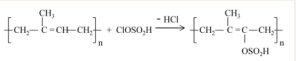Reaction Mechanism and Kinetics of Sulphonated Polyisoprene Elastomer for Proton Exchange Membrane
- 1. Department of Chemical Sciences, Edwin Clark University, Nigeria
ABSTRACT
The study on the reaction mechanism and kinetics of sulphonated polyisoprene elastomer for proton exchange membrane application was carefully carried out. Results obtained show that though the ion exchange capacity (IEC) and degree of sulphonation (DS) increase with sulphonation time, they were also found to be dependent on the concentration of acid used. While the lowest acid concentration (0.0004 mol/L) gave an IEC and DS of 0.4729 mmol/g and 1.278 % at 2 hrs of sulphonation, and 1.7550 mmol/g and 4.74 % at 18 hrs of sulphonation, the highest acid concentration (0.016 mol/L) used gave an IEC and DS of 2.7423 mmol/g and 7.41 % at 2 hrs of sulphonation and 10.711 mmol/g and 28.94 % at 18 hrs, respectively. Although with time, the rate of sulphonation gradually decreases especially after 12 hrs being the point of saturation of the polymer matrix with the acid. Similarly, the effect of temperature at constant concentration of acid (0.0016 mol/L) as investigated also showed that IEC and DS are temperature dependent as there was a 2fold increment in both IEC and DS. Synthesis of proton conductive membrane from polyisoprene in chlorosulphonic acid was found to involve an electrophilic substitution reaction, where the substitution reaction involves only the carbon at the double bond of each polyisoprene repeat unit. The kinetics of the reaction obeyed a first-order rate reaction with HCl produced as a by-product having a desulphonation effect as time of the reaction progressed. It was, however, noted that at higher temperature (> 333 K), the kinetics did not favour the rate law, and as such, sulphonation of polyisoprene should be carried out at temperature below 333 K.
KEYWORDS
• Sulphonation
• Polyisoprene
• Chlorosulphonic acid
• Ion exchange capacity
• Degree of sulphonation
CITATION
Christopher IA (2017) Reaction Mechanism and Kinetics of Sulphonated Polyisoprene Elastomer for Proton Exchange Membrane. Chem Eng Process Tech 3(2): 1041.
INTRODUCTION
The over dependency on fossil fuels, which are finite, particularly crude oil as a primary source of energy, has often time resulted into scarcity, high and unstable prices including energy crises. Aside, environmental pollution arising from the burning of these fossil fuels for energy has been a major challenge. Against this entire backdrop, efforts are committed towards getting alternative sources that are renewable, dependable, reliable and environmentally friendly. Fuel cell technology which involves electrochemical devices that convert chemical energy of the reactants directly into electrical energy [1] is now seriously considered in which, proton exchange membrane fuel cells (PEMFCs) amongst its counterparts is seen as the most promising because, it is efficient and potable power sources that is convenient for vehicular transportation, institutions, residences, home devices, mobile electronic devices and industrial applications [1-4]. All of this is attributed to their high-power density, making them compact and lightweight, rapid response to varying load, relatively quick start up, low operating temperature as well as environmental friendliness [3,5].
However, the central component of the PEMFCs which is the solid electrolyte membrane, derived from polymers and commonly referred to as the proton exchange membrane (PEM) has been a challenge. It functions as a conductor of protons generated at the anode and simultaneously opposing the direct contact between the fuel and the oxidant [6] and thus preventing fuel oxidation and cell over-potential. However, the current state-of-the-art PEM which is a perfluorinated ionomer Nafion®, although it exhibits high proton conductivity (σ ≥ 10-2 S cm-1) and excellent durability in fuel cell operating conditions (a life time of 50,000 hrs) [7] but, large scale application is hindered, particularly by its very high cost and monopoly. Other negative factors which militate against it include, high methanol permeation, and dehydration at high temperature (>80°C) [1] and thus leading to loss of conductivity. Hence efforts are seriously committed towards getting an alternative PEM for proton exchange membrane fuel cell. As a result, study on the modification of synthetic polyisoprene in chlorosulphonic acid medium was carried out via sulphonation, and was found to be proton conductive. This work, therefore, focuses on the reaction mechanism and kinetics of the sulphonation of polyisoprene in chlorosulphonic acid. Few kinetic studies of polymeric materials sulphonated in acid medium have been reported. For example, the kinetics of the sulphonation of polystyrene (PS) using concentrated sulphuric acid was reported by Akovali and Özkan [7,8], where they treated the phenyl ring of each repeat unit of the polymer as the substrate for the kinetics. The kinetics study of polystyrene butadiene rubber (PSBR) and polychlorprene (PCR) have also been carried out by Idibie et al. [9,10], and Idibie and Ogboru [11] where each of the repeat unit of the polymers were treated as the substrates for the kinetics. Others are the sulphonation of polyether-ether-ketone (PEEK) in the mixture of sulphuric acid and methane sulfuric acid, where only the phenyl ring flanked by two ether groups in the repeat unit that has only one of the four protons substituted by sulphuric acid group was considered as the substrate [12], and Baily et al., showed that the kinetics study of PEEK in the mixture of methane sulfuric acid and sulphuric acid, the degree of sulphonation was found to be a function of the fourth power of the sulphuric acid concentration [13].
Experimental
Materials and equipment: All the chemicals used in this study are of analytical grade (> 98 %). They are chlorosulphonic acid (Charlec, Nigeria), toluene (Steve Nicholas, Nigeria), ethanol (Charlec, Nigeria), commercial polyisoprene (Karbochem RSA), and a fabricated four neck reactor.
Sulphonation of polyisoprene elastomer with chlorosulphonic acid: This was done according to the method described by Idibie et al. [11], Here, 10 g of polyisoprene (PI) was dissolved in a beaker using 250 ml toluene. This was followed by the gradual addition (dropwise) of concentrated chlorosulphonic acid of different initial concentrations (0.0004, 0.0006, 0.0008, 0.0013, 0.0016 mol/L) that was initially chilled in an iced bath (in order to remove content heat) to toluene containing the PI solution undergoing a vigorously stirring in a four-neck round bottom flask reactor, under argon atmosphere at room temperature. The sulphonation reaction was allowed to run for 2, 4, 6, 9, 12, 15 and 18 hours, respectively. Thereafter the reaction was terminated with the addition of ethanol, where the precipitated sulphonated polyisoprene (SPI) was recovered and washed with deionised water until the pH of wash reached values of 6-7. Finally the sulphonated product was dried in an oven at about 80°C for 2–3 hours.
Determination of ion exchange capacity and degree of sulphonation: Percent measurement of sulphur attached to the oven-dried sample via elemental analysis was used to determine the ion exchange capacity (IEC) and degree of sulphonation (DS). From the elemental analysis of sulphur, the IEC was thus calculated using Equation (1) [14] below:
(1)
Where: Sc denotes the sulphur content in percentage weight rate, MWs is the molecular weight of sulphur and 1000 is the multiplication factor to have IEC value in mmol/g. The value of IEC obtained from Equation 1 was used to determine the degree of sulphonation of sulphonated polyisoprene (SPI) using the relationship as shown in Equation MWOSO H2 2:
(2)
Where: MWSPI = the molecular weight of the sulphonated polyisoprene, IEC = ion exchange capacity, and MWPI = the molecular weight of unsulphonated polyisoprene and MWOSO H2 = the molecular weight of OSO2 H
RESULTS AND DISCUSSION
Ion exchange capacity and degree of sulphonation
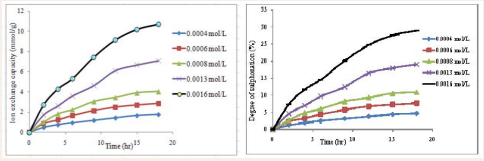
The ion exchange capacity of a membrane depends on the membrane’s acid concentration and is brought about via the sulphonation process. The acid concentration of the membrane is related to the amount of ionic groups in the membrane; hence it is referred to as the measure of the degree of sulphonation [15]. Thus ion exchange capacity (IEC) is an indicator of the proton conduction sites [16]. Results obtained from this study as presented in Figure 1
Figure 1: Ion exchange capacity and degree of sulphonation against time.
shows that though IEC and DS increase with time, they were also found to be dependent on the concentration of acid used, as the lowest acid concentration of 0.0004 mol/L gave an IEC and DS of 0.4729 mmol/g and 1.278 % at 2 hrs of sulphonation and 1.7550 mmol/g and 4.74 % at 18 hrs of sulphonation, while the highest acid concentration (0.016 mol/L) used gave an IEC and DS of 2.7423 mmol/g and 7.41 % at 2 hrs of sulphonation and 10.711 mmol/g and 28.94 % at 18 hrs, respectively. The increase in degree of sulphonation from the results shows that the content of the acid groups present in the polymer matrix is proportional to the ion exchange capacity of the resulting sulphonated polymer, which is known to increase with time [9], although it can also be observed from the shape of the curves that the rate of sulphonation gradually decreases with reaction time especially after 12 hrs being the point of saturation of the polymer matrix with the acid.
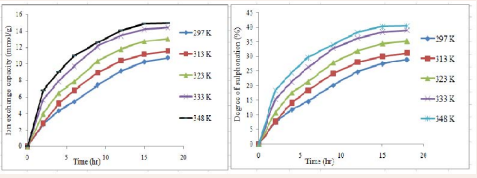
Similarly, the effect of temperature at constant concentration of acid (0.0016 mol/L) as investigated also showed that IEC and DS are temperature dependent (Figure 2).
Figure 2: Effect of temperature on IEC and DS at constant acid concentration (0.0016 mol/L).
At 297 K, IEC and DS are 2.7423 mmol/g and 7.41 % at 2 hrs, and at 348 K while IEC obtained was 6.8048 mmol/g DS was 18.39 %. However, increase in IEC and DS were recorded with increasing time, as 18 hrs gave an IEC of 10.71 mmol/g and DS of 28.94 at 297 K, and at 348 K an IEC of 14.9757 mmol/g and a DS of 40.47 % were obtained. In other words, there is about 2 fold increment in both IEC and DS with the effect of temperature.
Reaction mechanism of polyisoprene in chlorosulphonic acid
Figure 3
Figure 3: Reaction of polyisoprene in chlorosulphonic acid medium.
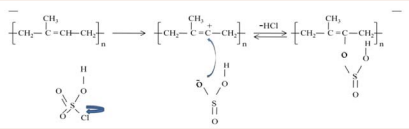
presents the reaction of polyisoprene with chlorosulphonic acid. The proposed reaction mechanism is that of electrophilic substitution as shown in Figure 4.
Figure 4: Proposed reaction mechanism of electrophilic substitution reaction of polyisoprene in chlorosulphonic acid medium.
The first stage involves the breaking of the S-Cl sigma (σ) bond to release chlorine atom. Following this is the second stage which is the carbocation (intermediate stage) as a result of attack of the nucleophile on the carbon atom, thereby displacing the hydrogen atom which eventually combines with Cl atom that broke away at the first stage. The third stage involves the stability of the molecule by the formation of the C-O sigma bond.
Sulphonation being an electrophilic substitution reaction involves the active site for substitution being determined mainly by the electron density of the site [17]. Considering Figure 4 for the substitution reaction, there is high electron density at the double bond and as such, the carbon at the double bond thus becomes a point for the substitution reaction. It can be assumed that only one -OSO2 H group can be attached to each of the repeat units of sulphonated polyisoprene (SPI). Taking this into consideration therefore, the following assumptions can be deduced:
1. Only one of the repeat units of polyisoprene (PI) can be sulphonated at a time.
2. The electrophilic substitution reaction involves only the carbon at the double bond.
3. Probable volume change is ignored.
4. By-product HCl could or could not have effect on the reaction. Based on the above stated assumptions, particularly taking into cognisant the 4th assumption, two reaction mechanisms are hereby proposed:
A. Sulphonation of PI with chlorosulphonic acid has HCl having no effect and hence no desulphonation.
B. Sulphonation of PI with chlorosulphonic acid has HCl having effect and hence there is occurrence of desulphonation.
Kinetic study of sulphonation: case A mechanism
First-order rate law is proposed;
Taking mass balance of the sulphonation of PI in the absence of desulphonation, then
(3)
Mass balance assuming a first-order reaction with respect to PI repeat unit concentration (C) in a batch reactor system with HCl not having effect
(4)
Where: t and k1 denote the reaction time and the rate constant, respectively. Integrating Equation (3) from the beginning of the reaction (C = C0 at t = 0) to a concentration (C at t = t), gives
(5)
(6)
Below Equation 7 describes the substrate concentration
(7)
Where X = reaction conversion
Substituting Equation 7 into Equation 6 gives
(8)
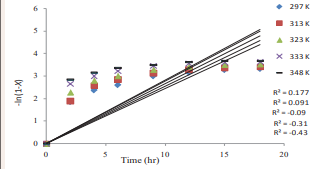
A first-order reaction without the effect of HCl on the sulphonation will give a plot of –l(1-X) against t a straight line otherwise HCl produced has effect on the sulphonation. But the test of –l(1-X) against t as presented in Figure 5
Figure 5: Kinetics of PI sulphonation in chlorosulphonic acid; firstorder rate in respect to non effect of HCl on the substrate concentration.
failed, as it couldn’t give a straight line. In other words, since chlorosulphonation of PI doesn’t obey mechanism A, it is therefore, not a firs-order reaction where HCl doesn’t have effect.
The likeliness of HCl having an effect or desulphonation effect on the substrate can now be considered. This brings us to testing mechanism B as proposed earlier.
Kinetic study of sulphonation: case B mechanism
Possible effect of HCl inducing desulphonation
(9)
Where: k2 and k3 are the rate constants for both forward and backward reactions, respectively.
Mass balance assuming a first-order reaction with respect to PI repeat unit concentration (C) in a batch reactor system having effect of HCl
(10)
Equation 10 is integrated to achieve:
(11)
(12)
Substituting substrate concentration in Equation (7) into Equation 12 gives
(13)
(14)
Therefore Equation (14) becomes
(15)
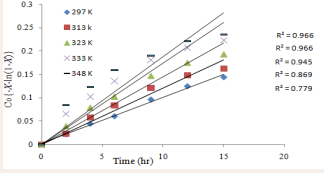
A plot of vs t (time) gives a straight lines as shown in Figure 6.
Figure 6: Kinetics of PI sulphonation in chlorosulphonic acid; firstorder rate in respect to effect of HCl on the substrate concentration.
It is interesting to note here that Figure 6 fits a straight line as each of the least square fits of the plots intersected (0,0), with a linear regression ( R2 ) values > 0.9 except for the last two (333 K [0.869] and 348 K [0.779]), which on overall, indicates that HCl produced has effect on the sulphonation of PI in chlorosulphonic acid by causing desulphonation, and thus, inducing reversibility of the process. This is in line with previous work carried out on some polymeric materials such as polystyrene butadiene rubber [9], polychloroprene [10], poly (Ether Ether Keton) PEEK) [17].
CONCLUSION
Synthesis of proton conductive membrane from polyisoprene using chlorosulphonic acid was found to involve an electrophilic substitution reaction, where the substitution reaction involves only the carbon at the double bond of each polyisoprene repeat unit. The kinetics of the reaction obeyed a first-order rate reaction with HCl produced as a by-product having a desulphonation effect as time of the reaction progressed. It was, however, noted that at higher temperature (> 333 K), the kinetics doesn’t favour the rate law, and as such, sulphonation of polyisoprene should be carried out at temperature below 333 K.
ACKNOWLEDGMENT
University of the Witwatersrand, Johannesburg, South Africa for their assistance
REFERENCES
- Gao Y, Gilles PR, Guiver MD, Jian X, Mikhailenko SD, Wang KaliaguineS. Sulfonation of poly (phthalazinones) with fuming sulfuric acid mixtures for proton exchange membrane materials. J Membr Sci. 2003; 227: 39-50.
- Steele BCH, Heinzel A. Materials for fuel-cell technologies. Nature. 2001; 414: 345-352.
- Quan P, Zhou B, Sobiesiak A, Liu Z. Water behavior in serpentine micro-channel for proton exchange membrane fuel cell cathode. J Pow Sour. 2005; 150: 131-144.
- Cheddie D, Munroe N. Review and comparison of approaches to proton exchange membrane fuel cell modeling. J Pow Sour. 2005; 147: 72-84.
- Shibasaki M, Yachi T, Tatsuo T. A new direct methanol fuel cell with azigzag folded membrane electrode assembly. J Pow Sour. 2005; 145: 477-484.
- Bai Y, Wu C, Wu F, Yi B. Carbon-supported platinum catalysts for onsite hydrogen generation from NaBH4 solution, Material letter. 2006; 60: 2236-2239.
- Akovali G, Özkan A. Converting waste polystyrene into a polymer flocculant for waste water treatment. Polymer. 1998; 27: 1277.
- Ralp TR. Proton exchange membrane fuel cells. Progress in cost reduction of the key components. Plantinum Metals Review. 1997; 41: 102-113.
- Idibie CA, Abdulkareem SA, Pienar HvZ, Iyuke SE, Lizelle VD. Mechanism and kinetics of sulphonation of polystyrene-butadiene rubber with chlorosulphonic acid. J Ind Eng Chem Res. 2010; 49: 1600-1604.
- Idibie CA, Ogboru RO. Kinetics of a functionalised polychloroprene for ionic exchange membrane. Inter J Appl Res Tech. 2016; 5: 84-90.
- Idibie CA, Abdulkareem AS, Pienaar HCvZ, Iyuke SE, Dyk L. Van. Synthesis of low methanol permeation polymer electrolyte membrane from polystyrene-butadiene rubber. Polym Plast Technol Eng. 2009; 48: 1121-1129.
- Bishop MT, Karasz FE, Russo PS, Langley KH. Solubility and properties of a polyarylether keton in strong acids. Macromolecules. 1985; 18: 86-93.
- Bailly C, Williams D, Karasz FE, Macknight WJ. The sodium salts of sulphonated poly(aryl-ether-ether-ketone) (PEEK): Preparation and Characterization. Polymer. 1987; 28: 1009-1016.
- Bebin P, Caravanier M, Galiano H. Nafion/Clay –SO3H membrane for proton exchange membrane fuel cell application. J Memb Sci. 2005; 278: 35- 42.
- Sageetha D. Conductivity and solvent uptake of proton exchange membrane based on polystyrene (ethelene-butadiene) polystyrene triblock polymer. Eur Polym J. 2005; 41: 2644-2652.
- Parnian MJ, Gashoul F, Rowshanzamir S. Studies on the SPEEK membrane with low degree of sulfonation as astable proton exchange membrane for fuel cell applications. IJHFC. 3; 3: 221-232.
- Huange RYM, Shao P, Burns CM, Feeng X. Sulfonation of poly (Ether Ether Keton) PEEK): Kinetic study and characterization. J Appl Polym Sci. 2001; 82: 2651-2660.