Printed Carbon-Based Electrochemical Sensor for Sensitive Diuron Detection in Water Quality Assessment
- 1. Natural Sciences Department, Federal University of São João del Rei, Praça Dom Helvécio 74, São João del Rei 36301-160, MG, Brazil
Abstract
This work developed a printed electrochemical sensor for the detection of diuron, a persistent and toxic herbicide, utilizing the screen-printing technique and polyethylene terephthalate (PET) as the support material. The sensor employs a carbon conductive ink composed of graphite (1.875 g), carbon black (0.625 g), and nail polish (2.650 g) for electrode printing. The SPCE sensor was modified with Metallic Nanoparticles (MNP) and characterized using UV-Vis spectroscopy. The modification with gold nanoparticles (AuNPs) increased the electroactive area of the sensor, enhancing its electrochemical performance. The materials used and the fabricated sensor were characterized by Cyclic Voltammetry (CV) and Differential Pulse Voltammetry (DPV). The optimization of experimental conditions, such as the amount of AuNPs used in the modification, pH, and ionic strength of the supporting electrolyte, contributed to an increased sensitivity of the sensor, resulting in a low detection limit (0.15 µmol L-1). The sensor was employed in the determination of diuron, whose presence in aqueous matrices is concerning due to its persistence and toxicity. The SPCE-MNP-Au sensor showed high selectivity in the determination of the herbicide, with a Relative Standard Deviation (RSD) of 4.8%, no matrix effect, and recoveries between 95% and 97.9% in river water samples, allowing us to conclude that the sensor is suitable for the detection of diuron in environmental samples, such as water.
Keywords: Printed electrochemical sensor; Carbon black; AuNPs; Diuron; River water
Abbreviations: PET: Polyethylene Terephthalate; SPCE: Screen-Printed Carbon Electrode; MNP: Metallic Nanoparticles; AuNPs: Gold Nanoparticles; AgNPs: Silver Nanoparticles; CV: Cyclic Voltammetry; DPV: Differential Pulse Voltammetry; RSD: Relative Standard Deviation
Introduction
Herbicides are chemical substances applied in cultivated areas to inhibit the growth of weeds, increasing productivity and reducing production costs [1,2]. However, diuron (N-(3,4-dichlorophenyl)-N-dimethylurea), like other herbicides, although effective and used in various crops, has a long persistence in the soil, leading to environmental contamination, mainly through leaching into surface and groundwater [3,4].
Due to its high persistence and genotoxicity, continuous monitoring of diuron levels in aqueous matrices is fundamental [5]. Although traditional analytical techniques such as atomic absorption spectrometry, high-performance liquid chromatography, and fluorimetry are used, electrochemical methods emerge as promising alternatives due to their high sensitivity, simplicity, good reproducibility, and capacity for modification to detect diuron in complex samples [6].
Screen-printing, a promising printing technique, has demonstrated great potential in various analytical devices for environmental monitoring. It allows the production of standardized electrodes on various substrates, combined with ease of modification. This facilitates integration into portable and low-cost devices, enhancing the performance of electrochemical sensors [7,8].
Metallic nanoparticles, with their large surface area and catalytic properties, offer great potential for modifying printed sensors, significantly expanding their detection capability. Combining these nanoparticles with screen-printing technology enables the creation of customized and low-cost detection devices, with applications in various areas, from health to the environment [9,10].
Moço et al. [11] developed a screen-printed sensor with a conductive ink based on graphite, carbon black, vinyl, and butyl glycol acetate modified with 4-aminothiophenol (4-ATP) and gold nanoparticles for HIV RNA detection. The 4-ATP monolayer improved the sensor's electronic properties and constituted an anchoring network for thiolate-probe- functionalized gold nanoparticles. The constructed sensor showed good stability, sensitivity, specificity, and selectivity for viral RNA detection.
Qin et al. [12] developed an electrochemical sensor built from a Glassy Carbon Electrode (GCE) modified with a membrane composed of Nafion, Gold Nanoparticles (AuNPs), and electrochemically Reduced Graphene Oxide (rGO). The combination of rGO and AuNPs provided a synergistic effect, resulting in high diuron absorption capacity and high interfacial conductivity. This configuration allowed the detection of diuron in samples such as pear juice, tea, and mineral water, with a low detection limit, high selectivity, and stability.
In this sense, the present work aimed to evaluate the performance of an electrochemical sensor printed by the screen-printing method, modified with metallic nanoparticles, for the quantitative determination of diuron in river water samples for environmental monitoring.
Materials and methods
Reagents
Graphite powder (98% purity), acetone, nitric acid, acetic acid, boric acid, sodium phosphate dibasic heptahydrate, sodium phosphate monobasic monohydrate, sodium acetate, sodium carbonate, and magnesium sulfate were obtained from Synth®. Diuron, azithromycin, imidacloprid, silver Nitrate, Sodium Borohydride (NaBH4), sodium tetrachloroaurate (NaAuCl4), acetic acid, boric acid, and sodium hydroxide were acquired from Sigma-Aldrich®. TRIS-HCl was acquired from Dinâmica®. Sodium citrate (Na3C6H5O7) was acquired from Cinética® and methanol from Vetec®. Phosphoric acid, potassium ferricyanide K3[Fe(CN)6], and potassium ferrocyanide (K4[Fe(CN)6]) were obtained from Neon®. Carbon black was acquired from Cabot®. Silver powder was obtained from Purex ACS Científica®. Colorless nail polish from Cora®. A4 sheet/transparent crystal polyethylene terephthalate (PET) from BWB Embalagens. All reagents used were of analytical grade, diuron stock solutions were prepared in methanol, and buffer solutions were prepared in water purified by the Millipore Milli-QAs system.
Instrumentation
Electrochemical measurements were performed using an Autolab PGSTAT204 potentiostat/galvanostat and a FRA32M impedance module. The electrochemical techniques employed were cyclic voltammetry (CV) and differential pulse voltammetry (DPV). Data were acquired and analyzed using Nova 2.1 software and subsequently processed and visualized in OriginPro 8 software. The synthesized nanoparticles were characterized by UV-Vis spectra recorded on a UV-2550 spectrophotometer (SHIMADZU), in a wavelength range of 300–800 nm.
Development of the printed electrode
The disposable electrodes, called SPCEs, were fabricated by screen-printing. The molds were made on a Visutec MVSK800 cutting plotter and fixed on PET sheets. A conductive ink containing graphite (1.875 g), carbon black (0.625 g), and colorless nail polish (2.650 g) dispersed in acetone (5 mL) was applied to the molds. An insulating layer of colorless nail polish was applied to delimit the geometric area of the electrode. The silver ink for the quasi-reference electrode was prepared as described in the literature [13].
Synthesis of silver nanoparticles (AgNPs) and gold nanoparticles (AuNPs)
Silver nanoparticles were synthesized by chemical reduction of silver nitrate using sodium borohydride as a reducing agent. Solutions of silver nitrate (0.67 mmol L-1) and sodium citrate (0.67 mmol L-1) were mixed in equal parts (45 mL) and kept in an ice bath. Sodium borohydride, 10 mL (27 mmol L-1), was added dropwise under constant stirring, resulting in the formation of a colloidal dispersion with the characteristic yellow color of AgNPs [14].
AuNPs were synthesized by adapting the Turkevich method [15]. Sodium tetrachloroaurate (NaAuCl?, 0.125 mol L?¹) was heated in water (90°C) under constant stirring, and sodium citrate (Na?C?H?O?, 10 mg mL?¹) was added to reduce the gold ions and stabilize the nanoparticles. The reaction was maintained at 90°C for 20 minutes to ensure complete formation of the AuNPs. Both dispersions were stored under refrigeration.
Table 1
Table 1: Comparison of the analytical performance proposed by SPCE-MNP-Au with other electrochemical sensors for diuron determination
|
Proposed Sensor |
Technique |
Linear Range (μmol L-1) |
Detection Limit (μmol L-1) |
Reference |
|
GCE/rGO |
BIA-AMP |
5.0 - 50.0 |
0.36 |
[19] |
|
NC-CPE |
SWV |
4.2 - 47.0 |
0.35 |
[20] |
|
ZnONPs-CPE |
SWV |
1.3 - 7.7 |
0.22 |
[21] |
|
BaO-MWCNT/MCPE |
AMP |
1.0 - 10.0 |
0.31 |
[22] |
|
SPCE-MNP-Au |
DPV |
0.5 - 10.0 |
0.15 |
This work |
Abbreviations: GCE/rGO: Glassy Carbon Electrode modified with reduced graphene oxide; NC-CPE: nanocellulose modified carbon paste electrode; ZnONPs-CPE: Zinc Oxide Nanoparticles Modified Carbon Paste Electrode; BaO-MWCNT/MCPE: Barium Oxide Decorated Multiwalled Carbon Nanotube Modified Carbon Paste Electrode
Table 2
Table 2: Determination of diuron in river water samples
|
Added Concentration (μmol L-1) |
Concentration Detected ± RSD (μmol L-1) |
Recovery (%) |
|
0.0 |
Nd |
- |
|
2.0 |
1.45 ± 0.67 |
96.4 |
|
5.0 |
4.62 ± 0.90 |
97.9 |
|
8.0 |
6.7 ± 0.57 |
95.0 |
Abbreviations: RSD: Relative Standard Deviation; Nd: Not detected, considering the method's detection limit
Figure 1
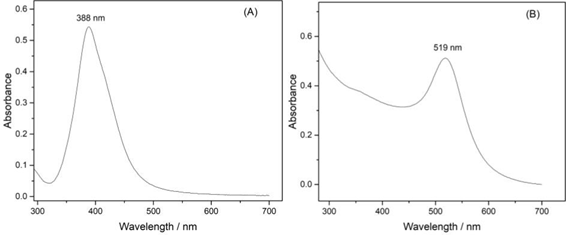
Figure 1: UV-VIS spectra of colloidal dispersions of (A) – AgNPs and (B) – AuNPs.
Figure 2
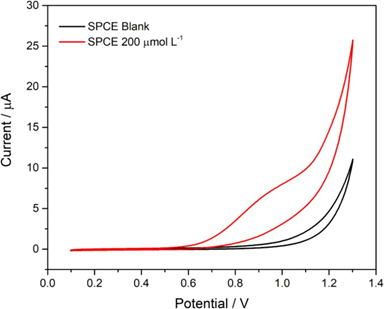
Figure 2: Cyclic voltammograms obtained for the unmodified printed electrode (SPCE). Reaction medium: 0.1 mol L-1 phosphate buffer, pH 7.00, in the presence of 200 µmol L-1 diuron. Scan rate 50 mVs-1.
Figure 3
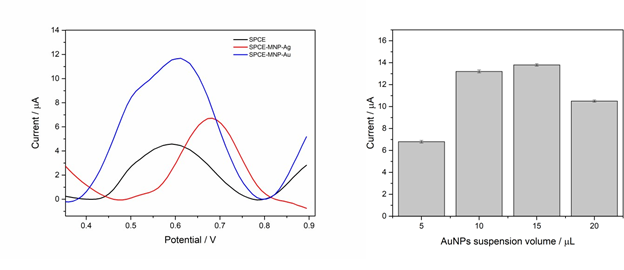
Figure 3: (A) – Differential pulse voltammograms obtained for different configurations of the printed working electrode. (B) – Variation of the anodic peak current about the volume of the AuNPs suspension used in the electrode. Reaction medium: 0.1 mol L-1 phosphate buffer, pH 7.00, containing 200 µmol L-1 diuron. Operating conditions: pulse amplitude 40 mV, scan rate 20 mV s-1.
Figure 4
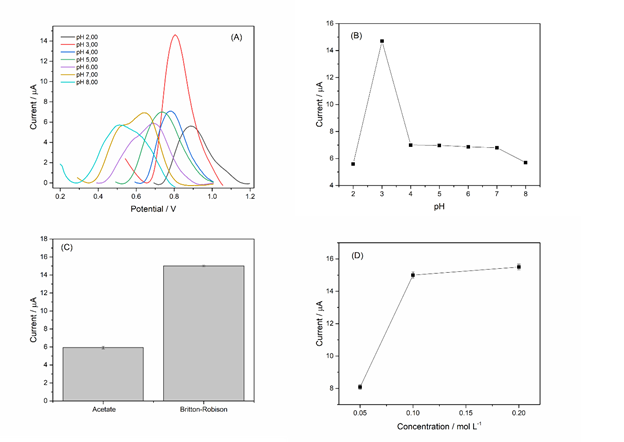
Figure 4: (A) – Influence of pH on the response of the proposed sensor. (B) – Bar graph of anodic current variation for pH. (C) – Influence of different buffer solutions on the anodic peak current variation of diuron. (D) – Influence of ionic strength on the anodic peak current of diuron. Reaction medium: 200 µmol L-1 diuron. Operating conditions: pulse amplitude 40 mV, scan rate 20 mV s-1.
Figure 5
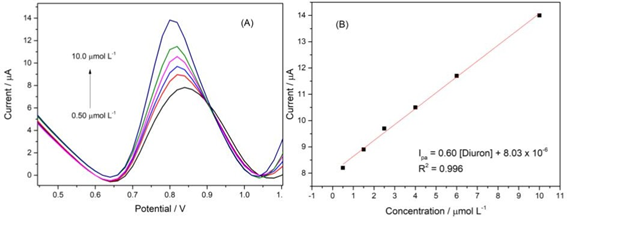
Figure 5: (A) – Differential pulse voltammograms obtained with successive additions of diuron. (B) – Analytical curve obtained from the voltammograms. Reaction medium: 0.1 mol L-1 Britton-Robinson buffer, pH 3.00. Operating conditions: pulse amplitude 60 mV, scan rate 40 mV s-1.
Figure 6
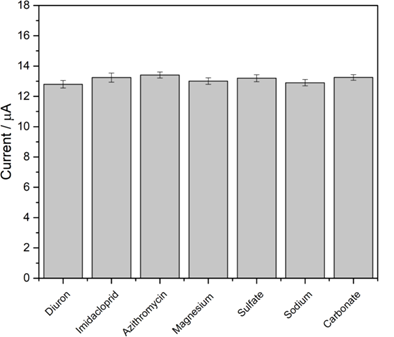
Figure 6: Study of possible interferents for the proposed method. Reaction medium: 0.1 mol L-1 Britton-Robinson buffer, pH 3.00, containing 8.0 µmol L-1 diuron. Operating conditions: pulse amplitude 60 mV, scan rate 40 mV s-1.
Scheme 1
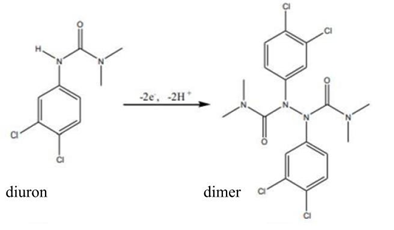
Scheme 1: Diuron oxidation process through an electrochemical method.
Results and Discussion
Spectroscopy UV-Vis
The absorption spectra in the UV-Vis region of AgNPs (Figure 1A) and AuNPs (Figure 1B) show a characteristic absorption peak at 388 nm and 519 nm, respectively. Each peak is attributed to the Surface Plasmon Resonance (SPR) of the metallic nanoparticles, which results in intense light absorption at a specific wavelength, confirming the successful formation of AgNPs and AuNPs. The position and intensity of these peaks are consistent with the literature, indicating an average particle size between 10 and 15 nm [16].
Electrochemical behavior of the printed electrode for diuron detection
The cyclic voltammogram obtained for diuron oxidation at the printed electrode (Figure 2) shows an oxidation peak at a potential of +0.85 V. The absence of a reduction peak in the cathodic scan suggests an irreversible electrochemical process, possibly involving the formation of a diuron dimer, favored by the high reactivity of the radicals formed after the loss of an electron by diuron (Scheme 1), as proposed in previous studies [17].
Study of the working electrode configuration in diuron oxidation
Figure 3A shows the differential pulse voltammograms obtained for diuron oxidation on different electrodes. The SPCE-MNP-Au electrode exhibited the highest anodic peak current intensity, approximately 158% higher compared to the unmodified SPCE electrode. These results suggest that gold nanoparticles increased the surface area and facilitated electron transfer, resulting in more sensitive diuron detection. Although the electrode modified with silver nanoparticles (SPCE-MNP-Ag) also showed electrocatalytic activity, its efficiency was lower, possibly due to the formation of oxidation products that block the active sites of the electrode. Therefore, the SPCE-MNP-Au electrode was selected for further optimizations.
The ideal volume of AuNPs deposited on the electrode was determined by analyzing the peak current variation (Figure 3B). Upon reaching a volume of 20.0 μL, there was a decrease in current, possibly due to the formation of a thick layer of nanoparticles that hinders electron transfer and leads to aggregation. The volume of 10.0 μL was chosen as the ideal, as it offered the best relationship between the amount of AuNPs and signal intensity, indicating a larger surface area for the diuron oxidation reaction, resulting in a sensor with high sensitivity and reproducibility.
Evaluation of pH and supporting electrolyte composition on diuron oxidation
Figure 4A and 4B illustrate the influence of the electrolytic solution's pH on the electrochemical behavior of diuron in a Britton-Robinson buffer solution. A pH of 3.00 provided the highest anodic current intensity, indicating that the kinetics of the oxidation reaction are favored at this pH. Therefore, it was selected as the optimal pH for the electroanalytical determination of diuron, as it provides the best sensitivity and reproducibility.
The evaluation of acetate and Britton-Robinson buffer solutions (0.1 mol L-1, pH 3.00) in the oxidation of diuron (Figure 4C) shows that the Britton-Robinson buffer provided a superior oxidation current intensity. This higher intensity is attributed to the Britton-Robinson buffer's ability to maintain a stable pH, create a favorable environment for oxidation, and have high ionic conductivity and the ability to support an acidic pH.
The ionic strength of the Britton-Robinson buffer solution at pH 3.00 significantly influences the electrochemical sensor's response in diuron detection (Figure 4D). Optimizing ionic strength is crucial, as an increase in buffer concentration can enhance conductivity and facilitate charge transport, but an excess of ions can cause compression of the electrical double layer, hindering electron transfer.
A concentration of 0.1 mol L?¹ demonstrated the highest anodic peak current. Lower concentrations, such as 0.05 mol L?¹, resulted in reduced oxidation currents, possibly due to the low ionic strength that compromises the stability of the electrical double layer and charge transport. On the other hand, higher concentrations, such as 0.2 mol L?¹, did not provide significant increases in current, suggesting that the ideal ionic strength was achieved with 0.1 mol L?¹.
Analytical curve
The analytical curve was constructed by successively adding aliquots of a standard diuron solution (Figure 5). The curve exhibits linearity in the concentration range of 0.5 to
10.0 μmol L-1, and the limits of detection (LOD) and quantification (LOQ) were estimated to be 0.15 μmol L-1 and 0.5 μmol L-1, respectively.
As shown in Table 1, it values for the linear range and LOD are close to those found in the literature. This demonstrates the high sensitivity of the proposed sensor, which is associated with the simplicity of manufacturing printed electrodes with gold nanoparticles. This produces costs and enables the use of disposable devices, surpassing other analytical methods in diuron detection [18]. This combination of factors makes the device suitable for applications in environmental analysis, where the demand for rapid and accurate methods is increasing.
Evaluation of sensor performance against possible interfering species
The selectivity of the printed sensor was evaluated in the presence of several substances that could potentially interfere with the analysis, including imidacloprid, azithromycin, Mg²?, Na?, SO?²?, and CO?²? ions. The results show that the addition of these species (concentration 100 times higher) to the solution containing diuron did not cause significant changes in the sensor's analytical signal (Figure 6). The maximum relative standard deviation observed was only 4.8%, indicating that the sensor maintains its accuracy and selectivity in the presence of compounds commonly found in water samples. This selectivity allows the sensor to be applied in the analysis of diuron in real environments, such as river water samples, without significant interference from the species evaluated.
Application of the method
The applicability of the SPCE-MNP-Au sensor was evaluated in the determination of diuron in real river water samples, using the standard addition method. Samples fortified with three different concentration levels of diuron were analyzed in triplicate. The results, presented in Table 2, reveal excellent recovery percentages, ranging from 95% to 97.9%. These results demonstrate the precision and accuracy of the proposed method for the quantification of diuron in matrices such as river water, demonstrating its application in environmental analyses.
Conclusion
A low-cost and highly sensitive electrochemical sensor was developed for the detection of diuron in water samples. The sensor, based on printed electrodes modified with gold nanoparticles (SPCE-MNP-Au), demonstrated excellent performance in the determination of the herbicide, with a low detection limit (0.15 µmol L-1) and high recovery in real samples (95% and 97.9%). The optimized experimental conditions, including the amount of nanoparticles, pH, and ionic strength of the supporting electrolyte, contributed to obtaining accurate (RSD = 4.8%) and reliable results. The selectivity of the sensor was confirmed by the absence of interference from other species that may be present in river water samples. The results obtained demonstrate the potential of the proposed sensor for environmental monitoring of diuron in river waters.
Acknowledgments
The authors gratefully acknowledge the support provided by Fundação de Amparo À Pesquisa do Estado de Minas Gerais (FAPEMIG), Conselho Nacional de Desenvolvimento Científico e Tecno-lógico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and INCT-DATREM.








































































































































