-183.
- Oku H, Shiraishi T, Ouchi S, Kurozumi S, Ohta H. Pine wilt toxin, the metabolite of a bacterium associated with a nematode. Naturwissenschaften. 1980; 67: 198-199.
- Kuroda K. Terpenoids causing tracheid-cavitation in Pinus thunbergii infected by the pine wood nematode (Bursaphelenchus xylophilus). Ann Phytopath Soc Japan. 1980; 55: 170-178.
- Yik CP, Birchfield W. Observations on the morphology of the pine wood nematode, Bursaphelenchus xylophilus. J Nematol. 1981; 13: 376-384.
- Lee SM, Chung YJ, Moon YS, Lee DW, Choo HY, Lee CK, et al. Insecticidalactivity and fumigation conditions of several insecticides against Japanese pine sawyer (Monochamus alternatus) larvae. Jour Korean For Soc. 2003; 92: 191-198.
- Kim JG, Kim BK, Lee SK, Kim JC, Hang SS, Cha BJ, et al. Distribution of
-
Bursaphelenchus xylophilus in naturally infected Pinus densiflora and P. koraiensis and migration of B. xylophilus in artificially inoculated P. densiflora seedlings. Res Plant Dis. 2012; 18: 101-108.
- Kishi Y. The pine wood nematode and the Japanese pine sawyer. Thomas Company Limited. Japan. 1995; 302.
- Moon YS, Lee SM, Park JD, Yeo WH. Distribution and control of the pine wood nematode, Bursaphelenchus xylophilus and its vector Japanese pine sawyer, Monochamus alternatus. Fri J For Sci. 1995; 51: 119-126.
- Lee SM, Kim DS, Kim CS, Choo HY, Lee DW. Possibility of simultaneous control of pine wilt disease and Thecodiplosis japonensis and or Matsucoccus thunbergianae on black pine (Pinus thunbergii) by abamectin and emamectin benzoate. Korean J Pestic Sci. 2018; 12: 363-367.
- Kamata N. Integrated Pest Management of Pine Wilt Disease in Japan: Tactics and Strategies. In: Zhao BG, Futai K, Sutherland JR, Takeuchi Y, editors. Pine Wilt Disease. Tokyo. 2008; 304-322.
- Park IK, Kim J, Lee SG, Shin SC. Nematicidal activity of plant essential oils and components from ajowan (Trachyspermum ammi), allspice (Pimenta dioica) and litsea (Litsea cubeba) essential oils against pine wood nematode (Bursaphelenchus xylophilus). J Nematol. 2007; 39: 275-279.
- Lee CM, Lim TH, Lee SM, Mun LS, Han SS, Lee DW. Nematicidal and reproduction suppression activity of actinomyces isolates against pine wood nematode, Bursaphelenchus xylophilus. Korea J Pestic Sci. 2015; 19: 141-150.
- Tomlin CDS. A world compendium the pesticide manual. British Crop Protection Council. Hampshire, UK. 2006.
- Lange L, Breinholt J, Rasmussen FW, Nielsen RI. Microbial fungicide the natural choice. Pestic Sci. 1993; 39: 155-160.
- Katz E, Demain A. The peptide antibiotics of Bacillus: chemistry, biogenesis and possible functions. Bacteriol Rev. 1997; 41: 449-1474.
- Shin SC. Pine wilt disease in Korea. In: Zhao BG, Futai K, Sutherland JR, Takeuchi Y, editors. Pine Wilt Disease. Springer, Japan, 2008; 26-32.
- KFS (Korea Forest Service). Statistical Yearbook of Forestry 2013. Daejeon, Korea. 2013.
- Takai K, Soejima T, Suzuki T, Kawazu K. Emamectin benzoate as a candidate for a trunk-injection agent against the pine wood nematode, Bursaphelenchus xylophilus. Pest Manag Sci. 2000; 56: 937-941.
- Kang JS, Kim E, Lee SH. Il-Kwon P. Inhibition of acetylcholinesterases of the pinewood nematode, Bursaphelenchus xylophilus, by phytochemicals from plant essential oils. Pestic Biochem Physiol. 2013; 105: 50-6.
- Kong JO, Lee SM, Moon YS, Lee SG, Ahn YJ. Nematicidal activity of plant essential oils against Bursaphelenchus xylophilus (Nematoda: Aphelenchoididae). J Asia Pac Entomol. 2006; 9: 173-178.
- Kwon HR, Son SW, Han HR, Choi GJ, Jang KS, Choi YH, et al. Nematicidal activity of bikaverin and fusaric acid isolated from Fusarium oxysporum against pine wood nematode, Bursaphelenchus xylophilus. Plant Pathol J. 2007; 23: 318-321.
- Park DJ, Lee JC, Kim PK, Kim CJ. Isolation and identification of Micromonospora sp. showing nematocidal activity against pine wood nematode. Korean J Pestic Sci. 2008; 12: 97-101.
- Tomlin CDS. The pesticide manual, A World Compendium, BCPC. Hampshire, UK.Siemann E, Carson WP, Rogers WE, Weisser WW. Reducing herbivory using insecticides, In Insects and Ecosystem Function; Weisser WW, editors. Springer, Germany, 2004; 303-327.
- Lim TH, Cho SH, Kim JH. Effects of Streptomyces sp. MG 121 on growth of pepper plants and antifungal activity. Res Plant Dis. 2007; 13: 93- 97.
- Liu MJ, Hwang BS, Jin CZ, Li WJ, Park DJ, Seo ST, et al. Screening, isolation and evaluation of a nematicidal compound from actinomycetes against the pine wood nematode, Bursaphelenchus xylophilus. Pest Manag Sci. 2019; 75: 1585–1593.
- Kang MK, Kim MH, Liu MJ, Jin CZ, Park SH, Lee JM, et al. Nematicidal activity of teleocidin B4 isolated from Streptomyces sp. against pine wood nematode, Bursaphelenchus xylophilus. Pest Manag Sci. 2021; 77: 1607-1615.
- Wei Y, Fang W, Wan Z, Wang K, Yang Q, Cai X, et al. Antiviral effects against EV71 of pimprinine and its derivatives isolated from Streptomyces sp. Virol J. 2014; 11: 195.
- Fan XY, Hao XQ, Wei J, Liu W, Yu LY, Zhang Y, et al. Isolation, structure identification and anti-TB activity of antibiotics 9792A and B. Chinese J Antibiot. 2010; 35: 346-348+356.
- Zhang MZ, Mulholland N, Seville A, Hough G, Smith N, Dong HQ, et al. First discovery of pimprinine derivatives and analogs as novel potential herbicidal, insecticidal and nematicidal agents. Tetrahedron. 2021; 79: 131835.