Shear Mixer-Assisted Oxidative Desulfurization of Natural Gas Condensate in a Gas-Liquid Oxidation System
- 1. Department of Chemical Engineering, Faculty of Engineering, Ferdowsi University of Mashhad, Iran
Abstract
In this study, desulfurization of Natural gas condensate was carried out using a novel method consisting of a shear mixer in the gas-liquid oxidation system. The desulfurization process was performed in two stages of oxidation and extraction. In the oxidation step, a mixture of HNO3 , H2 SO4 and NO2 with different amounts was used simultaneously as oxidizing agent and a shear mixer with 5000 rpm for 30 minutes was used to increase mass transfer between gas and liquid phases (between gas and immisible liquid phases). The extraction step was performed by water and caustic solution. In experiments, very low amounts of oxidants (mole basis); HNO3 :0.031 to 0.619, NO2 : 0.031 to 0.619 and H2 SO4 : 0.45 to 2.25; were used and the mixing was carried out by the shear mixer. The results show that even with very low amounts of oxidants, the effect of the shear mixer is very significant, so that the amount of sulfur in condensate decreased from 2300 ppm to 201 ppm, its conversion is equal to 91%. It is worth mentioning that the effect of simultaneous use of HNO3 , H2 SO4 and NO2 as oxidant in the process of sulfurization, is much more than single or double use of these oxidants. In addition, the use of shear mixer in this system, has a significant effect on increasing the mass transfer rate and reducing sulfur content than conventional mixer.
Keywords
• Oxidation System; Heterogeneous Liquid; Desulfurization; Organic Hydro Peroxides; Octane Number
Citation
Pouladi B, Fanaei MA, Baghmisheh G (2025) Shear Mixer-Assisted Oxidative Desulfurization of Natural Gas Condensate in a Gas-Liquid Oxidation System. Chem Eng Process Tech 10(3): 1107.
INTRODUCTION
Sulfur content of petroleum products is highly undesirable and must be strictly regulated. The combustion of fuels with sulfur results in sulfur oxide release which is toxic, corrosive and pollutes the atmosphere. Among other sulfur oxides, SO2 is more abundant and can cause sulfate aerosol formation in the atmosphere. Crude oil contains sulfur in the form of sulfides such as thiols, thiophenes, substituted benzo- and dibenzothiophenes, etc [1].
Hydro-desulfurization (HDS) process have been applied in the refinery industry for removing sulfur- containing compounds from fuels. However, one of the important challenges in the conventional refinery hydrogenation plants is removing the aromatic sulfur compounds such as thiophene (T), benzothiophene (BT), dibenzothiophene (DBT), 4,6-dimethyldibenzothiophene (4,6-DMDBT), and their derivatives which are cannot be separated with HDS [2,3].
It is necessary to say that HDS requires high temperatures (300–400oC), high pressures (up to 100 atm), and high hydrogen consumption. Therefore, this process needs high investment and operating cost [2,4]. In addition to the HDS, several new processes have been developed as HDS alternatives or complementary processes to remove sulfur-containing compounds such as electrochemical oxidation, extraction with ionic liquids, oxidative desulfurization (ODS), selective adsorption, bio desulfurization, extractive desulfurization, adsorptive desulfurization and desulfurization by supercritical water. Most of these alternative methods have not been economically justified on a commercial scale [5,6]. Among different HDS alternative processes, ODS process is more attractive because of mild operating conditions and high ability in sulfur removal [7].
ODS process has two stages: i) the sulfur-containing compounds in the fuel are oxidized to their corresponding sulfoxides and sulfones, and ii) the polar oxidized products are separated using different separation processes such as extraction and adsorption [8]. In order to prevent the oxidation of other compounds of the feedstock, which lead to an increase in the hydrocarbon loss and reduction of cetane and octane number, the oxidation system should be selective toward sulfur-containing compounds [7]. In the ODS process, several types of oxidation systems have been considered and can be categorized into four groups: heterogeneous liquid, homogeneous liquid, gas-liquid and solid oxidants [3, 8-11]. In this study the gas-liquid system is used. In the ODS process, single oxidants were used by some researchers, such as nitric acid [12-15], sulfuric acid [10, 16], hydrogen peroxide [17], ozone [18], organic hydro peroxides [3], molecular oxygen [19], air [20], potassium ferrate [21], and Fenton [22]. Combination of two agents was used in some other ODS process system, one as oxidant and another as catalyst [9]. Using of hydrogen peroxide with formic, nitric, acetic, and phosphoric acids [23-26], are the general examples of mentioned binary agents. Furthermore, for oxidation yield improvement via hydrogen peroxide, several solid catalysts have been used such as vanadium oxide/aluminum oxide [9], or alumina-supported polymolybdates [27,28]. Moreover, to improve the sulfur removal efficiency through activation of oxidation process, high energy radiations such as UV, plasma and microwave may be employed [9,29-32]. Shear mixers (SM), also well-known as rotor–stator mixers, shear reactors and shear homogenizers, are typical of high shear rates, high rotor tip speeds, highly localized energy loss rates near the mixing head, and relatively higher power consumptions than conventional mechanically stirred vessels. Shear mixers are attributed to the centrifugal forces generated from the relative motion between the rotor and the stator equipped with narrow spacing and have been widely used in processes with energy consumption such as dispersion, homogenization, grinding, emulsification and dissolving in the fields of food-manufacturing, agricultural and, chemical reaction processes, etc. There are various geometries in the SMs design, which can be mainly classified into batch units and in-line. Batch units have either radial-discharged or axial- discharged types while the commercial in-line SMs are usually designed as either the rotor–stator teethed or the blade-screen configuration [33-36]. One of the advantages of SMs is intensification of chemical reaction processes with fast inherent reaction rates, but somewhat slow mass transfer rates, because of their local intense turbulence in the small shear gap with a short residence time. Heat and mass transfer for heterogeneous multiphase reactions can be boosted as a result of the nearly large interphase areas provided by shear mixing [33].
In this study, desulphurization of gas condensate (South Pars Gas Field in IRAN) in a gas-liquid system was investigated. Sulfuric and nitric acid as a liquid oxidants in the presence of NO2 as a gas oxidant were used. The effective mixing of these powerful, effective and inexpensive oxidants was done using a shear mixer as a novel method. First, desulfurization of sour gas condensates was performed with different amounts of oxidant mixtures with a conventional mixer and shear mixer to evaluate the effect of each mixing method in the same amount of oxidants. After performing various experiments in the presence of oxidants, as shown in the figure, with respect to total sulfur comparison, the use of shear mixer was more efficient and had a significant effect on total sulfur reduction, and it was determined that due to the limitation of mass transfer in The oxidation section, the shear mixer, has been able to significantly remedy this problem.
During the experiments, after adding H2 SO4 and HNO3 to the condensate, the mixture was converted into a two phase mixture and the oxidants immediately accumulated at the bottom of the reactor and there was not enough time to contact with condensate to polarize the sulfur compounds. Therefore, the use of a shear mixer in this oxidation system, due to its proper function, resulted in suitable mixing and rotation, without vortex and also with uniformity of mixture, due to their high- density difference at the top and bottom of the mixture .By increasing the collision between two phases, the amount of mass transfer in the oxidation section was increased and with the least amount of oxidants, the maximum efficiency could be generated in this system.
After selecting the appropriate contactor according to the gas-liquid oxidation system used, sulfuric initial tests began by using sulfuric acid. But no significant success was achieved in the removal of sulfur compounds. The same experiments were performed with nitric acid and still failed to reduce the total sulfur content. After that, a mixture of the nitric acid with sulfuric acid was studied and to some extent reduces total sulfur reduced. Subsequently, desulfurization of condensation was performed with a small amount of sulfuric acid and nitric acid in the presence of a very low NO2 content, which resulted in very good result due to the low amounts of oxidants, which is discussed below.
EXPERIMENTAL SECTION
Materials
Required materials include Industial H2 SO4 (98 wt %) and Ethanol (96.5 vol%) (Iran, Taghtir-Khorasan Co) on available, Merck HNO3 (65 wt %) and NO2 in situ provided and used in all experiments. Distilled water was used in all tests for first stage wash and NaOH (10 wt %) was used in second stage wash.Sure Natural Gas Condensate as the feedstock with total sulfur content of 2300 ppmw with physical specifications according to Table1, was obtained from the South Pars Gas Complex Co Laboratory. (Assaluyeh, boushehr, Iran). The specifications of Sure Natural Gas Condensate are shown in Table1.
Table 1: Specifications of Sure Natural Gas Condensate.
|
CHARACTERISTICS |
UNITS |
RESULT |
TEST METHOD |
|
Specific Gravity @ 15.56 /15.56 °C |
--- |
0.7358 |
ASTM D4052 |
|
API Gravity |
°API |
60.8 |
|
|
Sulfur Content (Total) |
wt.% |
0.271 |
ASTM D4294 |
|
H2S Content |
ppm |
<1 |
UOP 163 |
|
Mercaptan Content |
wt.% |
0.13 |
|
|
Nitrogen Content (Total) |
ppm |
<10 |
ASTM D4629 |
|
Water Content |
vol.% |
<0.025 |
ASTM D4006 |
|
Salt Content |
P.T.B |
<1 |
ASTM D3230 |
|
Hydrocarbon Types: |
|
|
|
|
Saturates |
vol.% |
88.4 |
ASTM D1319 |
|
Olefins |
vol.% |
1.2 |
|
|
Aromatics |
vol.% |
10.4 |
|
|
Kinematic Viscosity @ 0 °C |
mm2 /s |
1.095 |
|
|
Kinematic Viscosity @ 10 °C |
mm2 /s |
0.944 |
ASTM D445 |
|
Kinematic Viscosity @ 20 °C |
mm2 /s |
0.835 |
|
|
Cloud Point |
°C |
-31 |
ASTM D2500 |
|
Pour Point (Upper) |
°C |
-54 |
ASTM D97 |
|
Reid Vapor Pressure |
psi |
9.92 |
ASTM D5191 |
|
Wax Content |
wt.% |
0.3 |
BP 237 |
|
Corrosion Copper Strip (3h/50°C) |
--- |
1b |
ASTM D130 |
|
Total Acid Number |
mg KOH/g |
<0.05 |
ASTM D 664 |
|
Aniline Point |
°C |
61 |
IP2 |
|
Molecular Weight |
g/mol |
136.3 |
Osmomat |
|
Saybolt Color |
--- |
20.6 |
ASTM D156 |
|
Bromine Index |
mg Br2/100 g |
743 |
IP 130 |
|
Lead Content |
mg/kg |
<1 |
ASTM D 5863 |
|
Distillation I.B.P (oC) |
°C |
30.1 |
ASTM D86 |
|
10% Evaporated (oC) |
°C |
58.9 |
ASTM D86 |
|
20% Evaporated (oC) |
°C |
78.8 |
ASTM D86 |
|
50% Evaporated (oC) |
°C |
131 |
ASTM D86 |
|
90% Evaporated (oC) |
°C |
272.3 |
ASTM D86 |
Method of the analysis
Determination of total sulfur content of sour natural gas condensate done by Varian CP-3800. the total sulfur detection range of CP-3800 is 0 –5 wt %. CP-3800 determines total sulfur in petroleum products by using energy dispersive X-ray fluorescence (EDXRF) method, which is an accurate non-destructive economical and quick method assigned in ISO 8754 and ASTM D4294-03. All total sulfur analysis was carried out in the central laboratory of South pars gas complex Refinery (asalouyeh, Iran).Varian CP-3800 gas-chromatography with specification as shown in Table 2 is used:
Table 2: Specification of gas-chromatography.
|
Parameter |
Condition |
|
Column Column Type Column Length |
VF-5ms 60 m |
|
Columnd ID (Internal Diameter) Column ODOutside Diameter Column Film Thickness flow Detector PFPD Injector split/splitless split ratio Injection volume Oven temperature |
0.25 mm 0.39 mm 0.25 µm 2 ml/min 280 oC 280 oC 50 to 1 1µl
80 oC 10 oC/min 280 oC (15 min hold) |
SYSTEM AND PROCEDURE
Combination of Sulfuric and Nitric Acids as liquid reagents in presence of NO2 as gas oxidant was used. Due to the high density of sulfuric and nitric acids to the condensate and the nature of two-phase mixtures and also the problem of mass transfer in the oxidation stage, Shear mixer used to overcome the problems of phase separation and mass transfer to achieve an appropriate contact surface between oxidants and Natural Gas Condensate. In previous studies, ultrasonic and microwave have been used to increase mass transfer between the fuel and catalyst (acids as catalyst) used (moaseri and et.) but in this experimental study, shear mixer is used with the aim of mass transfer increasing.
Shear mixer at high speed rotation of the rotor, creates a powerful suction that can pull the condensate and oxidants into the work head. Due to exit of mixture from the grids of work head, an extreme hydraulic shear force created into the mixture. High speed extruding of the mixture from the grids and severity of the collision with the reactor wall, increase the temperature of the mixture about 50 55°C. this process occurs continuously and simultaneous rotation at the top (without Vortex) and low level of mixture Leads to a circle mixing. the use of shear mixer strongly increases the contact surface and consequently mass transfer surface in constant volume will increase. Increase in mass transfer surface between condensate and oxidants is the amazing result of this method. The ODS process chemistry is oxidizing divalent sulfur by the electrophilic addition reaction of oxygen atoms to form the hexavalent sulfur in a sulfone functionality. The different sulfur-containing compounds reactivity in this oxidation system is dependent on the electron densities on the sulfur atom of these compounds. The electron density on the sulfur atom of typical sulfur-containing compounds exist in the condensate is high enough to oxidize these compounds in the sulfuric acid-nitric acid oxidation system. Component analysis of gas oil is similar to sure natural gas condensate and Otsuki, S presented above system for chemistry of ods process for gas oil.
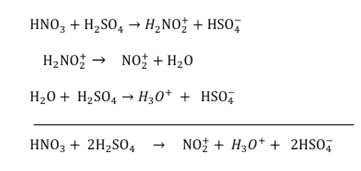
The data presents in Table 3 shows the sulfur containing compounds that can be exist in gas oil [36]. high standard reduction potential of nitronium cation produced during mixing of HNO3 with H2 SO4 is the main the reason of high efficiency of the HNO3 /H2 SO4 solution that provides larger oxidative tendency. the standard reduction potentials of H2 SO4 and HNO3 are 0.16 and 0.957, respectively [37,38]. Formation of nitronium cation (NO2 +) is the result of Addition of HNO3 to H2 SO4 based on the following reaction:.
The nitronium cation is hardly strong oxidation agent with the standard reduction potential of +1.6 V at pH = 7.0 [39]. The main ability of this cation is oxidizing sulfur component even strictly hindered and stable one such as benzene ring with an attached sulfur therefore, this strong cation can extremely increase the efficiency.
Table 3: Different sulfur-containing compounds present in the Agas oil feedstock [36].
|
Sulfur-containing Compounds |
MW |
|
1,3-Propanedithiol |
108 |
|
Methylphenyl sulfide |
124 |
|
Benzothiophene |
134 |
|
1,5-Pentanedithiol |
136 |
|
1-Octanethiol |
146 |
|
5-Methylbenzothiophene |
148 |
|
3-Methylbenzothiophene |
148 |
|
4-Methylthiophenol |
156 |
|
2-Naphtalenethiol |
160 |
|
3,5-Dimethyl benzothiophene |
162 |
|
Di-tert-butyl disulfide |
178 |
|
Dibenzothiophene |
184 |
|
2,3,4,6-Tetramethyl benzotiophene |
190 |
|
3-Methyl dibenzotiophene |
198 |
|
1-Methyl dibenzotiophene |
198 |
|
4-Methyl dibenzotiophene |
198 |
|
8-Methylnaphtho[2,1-b]thiophene |
198 |
|
1-Dodecanethiol |
202 |
|
2,8-Dimethyl dibenzotiophene |
212 |
|
1,6-Dimethyl dibenzotiophene |
212 |
|
4,6-Dimethyl dibenzothiophene |
212 |
|
3,4-Dimethyl dibenzotiophene |
212 |
|
4,9-Dimethyl [2,3-b] napthotiophene |
212 |
|
Thianthrene |
214 |
|
1-Octadecanethiol |
286 |
|
1-Docosanethiol |
342 |
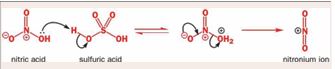
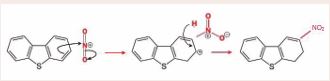
of desulfurization of H2 SO4 through both improving the efficiency of desulfurization reaction of H2 SO4 and oxidizing the sulfur compounds with high resistance to H2 SO4 [9]. According to Figure 1, The first steps are the formation of a very powerful electrophile, none other than NO2+, by the interaction of the two strong acids. Sulfuric acid is the stronger and it protonates the nitric acid on the OH group so that a molecule of water can leave [40], by nitration, aromatic components can be converted to nitrodibenzothiophene using NO2 + from HNO3 + H2 SO4 (Figure 2). Nitronium ion (NO2 +) is linear with an sp hybridized nitrogen at the centre. It is isoelectronic with CO2 . It is also very reactive and combines with dibenzothiophene according to the above picture. dibenzothiophene attacks the positively charged nitrogen atom but one of the N=O bonds must bebroken at the same time to avoid five-valent nitrogen.
Figure 1 Formation of the electrophile [40].
Figure 2 Nitration of dibenzothiophene.
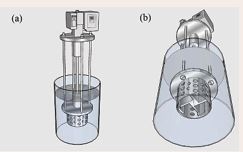
Figure 3 Schematic representation of the reactor and shear mixer applied in the experiments: (a) front view, and (b) bottom view.
Oxidation Procedure
The ODS experiments were performed by treating Sour Natural Gas Condensate with oxidative agents of H2 SO4 and HNO3 combination in presence of NO2 , under different Oxidant agent Ratio, with shear mixer assisted in batch class reactor. Figure 3 indicates a schematic of the applied reactor and shear mixer. The shear mixer consisted of a 330 W motor (HMA-22, Hertz) with a maximum speed of 18000 rpm and a high performance inverter (VFD-M, Delta). All the equipments installed in the shear mixer such as rotor, shaft, machine mounting, mixer frame, and stator were made of stainless steel. In each oxidation run, 1500 gr of Sour Natural Gas Condensate with a total sulfur content of 2300 ppmw was introduced into a glass reactor equipped with a shear mixer and thermometer. The shear mixer was placed in a reactor with ambient temperature and atmospheric pressure and an appropriate amount of H2 SO4 and HNO3 combination was added to the reactor in presence of NO2 . The reaction mixture was continuously mixed with shear mixer in 5000 rpm speed and during the reaction time for oxidation section was 30min and during the course of the reaction the temperature raise to about 60- 68oC due to performance of shear mixer and intensification touch glass reactor wall. Figure 4 shows using of shear mixer oxidation and its performance.
Extraction Procedure
Hydrocarbon from oxidation stage, was extracted in two stages in ambient temperature and atmospheric pressure 250 grams of oxidized condensate was used to extract. In each extraction stage. At first stage a solution containing oxidized condensate and Distilled water as solvent at solvent/condensate ratio equals to 0.25 by weight was prepared. This solution was shaken vigorously for 15 minutes assisted by a mixer.in the second stage the mixture was introduced to a separatory funnel for removing aqueous phase from solution. Then Costic at the concentration of 10 percent, was added to hydrocarbon phase 25% by weight and stirred by mixer for 15 minutes and then in a separatory funnel, 2 phases of solution separate and filtered. Figure 5 shows schematic of extraction stage. Total sulfure of hydrocarbon phase was measured in laboratory.
Figure 4 A: Before oxidation stage, B: After oxidation stage,C: flow patern of shear mixer performance.
Figure 5 Extraction stage.
RESULTS AND DISCUSSION
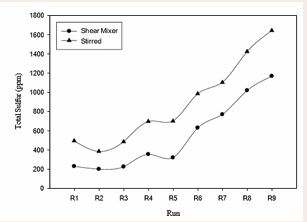
Figure 6 Comparison between shear mixer and stirred mixer
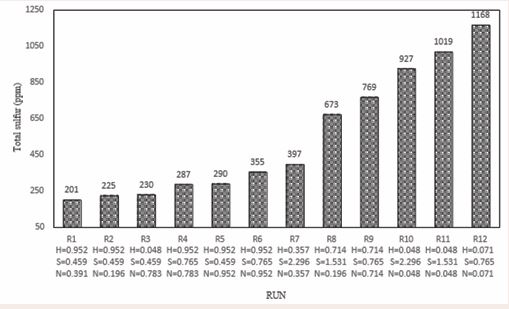
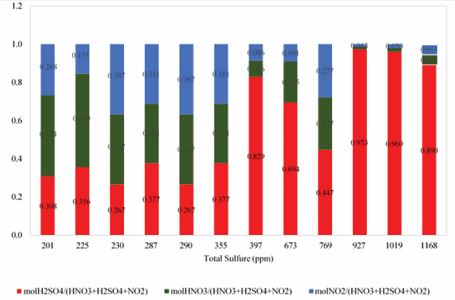
In Figure 6, number of 9 experiments with shear mixer at 5000rpm and with astirred mixer at 900 rpm with identical conditions and the same percentages of oxidants were investigated. In the R2 test, due to the proper amounts of oxidants, the removal of sulfur compounds with shear mixer was 91.26% with total sulfure equal to 201 ppm and with stirred mixer was 82.8% with total sulfure equal to 385 ppm. these results indicating the high impact of the shear mixer than the stirred mixer in sulfur removing. Figure 7 shows that various mole amounts of oxidants such as nitric acid (H), sulfuric acid (S) and nitrogen dioxide (N) in the oxidation system of this study have been used. As seen, in the R1 experiment (H = 0.952, S = 0.459, N = 0.391) and the R12 test (H = 0.071, S = 0.765, N = 0.071), the lowest and highest total sulfur and converting percent is observed. According to the diagram, the increase in the molar content of each oxidant will not have a significant positive effect on the reduction of total sulfur content and, given the simultaneous use of all three oxidants and their effects on each other, the appropriate and desirable values of each oxidant should be obtained. For example in the constant molar value H = 0.952 and S = 0.459 total sulfur variations can be investigated in different values of N. As it is evident, in the experiments R1, R2, R5, with the increase in values of N, the total amount of total sulfur did not decrease, as well as increase in total sulfur content with the decrease of N values. it seems that the optimum amount of molar N to reduce total sulfur to 200 ppm, is N = 0.391 in the R1 test. It is also observed in constant molar value of S = 0.459, in experiments R1, R2, R3, and R5, the increase and decrease in N and H values, both of which are nitrogenous, have a significant effect on the reduction of total sulfur content, so that in the R3 test, the lowest molecular value H = 0.048 was in comparison to other tests in the oxidation system in the same amount of H, with the increase of N to 0.783, total sulfur content was 230 ppm. By reviewing the R1 test, we can see that with decreasing N and H, we will have the highest total sulfur reduction. According to studies conducted in the presence of constant molar values of each of the oxidants, the N and H values depend on each other, and with each of them increasing, another reduction can be compensated. However, low N-values seem to have a more significant effect on total sulfur reduction.
Figure 7 The effect of different amounts of oxidant in the oxidation system in 12 test.
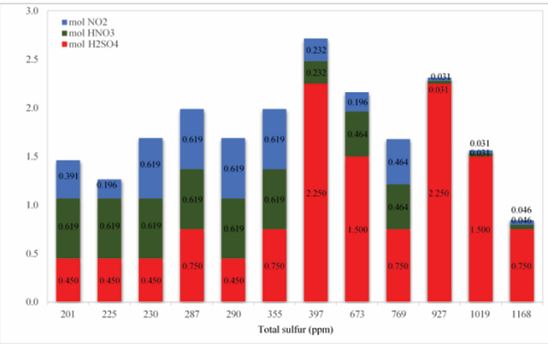
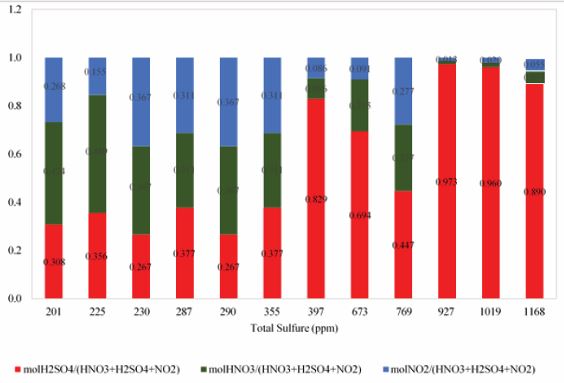
But deciding what to do about each one, will be based on price and accessibility for use in the oxidation system. Due to the presence of nitrogen oxides (N, H), the increase in S will reduce the oxidation efficiency of the oxidation system, and its molecular amount in this oxidation system should be chosen optimally, which is less than H and more than N. The presence of nitrogen oxidants can help to improve the solubility of various sulfur compounds oxidized in sulfuric acid. sulfuric acid plays a solvent role in the oxidation system in addition to its oxidizing properties, which also improves the oxidation process at extraction stage with lower amount of solvent. Based Figure 8 and 9, minimizing the total sulfur content does not require the use of maximum amounts of oxidant. As can be seen, with the lowest amount of sulfuric acid consumed along with the proper amounts of other oxidants, the best result is obtained in desulfurization. Figure 10 shows that with very low oxidant weight ratios versus total oxidant weight, we can achieve excellent results in desulphurization of condensate. At the lowest total sulfur content, the weight ratio of total oxidants to the weight of condensate was 0.082% by weight. these very low amounts of oxidants, in addition to maintaining oxidant properties, will also be economically feasible.
GC-PFPD CHROMATOGRAMS
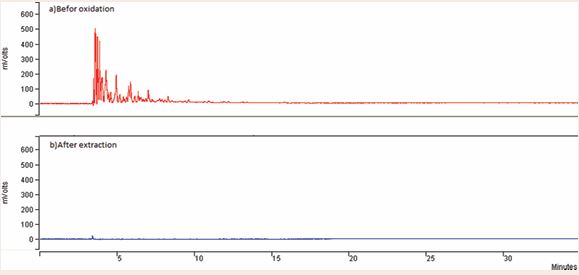
Figure 11 indicates the GC-PFPD chromatograms of gas condensate before and after the ODS process related to sample that its total sulfur after oxidation is equal to 201 ppm. As shown, the peaks of sulfur- containing components are obvious in the untreated sample. With respect to Figure 11(a) is the result of GC for untreated gas condensate. In Figure 11(b) the peaks related to these components disappeared in the treated gas condensate which implies that the process was fulfilled successfully. It should be noted that in order to ensure the accuracy of the GC results, 1 ml of benzothiophene solution at 100 ppm concentration was added to both untreated and treated samples as the internal standard. It was observed that the raw sample exhibited 70 peaks in addition to the peak assigned to the internal standard while only the internal standard peak appeared in the chromatogram of the treated sample.
Figure8 Variation of total sulfur with different amounts of mixture of oxidants.
Figure 9 Proportion of each of oxidant mole to total moles of oxidants.
Figure 10 Change of total sulfure versus change in weight of oxidant to weight of condensate.
Figure 11 GC-FPD chromatograms of the (a) untreated gas condensate, and (b) treated gas condensate after oxidation and subsequent extraction
CONCLUSION
In this research, a new method for the desulfurization of gas condensates was introduced. It was noted that the lime mixer has a higher efficiency in the oxidation process than the conventional mixer due to the increase of the mass transfer surface between the two organic and acid phases. 2. 2- Also desulphurization with high efficiency can be achieved by using a combination of oxidants including HNO3 , H2 SO4 and NO2 in very low amounts. 3. The results show that the maximum use of oxidants in the oxidation process does not necessarily have favorable results. 4. Also, the interaction effect of oxidant and their increasing and decreasing amount have significant effects on desulfurization of gas condensate. 5. The presence of nitrogenous compounds such as NO2 and HNO3 will have a significant effect on the desulphurization process.
REFERENCES
- Julião D, Gomes AC, Pillinger M, Cunha-Silva L, de Castro B, Gonçalves IS, et al. Desulfurization of model diesel by extraction/oxidation using a zinc-substituted polyoxometalate as catalyst under homogeneous and heterogeneous (MIL- 101(Cr) encapsulated) conditions. Fuel Processing Technology. 2015; 131: 78-86.
- Banisharif F, Dehghani MR, Capel-Sánchez M, Campos-Martin JM. Desulfurization of Fuel by Extraction and Catalytic Oxidation Using a Vanadium Substituted Dawson-Type Emulsion Catalyst. Ind Eng Chem Res. 2017; 56: 3839-3852.
- Jiang Z, Hongying L, ZHANG Y, Can L. Oxidative desulfurization of fuel oils. Chin J Catal. 2011; 32: 707- 715.
- Mafi M, Mokhtarani B, Dehghani MR. Removal of thiophene from model diesel oil with nitrate based ionic liquids at several temperatures. J Mol Liq. 2016; 221: 1104-1110.
- Jalali MR, Sobati MA. Intensification of oxidative desulfurization of gas oil by ultrasound irradiation: Optimization using Box–Behnken design (BBD). Appl Therm Eng. 2017; 111: 1158-1170.
- Srivastava VC. An evaluation of desulfurization technologies for sulfur removal from liquid fuels. RSC Advances. 2012; 2: 759-783.
- Campos-Martin JM, Capel-Sanchez MC, Perez-Presas P, Fierro J. Oxidative processes of desulfurization of liquid fuels. J Chem Technol Biotechnol. 2010; 85: 879-890.
- Khodaei B, Sobati MA, Shahhosseini S. Rapid oxidation of dibenzothiophene in model fuel under ultrasound irradiation. Monatshefte für Chemie - Chemical Monthly. 2016; 148: 387-396.
- Moaseri E, Shahsavand A, Bazubandi B. Microwave-Assisted Oxidative Desulfurization of Sour Natural Gas Condensate via Combination of Sulfuric and Nitric Acids. Energy & Fuels. 2014; 28: 825-831.
- Moaseri E, Mostaghisi O, Shahsavand A, Bazubandi B, Karimi M, Ahmadi J. Experimental study and techno- economical evaluation of Khangiran sour natural gas condensate desulfurization process. J Nat Gas Sci Eng. 2013; 12: 34-42.
- Carnaroglio D, Gaudino EC, Mantegna S, Moreira EM, Vicente de Castro A, Flores EM, et al. Ultrasound- assisted oxidative desulfurization/ denitrification of liquid fuels with solid oxidants. Energy & Fuels. 2014; 28: 1854-1859.
- Ehsani MR. Desulfurization of tabas coals using chemical reagents.Iran J Chem Chem Eng (IJCCE). 2006; 25: 59-66.
- Yu C, Fan X, Yu L, Bandosz TJ, Zhao Z, Qiu J. Adsorptive removal of thiophenic compounds from oils by activated carbon modified with concentrated nitric acid. Energy & Fuels. 2013; 27: 1499-1505.
- Yu G, Jin M, Sun J, Zhou X, Chen L, Wang J. Oxidative modifications of rice hull-based carbons for dibenzothiophene adsorptive removal. Catalysis today. 2013; 212: 31-37.
- Pietrzak R, Wachowska H. The influence of oxidation with HNO 3 on the surface composition of high-sulphur coals: XPS study. Fuel processing technology. 2006; 87: 1021-1029.
- Nehlsen J, Benziger J, Kevrekidis I. Oxidation of aliphatic and aromatic sulfides using sulfuric acid. Ind eng chem res. 2006; 45: 518-524.
- Sato K, Hyodo M, Aoki M, Zheng XQ, Noyori R. Oxidation of sulfides to sulfoxides and sulfones with 30% hydrogen peroxide under organic solvent-and halogen-free conditions. Tetrahedron. 2001; 57: 2469-2476.
- Wang J, Zhao D, Li K. Oxidative desulfurization of dibenzothiophene using ozone and hydrogen peroxide in ionic liquid. Energy & Fuels. 2010; 24: 2527-2529.
- Kharasch MS, Nudenberg W, Mantell GJ. REACTIONS OF ATOMS AND FREE RADICALS IN SOLUTION. XXV. THE REACTIONS OF OLEFINS WITH MERCAPTANS IN THE PRESENCE OF OXYGEN1. J Organic Chem. 1951; 16: 524-532.
- Guo W, Wang C, Lin P, Lu X. Oxidative desulfurization of diesel with TBHP/isobutyl aldehyde/air oxidation system. Applied energy. 2011; 88: 175-179.
- 21.Yanxiu L, Hua S, Wenchao Z. Oxidative desulfurization of model sulfur compound by potassium ferrate in the presence of phosphomolybdic acid catalyst. China Petroleum Processing and Petrochemical Technology. 2013; 15: 61-65.
- Dai Y, Qi Y, Zhao D, Zhang H. An oxidative desulfurization method using ultrasound/Fenton's reagent for obtaining low and/or ultra- low sulfur diesel fuel. Fuel processing technology. 2008; 89: 927-932.
- Otsuki S, Nonaka T, Takashima N, Qian W, Ishihara A, Imai T, et al. Oxidative Desulfurization of Light Gas Oil and Vacuum Gas Oil by Oxidation and Solvent Extraction. Energy & Fuels. 2000; 14: 1232- 1239.
- Ali MF, Al-Malki A, El-Ali B, Martinie G, Siddiqui MN. Deep desulphurization of gasoline and diesel fuels using non- hydrogen consuming techniques. Fuel. 2006; 85: 1354-1363.
- Yuping S, Jian S. STUDY ON THE CATALYTIC OXIDATIVE DESULFURIZATION OF MODEL GASOLINE OVER NIOBIC ACIDCATALYST. Petroleum Processing and Petrochemicals. 2011; 10: 024.
- Ali MF, Al-Malki A, Ahmed S. Chemical desulfurization of petroleum fractions for ultra-low sulfur fuels. Fuel processing technology. 2009; 90: 536-544.
- García-Gutiérrez JL, Fuentes GA, Hernández-Terán ME, Murrieta F, Navarrete J, Jiménez-Cruz F. Ultra-deep oxidative desulfurization of diesel fuel with H2O2 catalyzed under mild conditions by polymolybdates supported on Al2O3. Applied Catalysis A: General. 2006; 305: 15-20.
- Abdullah WNW, Ali R, Bakar WAWA. In depth investigation of Fe/ MoO3–PO4/Al2O3 catalyst in oxidative desulfurization of Malaysian diesel with TBHP-DMF system. J Taiwan Ins Chem Eng. 2016; 58: 344-350.
- Lu R, Yang J, Xu X, Gao J. Microwave-chemical desulphurization of sulfurous crude oil. Petroleum Science and Technology. 2009; 27: 1789-1799.
- Hirai T, Shiraishi Y, Komasawa I. Desulfurization Process for Light Oil by Photochemical Reaction and Liquid-Liquid Extraction: Removal of Benzothiophenes and Alkyl Sulfides. J chem eng Japan. 1997; 30: 173- 175.
- Matsuzawa S, Tanaka J, Sato S, Ibusuki T. Photocatalytic oxidation of dibenzothiophenes in acetonitrile using TiO2: effect of hydrogen peroxide and ultrasound irradiation. J Photochem Photobiol A Chem. 2002; 149: 183-189.
- Wang D, Qian EW, Amano H, Okata K, Ishihara A, Kabe T. Oxidative desulfurization of fuel oil: Part I. Oxidation of dibenzothiophenes using tert-butyl hydroperoxide. Appl Catal A General. 2003; 253: 91- 99.
- Zhang J, Xu S, Li W. High shear mixers: A review of typical applications and studies on power draw, flow pattern, energy dissipation and transfer properties. Chem Eng Pro. 2012; 57: 25–41.
- Paul EL, Atiemo-Obeng VA, Kresta SM. Handbook of industrial mixing: science and practice. John Wiley & Sons. 2004.
- Davies J. A physical interpretation of drop sizes in homogenizers and agitated tanks, including the dispersion of viscous oils. Chem Eng Sci. 1987; 42: 1671-1676.
- Sobati MA, Dehkordi AM, Shahrokhi M. Extraction of Oxidized Sulfur- Containing Compounds of Non-Hydrotreated Gas Oil. Chem Eng Technol. 2010; 33: 1515-1524.
- Gorensek MB, Staser JA, Stanford TG, Weidner JW. A thermodynamic analysis of the SO2/H2SO4 system in SO2-depolarized electrolysis. int j hyd ener. 2009; 34: 6089-6095.
- Sharma B. Nuclear and radiation chemistry. Krishna Prakashan Media. 1995.
- Lancaster JR. Nitric oxide: principles and actions. Academic Press. 1996.
- Clyden G. Warren and Wothers, Organic Chemistry, Oxford university. 2001.