Preparation and Characterization of 3-Amino- 1h-1, 2, 4-Triazole Grafted on the Surface of Silica Nanoparticles Support (SNPs-AT) for the Synthesis of Pyrano[2,3-C] Pyrazole Derivatives as the Novel, Effective, and Reclaimable Catalyst
- 1. Department of Chemistry, Yasouj University, Iran
Abstract
The main objective of the current research, 3-amino-1H?1,2,4?triazole immobilized on 3-chloropropyl joined SiO2 nanoparticles as a recyclable, environment-friendly, and novel heterogeneous base catalyst. It was characterized by fourier transform infrared, X-ray diffraction patterns, field emission scanning electron microscopes, and energy-dispersive X-ray spectroscopy. This new material as a green and efficient catalyst was used for the synthesis of pyrano [2, 3-c] pyrazoles derivatives through the reaction of aromatic aldehydes, malononitrile, and 3-methyl-1-phenyl-2-pyrazoline-5-one. The reactions did in solvent-free conditions at 60 °C and the related pyran derivatives prepared in excellent yields. also, this catalyst could be used several times without decreasing the activity.
Keywords: Nano-Silica; 3-Amino-1h?1, 2, 4?Triazole; Pyrano [2, 3-C] Pyrazole; Reusable Catalysts; Solvent-Free Condition
Introduction
About 1.5% of the Gross National Product (GNP) in the world is related to catalyst technology and plays a significant role in economic advancement and the promotion of the chemical industry [1]. On the other hand, in today's modern world, the inclination to use tinier and more efficient systems in the field of nanoscience and technology by researchers causes them to synthesize smaller materials with improved properties. The application of nanomaterials as heterogeneous catalysts has attracted a lot of consideration due to their economic, environmental, and structural specifications [2], and as the forerunner of green chemistry plays an important role in achieving selectivity, excellent activity, desired flexibility, chemical and thermal stabilization [3]. In recent research, attempts to the synthesis of supported nanocatalysts on various substrates such as charcoal, alumina, magnetic nanoparticles, silica, and polymers, as green materials have obtained great attention [4]. SiO2 nanomaterials have unique and adjustable physiochemical properties with a high surface area to volume ratio, excellent chemical, thermal, and mechanical stability. Hence, they have high potential chemical reactivity [5]. Former articles have authenticated that substituted pyrano [2,3-c] pyrazoles are an appealing category of heterocyclic compounds due to biological and medicinal activities [6]. In specific, their advantages have resulted in fungicidal, anticancer, antibacterial, antitumor, antiplatelet, and antioxidant of these compounds [7]. So far, there have been many reports for the synthesis of the pyrano [2,3-c] pyrazole. The common method is condensation reaction of aryl aldehydes, malononitrile, and 3-methyl-1-phenyl-2-pyrazoline-5-one in the presence of various catalysts including HDBAC, HTMAB, MDOs, D and L-Proline, [Dsim] AlCl4, silicotungstic acid, isonicotinic acid, gamma-Alumina. Each of the above methods has its own merits, while most of these techniques suffer from disadvantages such as long reaction times, low product yields, use of excess amounts of catalyst, application of hazardous solvents, and toxic effluents [8,9].
Results and Discussion
In continuation of research on developing supported catalysts done by Karami group, several green catalysts were reported such as STA [4], SSC [10] Fe3O4@TiO2@(CH2)3OWO3H [6], Fe3O4@SiO2@(CH2)3OMoO3H [11], MCM-41-HWO4 [12], phthalhydrazide-MCM-41 [13]. Herein, we have reported a new heterogeneous nanocatalyst using Azoles because other researcher groups have used them in the synthesis of catalysts, too [14, 15]. Accordingly, we grafted 3-amino-1H-1,2,4-triazole (3-AT) on 3-chloropropyl, which is coated on silica nanoparticles, and used in the multi-component reactions in organic chemistry. The SiO2 NPs were initially synthesized through the sol-gel process. Then, SiO2@(CH2)3Cl was formed via the reaction of SiO2 NPs with 3-chloropropyl tri methoxy silane according to the previous report [13]. Finally, as expected, in the reaction of SiO2@(CH2)3Cl with 3-amino-1H-1,2,4-triazole (3-AT), the chloride group was replaced by (3-AT), to obtain nano-catalyst SiO2@(CH2)3-(3-AT) (SNPs-AT) (Scheme 1). Chemical analysis of SiO2@(CH2)3(3-AT) bases catalyst was determined using fourier transform infrared (FT-IR), X-ray diffraction patterns (XRD), field emission scanning electron microscopes (FE-SEM), and energy-dispersive X-ray spectroscopy (EDS).
PREPARATION OF SNPs-AT
To prove the produced catalyst, FT-IR spectra of the synthesized particles (SiO2, SiO2@(CH2)3Cl and SNPs-AT) are from wavelength 400 to 4000 cm-1, shown in Figure 1. FT-IR spectra of SiO2 Figure 1a illustrate the absorption bands at the region 809, 1103 cm-1 assigned to Si–O–Si stretching symmetric and Si–O–Si stretching asymmetric, respectively. The bending vibrations Si–O–Si appear at 462 cm-1 [16]. FT-IR spectra of the SiO2 @ (CH2)3Cl structure Figure 1b in comparison with that of SiO2 Figure 1a confirm that the CH2 of the alkyl chains is in the 2854 cm-1 for symmetric CH2 and 2923 cm-1 for asymmetric stretching peaks [17]. Compare with FT-IR spectra of Figure 1b, SiO2 @ (CH2)3 (3-AT), shown in Figure 1c, has been formed. Moreover, two peaks that appear at 1442 cm-1 and 1645 cm-1are related to N=N and C=N stretching vibration bands of the (3-AT) material immobilized on the surface catalyst [15,18] (Scheme 1).
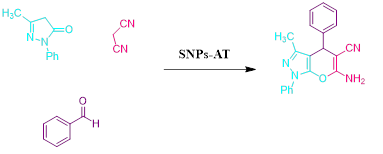
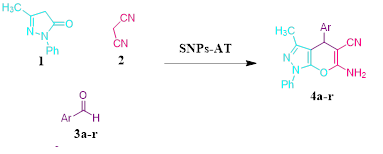
EDS is an analytical technique used for the elemental or chemical characterization of a sample. According to Figure 2, it shows the presence of elements Si, O, C, and N in the SNPs-AT that agrees with our predictions. also, the successful bonding of the 3-AT groups is fully confirmed by the presence of nitrogen. XRD is one of the most significant, non-destructive instruments used to study the structure, composition, and quality control. The presence of amorphous SiO2 structure with a wide scattering centered in the range of (20°< 2θ < 32°) was observed in Figure 3a. The XRD pattern in Figure 3b displays the nanoparticles SNPs-AT and in comparison with Figure 3a, the existance of nitrogen in the structure confirmed the formation of SNPs-AT [19]. FE-SEM images show the morphology and size of the novel catalyst SNPs-AT. The result proves that the nanoparticles are spherical with average diameters of 37.96 - 64.91 nm Figure 4. All this evidence demonstrates that the novel nanocatalyst SNPs-AT was produced successfully. Continuing this research, we have tested the catalytic activity through one-pot, the multicomponent reaction for the synthesis of pyrano [2,3-c] pyrazoles derivatives (4a-r). In this work, the one-pot reaction was performed between a mixture of 3-methyl-1-phenyl-2 pyrazoline -5-one (1) (1mmol, 0.174 g), malononitrile (2) (1.25 mmol, 0.08 g) and aromatic aldehydes (3a-r) (1 mmol, 0.14 g) and SiO2@(CH2)3 (3-AT) as catalyst under different conditions (Scheme 2).
4.2. Novel SNPs-AT as catalysts for the Synthesis of pyrano [2,3-c] pyrazoles
As a replacement of toxic organic solvents, solvent-free reactions were shown to be an impressive technique in chemist organic. frequently, solvent-free conditions lead to a significant decrease in reaction times, increased production, and regioselectivity, and stereoselectivity of reactions [20,21]. At first, the optimized conditions for the model reaction should be obtained. The model reaction in the absence of catalyst checked out but the favorable product was not created even after a long-time reaction. The model reaction was performed by various amounts of SNPs-AT at the range of 25–70 ºC. Results are shown in Table 1. According to (Table 1), the products were produced efficiently utilizing 0.003g of the catalyst (Table 1, entry 11). Also, maximized product yield obtained at 60 ºC (Table 1, entry 8). To investigate the solvent effect, the model reaction was performed using 0.003g nanocatalyst SNPs-AT in solvent-free conditions and several polar and nonpolar solvents including water, ethanol, carbon tetrachloride, tetrahydrofuran, and acetonitrile conditions (Table 1, entries 2-5). Using the solvents couldn’t increase the yields of the product, while under solvent-free conditions, product yield was in the range of 85-95 percentage (Table 1, entries 2, 8). In this way, the optimized conditions are shown in entry 11 of Table 1 for pyrano[2,3-c] pyrazoles derivatives which have been synthesized by SNPs-AT catalyst in solvent-free condition at 60 ºC. Under these conditions, the modal reaction was studied in an extensive range of aromatic aldehydes containing both electron-donating and electron-withdrawing groups completely. Acceptable efficiency of the products was obtained by both aromatic aldehydes groups in the short reaction times showed in Table 2. The main problem of some previous methods in the preparation of pyrano [2,3-c] pyrazoles is that the catalysts cannot be reused, but In our work, the recycled catalyst was used at least five times without loss of its activity Figure 5. According to (Scheme 3), a possible mechanism for the synthesis of product 4 is brought in the following steps. the first step includes the Knoevenagel condensation between aryl aldehyde (3a-o) and malononitrile (2) using a base catalyst. Then, Michael's addition occurred between intermediate (6) and 2-benzylidene malononitrile (5). Finally, cyclization and tautomerization of intermediates (7) and (8) give the expected product (4).
NOVEL SNPs-AT as Catalysts for the Synthesis ofF PYRANO [2,3-C] PYRAZOLES (Scheme 3).
Table 1
a benzaldehyde 1 mmol, 3-methyl-1-phenyl-2-pyrazoline-5-one 1mmol, malononitrile 1.25 mmol
b Isolated yield.
|
Entry
|
Catalyst (g)
|
Solvent
|
Time (min)
|
Temp (ºC)
|
Yield (%) b
|
|
1 |
No Catalyst |
No solvent |
240 |
25 |
No product |
|
2 |
0.005 |
EtOH |
120 |
Reflux |
85 |
|
3 |
0.005 |
H2O |
120 |
Reflux |
70 |
|
4 |
0.005 |
THF |
120 |
Reflux |
55 |
|
5 |
0.005 |
CCl4 |
120 |
Reflux |
48 |
|
6 |
0.005 |
CH3CN |
120 |
Reflux |
40 |
|
7 |
0.005 |
Solvent-free |
60 |
25 |
40 |
|
8 |
0.005 |
Solvent-free |
60 |
60 |
95 |
|
9 |
0.005 |
Solvent-free |
60 |
70 |
95 |
|
10 |
0.004 |
Solvent-free |
30 |
60 |
93 |
|
11 |
0.003 |
Solvent-free |
30 |
60 |
90 |
|
12 |
0.003 |
Solvent-free |
30 |
70 |
93 |
|
13 |
0.002 |
Solvent-free |
60 |
60 |
75 |
Table 2
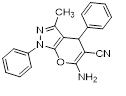
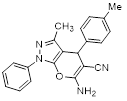
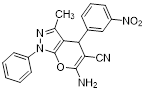
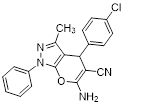
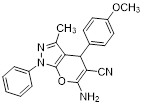
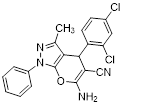
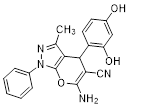
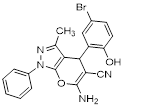
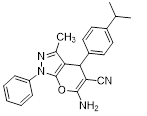
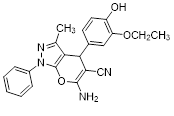
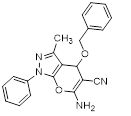
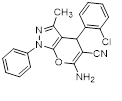
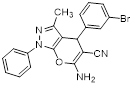
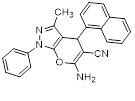
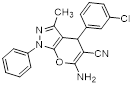
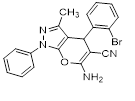
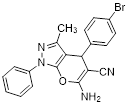
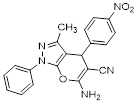
Synthesis of pyrano [2,3-c] pyrazoles derivatives 4a-r using SNPs-AT a
|
Entry |
Product |
Time (min) |
Yield (%)b |
Mp. (ºC) [lit] |
|
4a |
|
15 |
97 |
172-173 (171-173) [22]
|
|
4b |
|
25 |
86 |
175-177 (176-177) [22] |
|
4c |
|
20 |
88 |
190-192 (190-192) [22] |
|
4d |
|
20 |
90 |
171-173 (172-174) [23] |
|
4e |
|
25 |
90 |
174-175 (174-175)[23] |
|
4f |
|
30 |
92 |
186-188 (185-186)[23] |
|
4g |
|
40 |
80 |
322-325 (320-322)[6] |
|
4h |
|
50 |
90 |
316-318 (314-315)[6] |
|
4i |
|
35 |
88 |
169-170 (169-170)[23] |
|
4j |
|
30 |
80 |
169-171 (169-171)[23] |
|
4k |
|
25 |
89 |
160-162 (160-161)[23] |
|
4l |
|
35 |
80 |
144-146 (144-146)[23] |
|
4m |
|
30 |
80 |
159-161 (159-160)[24] |
|
4n |
|
30 |
92 |
159-160 (159-160)[25] |
|
4o |
|
25 |
85 |
158-159 (157-159)[26] |
|
4p |
|
30 |
82 |
166-168 (166-167)[26] |
|
4q |
|
30 |
89 |
183-185 (183-184)[23] |
|
4r |
|
20 |
94 |
193-195 (194-196)[23] |
aReaction conditions: 3-methyl-1-phenyl-2-pyrazolin-5-one (1 mmol), arylaldehyde (1 mmol), malononitrile (1.25 mmol), and SNPs-AT (0.003 g), solvent-free, 60 °C.
bIsolated yields.
Figure 1
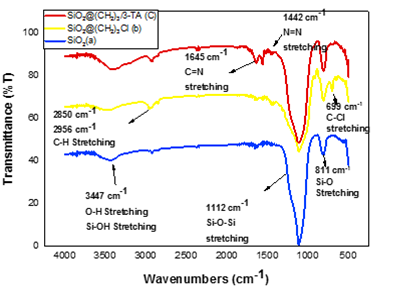
Figure 1: The FT-IR spectrum of SiO2 (a), SiO2@(CH2)3Cl (b), SNPs-AT (c).
Figure 2
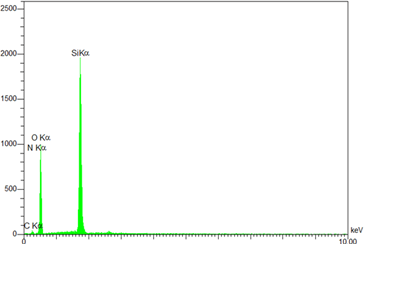
Figure 2: EDX spectrum a of SNPs-AT.
Figure 3
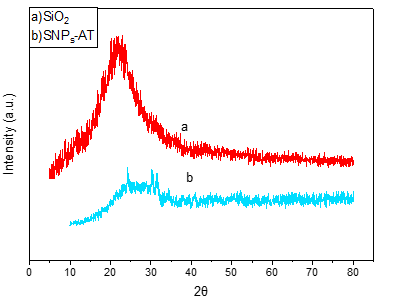
Figure 3: XRD patterns of SiO2 (a), SNPs-AT (b).
Figure 4
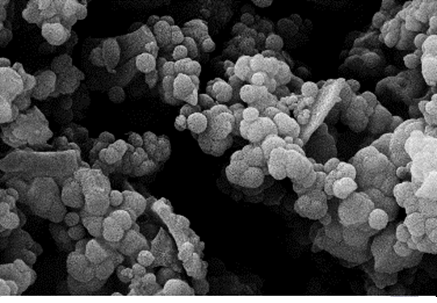
Figure 4: Fe-SEM image of SNPs-AT.
Figure 5
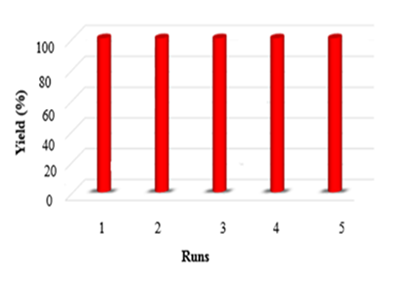
Figure 5: Reuse of the catalyst in the synthesis of pyrano [2,3-c] pyrazole derivatives.
Schema 1
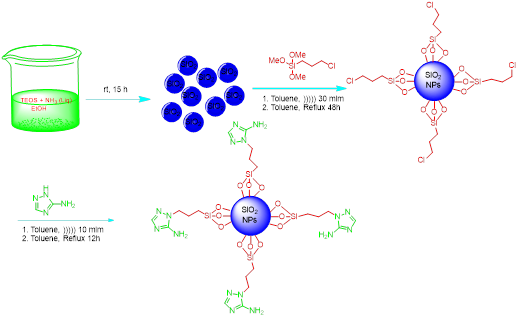
Scheme 1: Preparation of (NSAT) experimental
Schema 2
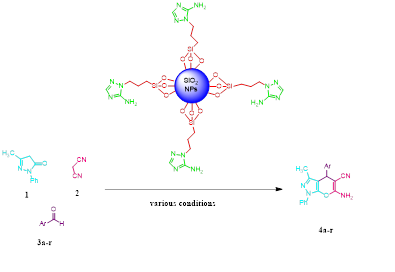
Scheme 2: Synthesis of pyrano [2,3-c] pyrazoles derivatives using (NSAT).
Schema 3
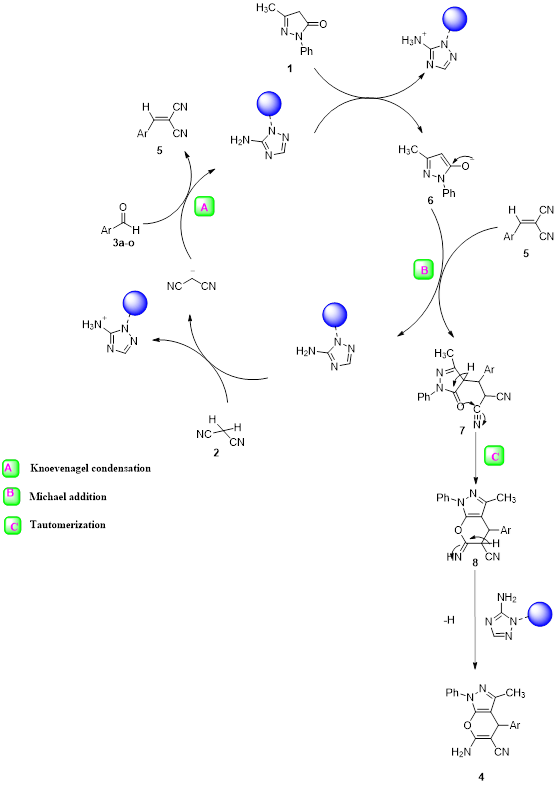
Scheme 3: An acceptable mechanism for reaction
Experimental
Materials and Methods
Tetraethyl orthosilicate (TEOS), 3-chloropropyl tri methoxy silane (CPTMO), 3-amino-1H-1,2,4-triazole (3-AT), and another chemical material in this research were buying from Fluka and Merck chemical companies in high-level purity. The reaction progress was controlled by utilizing thin layer chromatography (TLC) (silica-gel SIL G/UV254 plates, n-Hexane/ EtOAc: 2/1). Melting points were determined by a KRUSS model measuring device. 1H NMR spectra were recorded with a Bruker 400 Ultrashield and 13C NMR spectra were recorded at 100 MHz, with DMSO-d6 as the solvent and TMS as the internal standard. Fourier transform infrared (FT-IR) spectra were taken with a JASCO FT-IR/680 plus spectrometer, using KBr pills. Energy dispersive spectroscopy (EDS) was recorded exploitation TESCAN Vega model instrument. Energy dispersive X-ray (XRD) spectroscopy was performed using a Bruker AXS (D8 Advance) model instrument, with Cu-Ka radiation (λ = 0.15418 nm). The measurement in 2θ ranging from 10º to 80º at the rate of 0.05º min-1 was done. Field Emission Scanning Electron Microscopy determination for the particle size and morphology of the samples through a MIRA3TESCAN-XMU FE-SEM instrument under an acceleration voltage of 26 kV was observed.
A general manner for preparation of SiO2 nanoparticle. The SiO2 nanoparticles applied in this process are prepared using the sol-gel method. These nanoparticles were made by mixing TEOS (6.2 ml) with ethanol (100 ml) as the solvent and ammonium hydroxide (6.5 ml) as a catalyst to create alkaline conditions under magnetic stirring for 15 h at room temperature. The mixture was filtered by Centrifuge (4000 rpm, 30 min), washed three times with ethanol solution and water, and then white powder dried at 60 °C for 12 h in the vacuum [27].
Method for the synthesis of 3-chloropropyl-SiO2 NPs. (1g) SiO2 nanoparticles were dispersed in dried toluene (60 mL) by ultrasonic for 15 min. Then, in the reflux method, 3-chloropropyl tri methoxy silane (2 ml) was added dropwise into a round bottom flask under moderate stirring and argon atmosphere for 24 h. Finally, the mixture was cooled to room temperature, separated by Centrifugation (4000 rpm, 10 min), and washed with water and ethanol several times. The 3-chloropropyl-SiO2 NPs were dried at 80 °C for 6 h in the vacuum [13].
Synthesis of 3-amino 1H?1,2,4?triazole (3-AT) -3-chloropropyl- SiO2 NPs. 3-Amino-1H?1,2,4?triazole (3-AT) (0.42 g, 5 mmol) in the presence of K2CO3 (0.69 g, 5 mmol) was added into a suspension of SiO2@(CH2)3Cl powder (1.0 g) and dry toluene (30 mL) under stirring and refluxed at 110°C for 12 h. After completion of the reaction, the mixture was filtered and the obtained powder washed several times with ethanol and water, then the solid powder was dried at 100 °C in the Vacuum for 24 h.
The general procedure for the synthesis of pyranopyrazole derivatives using SiO2@ (CH2)3- (3-AT)(SNPs-AT) as nanocatalyst. Aryl aldehydes (1 mmol), 3-methyl-1-phenyl-2-pyrazoline-5-one (1mmol), and malononitrile (1.25 mmol) in the presence (0.003 g) of SNPs-AT catalyst were combined under solvent-free conditions and heated at 60°C in an oil bath. The reaction completion was followed using TLC (n?hexane: ethyl acetate, 2:1). At the end of the reaction, the mixture was cooled to room temperature, then hot ethyl acetate (5 mL) was added, and the catalyst was collected by Centrifuge instrument. Extra purification and recrystallization were obtained by adding hot ethanol. Several selected spectral data of compounds (4c-4h) are given below.
6-amino-3-methyl-4-(3-nitrophenyl)-1-phenyl-1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile (4c). Mp. 190–192°C, White crystals. IR spectrum, ?, cm–1:3454, 3359 (NH2), 2923 (CH arom.), 2854 (CH, CH3), 2190 (CN), 1653, 1594 (C=N), 1446, 1351, 1267, 1184, 1069 (C-O, CN).
6-amino-4-(4-chlorophenyl)-3-methyl-1-phenyl-1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile (4d). Mp. 171–173°C, White crystals. IR spectrum, ?, cm–1:3454, 3330 (NH2), 3068 (CH arom.), 2883 (CH, CH3), 2195 (CN), 1655, 1594 (C=N), 1455, 1396, 1285, 1177, 1069 (C-O, CN), 692 (C-Cl).
6-amino-4-(4-methoxyphenyl)-3-methyl-1-phenyl-1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile (4e). Mp. 174–175 °C, White crystals. IR spectrum, ?, cm–1: 3392, 3321 (NH2), 3023 (CH arom.), 2883 (CH, CH3), 2197 (CN), 1658, 1590 (C=N), 1455, 1392, 1250, 1173, 1128 (C-O, CN).
6-amino-4-(2,4-dichlorophenyl)-3-methyl-1-phenyl-1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile (4f). Mp. 186–188 °C, White crystals. IR spectrum, ?, cm–1: 3457, 3324 (NH2), 3023 (CH arom.), 2883 (CH, CH3), 2191 (CN), 1660, 1595 (C=N), 1469, 1394, 1267, 1167, 1126 (C-O, CN), 756 (C-Cl).
6-amino-4-(2,4-dihydroxyphenyl)-3-methyl-1-phenyl-1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile (4g). Mp. 320–322°C, White crystals. IR spectrum, ?, cm–1:3424, 3309, 3183 (OH, NH2), 2923 (CH arom.), 2854 (CH, CH3), 2211 (CN), 1590, 1511 (C=N), 1400, 1351, 1255, 1189, 1045 (C-O, CN), 842, 800. 1H NMR spectrum (DMSO-d6), δ, ppm: 9.68 s (1H, OH), 7.92 s (1H, OH), 7.76 s (2H), 7.52–7.75 m (2H), 7.25–7.27 m (2H), 6.89 s (2H, NH2), 6.45 d (J = 4 Hz, 2H), 6.32 s (1H), 5.53 s (1H, CH), 1.82 s (3H, ring CH3). 13C NMR spectrum, δ, ppm: 166.6, 162.2, 161.9, 159.7, 155.8, 153.6, 138.2, 126.3, 123.1, 117.1, 114.3, 108.8, 107.1, 103.8, 101.3, 75.7, 42.1, 26.1. Anal. Calcd. for C20H16N4O3: C, 66.66; H, 4.48; N, 15.55. Found: C, 66.58; H, 4.53; N, 15.60.
6-amino-4-(5-bromo-2-hydroxyphenyl)-3-methyl-1-phenyl-1,4-dihydropyrano[2,3-c]pyrazole-5 carbonitrile (4h). Mp. 314–315°C, Yellow crystals, IR spectrum, ?, cm–1: 3455, 3347, 3212 (OH, NH2), 2935 (CH arom.), 2210 (CN), 1646, 1608, 1554 (C=N), 1477, 1388, 1280 (CN), 1222, 1087 (C-O), 802, 470 (C-Br). 1H NMR spectrum (DMSO-d6), δ, ppm: 8.71 s (1H, OH), 7.71 d (J=4 Hz, 2H), 7.52 t (J=4 Hz, 2H), 7.36 s (1H), 7.27–7.30 m (2H), 6.83–6.93 m (2H, NH2), 6.61 d (J= 4 Hz,1H), 5.46 s (1H, CH), 2.04 s (3H, CH3). 13C NMR spectrum, δ, ppm: 162.9, 162.3, 158.2, 158.1, 151.6, 140.9, 137.2, 127.6, 121.3, 118.3, 118.0, 118.0, 117.2, 116.7, 97.6, 75.2, 47.5, 23.8. Anal. Calcd. for C20H15BrN4O2: C, 56.75; H, 3.57; N, 13.24. Found: C, 56.70; H, 3.51; N, 13.29.
Conclusion
In this study, SNPs-AT as a novel, eco-friendly and efficient nanocatalyst was synthesized. The characterization and catalytic activity were done by FT-IR, EDS, XRD, Fe-SEM analysis. SNPs-AT has used for the synthesis of pyrano [2,3-c] pyrazoles derivatives. This procedure has unique advantages including excellent yields, reusability of the catalyst, short reaction times, simple protocol, that cause the present methodology will be effective.
Acknowledgments
We gratitude to thank the research council of the Yasouj University of Iran for the financial support of this project.
References
- Wilson K, Clark JH. Solid acids and their use as environmentally friendly catalysts in organic synthesis. Pure Appl. 2000; 72: 1313-1319.
- Amarloo, F, Zhiani R, Mehrzad J. Novel Fe3O4/SiO2/PPA Magnetic Nanoparticles: Preparation, Characterization, and First Catalytic Application to the Solvent- Free Synthesis of Tetrahydrobenzo [a]xanthene-11-ones. Russ J Org. 2019; 55: 1584-1590.
- Karami B, Kiani M, Hosseini SJ, Bahrami M. Synthesis and characterization of novel nanosilica molybdic acid and its first catalytic application in the synthesis of new and known pyranocoumarins New. J Chem. 2015; 39: 8576-8581.
- Karami B, Ghashghaee V, Khodabakhshi S. Novel silica tungstic acid (STA): Preparation, characterization and its first catalytic application in synthesis of new benzimidazoles. Catal Commun. 2012; 20: 71-75.
- Biricik H, Sarier N. Comparative study of the characteristics of nano silica - , silica fume - and fly ash - incorporated cement mortars. Mater Res. 2014; 17: 570-582.
- Gholtash JE, Farahi M. Tungstic acid-functionalized Fe3O4@TiO2: preparation, characterization and its application for the synthesis of pyrano[2,3-c]pyrazole derivatives as a reusable magnetic nanocatalyst. RSC Adv. 2018; 8: 40962-40967.
- Nasr MN, and Gineinah MM. Pyrido [2, 3-d] pyrimidines and Pyrimido[5’,4’:5, 6]pyrido[2, 3-d]pyrimidines as New Antiviral Agents: Synthesis and Biological Activity. Archiv der Pharmazie. 2002; 335: 289-295.
- Mishra M, Nizam A, Jomon, KJ, Tadaparthi, K. A New Facile Ultrasound-Assisted Magnetic Nano-[CoFe2O4]-Catalyzed One-Pot Synthesis of Pyrano[2,3-c]pyrazoles. J Org Chem. 2019; 55: 1925-1928.
- Moosavi-Zare AR, Zolfigol MA, Mousavi-Tashar A. Synthesis of pyranopyrazole derivatives by in situ generation of trityl carbocation under mild and neutral media Res. Chem Intermed. 2016; 42: 7305-7312.
- Eskandari K, Karami B, Khodabakhshi S. Eskandari. Catal Commun. 2014; 54: 124-130.
- Khosravian F, Karami B, Farahi M. Synthesis and characterization of molybdic acid immobilized on modified magnetic nanoparticles as a new and recyclable catalyst for the synthesis of chromeno[4,3-b]chromenes. New J Chem. 2017; 41: 11584-11590.
- Karami B, Farahi M, Akrami S, Elhamifar D. Functionalized MCM-41: Versatile Catalysts for Organic Transformations. New J Chem. 2018; 42: 12811-12816.
- Akrami S, Karami B, Farahi M. Preparation and characterization of novel phthalhydrazide-functionalized MCM-41 and its application in the one-pot synthesis of coumarin-fused triazolopyrimidines. RSC adv. 2017; 7: 34315-34320.
- Garkoti C, Shabir J, Mozumdar S. An imidazolium based ionic liquid supported on Fe3O4@SiO2 nanoparticles as an efficient heterogeneous catalyst for N-formylation of amines. New J Chem. 2017; 41: 9291-9298.
- Nasrollahzadeh M, Sajjadi M, Khonakdar HA. Synthesis and characterization of novel Cu(II) complex coated Fe3O4@SiO2 nanoparticles for catalytic performance. J Mol Struct. 2018; 1161: 453-463.
- Azarshin S, Moghadasi J, AAboosadi, Z. Surface functionalization of silica nanoparticles to improve the performance of water flooding in oil wet reservoirs. Energy Explor Exploit. 2017; 35: 685-697.
- Nasseri MA, Zakerinasab B, Samieadel MM. Sulfamic acid supported on Fe3O4@SiO2 superpara magnetic nanoparticles as a recyclable heterogeneous catalyst for the synthesis of quinolones. RSC Adv. 2014; 4: 41753-41762.
- Wang J, Zheng S, Shao Y, Liu J, Xu Z, Zhu D. Amino-functionalized Fe3O4@SiO2 core–shell magnetic nanomaterial as a novel adsorbent for aqueous heavy metals removal. J Colloid Interface Sci. 2010; 349: 293-299.
- Márquez F, Herrera GM, Campo T, Cotto M, Ducongé J, Sanz JM, et al. Preparation of hollow magnetite microspheres and their applications as drugs carriers. Nanoscale Res Lett. 2012; 7: 210.
- Khazaei A, Zolfigol MA, Moosavi-Zare AR, Zare A, Khojasteh M, Asgari Z, et al. Organocatalyst trityl chloride efficiently promoted the solvent-free synthesis of 12-aryl-8,9,10,12-tetrahydrobenzo[a]-xanthen-11-ones by in situ formation of carbocationic system in neutral media. Catal Commun. 2012; 20: 54-57.
- Imanzadeh GH, Zare A, Khalafi-Nezhad A, Hasaninejad A, Zare AM, Parhami A. Microwave-assisted Michael addition of sulfonamides to α, β-unsaturated esters: A rapid entry to protected β-amino acid synthesis. J Iran Chem Soc. 2007; 4: 467-475.
- Jin TS, Zhao RQ, Li TS. An one-pot three-component process for the synthesis of 6-amino-4-aryl-5-cyano-3-methyl-1-phenyl-1,4-dihydropyrano[2,3-c]pyrazoles in aqueous media (06-1864DP) Arkivoc. 2006; 11: 176-182.
- Reddy GM, Raul Garcia J. Synthesis of Pyranopyrazoles under Eco-friendly Approach by Using Acid Catalysis. J Heterocycl Chem. 2017; 54: 89-94.
- Niknam K, Borazjani N, Rashidian R, Jamali A. Silica-bonded N-propylpiperazine sodium n-propionate as recyclable catalyst for synthesis of 4H-pyran derivatives. Chin J Catal. 2013; 34: 2245-2254.
- Farahi M, Karami B, Sedighimehr I, Tanuraghaj HM. An environmentally friendly synthesis of 1,4-dihydropyrano[2,3-c]pyrazole derivatives catalyzed by tungstate sulfuric acid. Chinese Chem Lett. 2014; 25: 1580-1582.
- Aliabadi RS, Mahmoodi NO. Green and efficient synthesis of pyranopyrazoles using [bmim][OH−] as an ionic liquid catalyst in water under microwave irradiation and investigation of their antioxidant activity RSC adv. 2016; 6: 85877-58884.
- Zhang YP Lee, SH Reddy, Gopalan AI, Lee KP. Synthesis and characterization of core-shell SiO2 nanoparticles/poly (3-aminophenylboronic acid) composites. J Appl Polym Sci. 2007; 104: 2743-2750.