Should “Ionic Liquids” also be used for Solid Ionic Compounds? Synthesis and Characterization of p-Anisidinium-Based Ionic Compounds
- 1. Department of Applied Sciences, Durban University of Technology, South Africa
Keywords
• Ionic Liquids
• Ionic Solids
• Melting Point
• p-Anisidinium
Citation
Tokoyi V, Kabane B, Deenadayalu N (2025) Should “Ionic Liquids” also be used for Solid Ionic Compounds? Synthesis and Characterization of p-Anisidinium-Based Ionic Compounds. Chem Eng Process Tech 10(1): 1097.
INTRODUCTION
ILs are continuously being explored because of their beneficial and tuneable physicochemical properties like excellent chemical stability, thermal stability, electrochemical stability, nonflammability, and negligible volatility [1]. They are composed of organic cations coupled with organic or inorganic anions producing organic molten salts in both liquid and solid forms (Figure 1), with a melting point that is usually below 100°C [2].
Figure 1: Common anions and cations used for producing ILs/ISs: [OAc] = Acetate, [N(CN)2] = Dicyanamide, [OTf] = Trifluoromethanesulfonate, [NTf2]= Bis(trifluoromethylsulfonyl)imide, [CF3CO2] = Trifluoroacetate, [BF4] = Tetrafluoroborate, [PF6] = Hexafluorophosphonate [1,2]
They are called ‘green’ and alternatives to organic solvents usually because of their properties being in line with the principles of Green Chemistry. However, there have been a lot of studies that reported potential toxicity [4], environmental hazards, and non-biodegradability of ILs [3,5,6], and this results from certain anions with cations interactions. Owing to their unique properties (especially the flowability of the liquid-state ILs and the commonly produced solid-state), they are versatile and widely used in chemical reaction processes as solvents and extractants [7], catalysts [8], in reaction media [9], lubricants, and batteries [3,10,11].
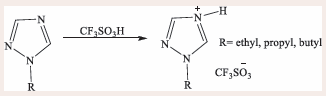
Several studies have been mostly focussing on the syntheses of imidazolium [12-14], pyridinium [4,13], phosphonium [14], and ammonium-based [4,15-18] ILs. Coupling of anions with these types of cations have been reported to produce both solid and liquid states of ILs, and this can be better understood using the structure-activity and cation-anion relationships when designing these ionic compounds. Nagarajan, Shaikh [19], synthesized three triazolium triflate-based acidic ILs with different alkyl chain substituents which resulted into colourless liquid products [Scheme 1].
Scheme 1: Synthesis of 1-alkyl-1,2,4-triazolium triflate.
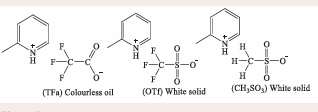
Duan, Gu [13], explored ILs by synthesizing and characterizing based three 2-methylpyridinium compounds with trifluoroacetate (TFa), trifluoromethanesulfonate (OTf) and methanesulfonate (CH3 SO3 ) anions, producing both solid and liquid products shown in Figure 2.
Figure 2: 2-methylpyridinium-based ILs.
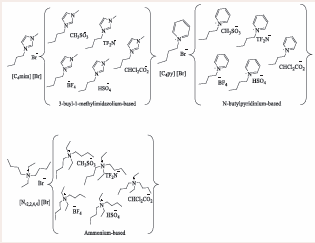
Hassan, Nazir [4], developed a series of ILs using 3-butylimidazolium, butylpyridinium and diethyldibutylammonium as cations, and bromide, methanesulfonate, bis(trifluoromethanesulfonyl) imide and dichloroacetate as ions. A metathesis method was used to produce these compounds and the resulting compounds were either in a liquid or a solid form and are presented in Figure 3.
Figure 3: Series of ionic compounds developed from 3-butyl-1 methylimidazolium, N-butylpyridinium and ammonium-based bromide ILs.
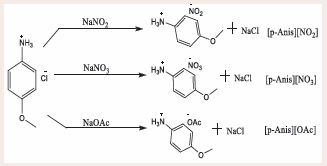
From the previously reported compounds that have been synthesized and characterized, it is quite evident that several cations interact with anions differently, and a variety of anions coupled with the same cation have an overall influence on the state of the compounds [2]. In this study, three ionic compounds bearing a p-anisidinium cation were synthesized using a metathesis approach to produce p-anisidinium nitrite [p-Anis][NO2 ], p-anisidinium nitrate [p-Anis][NO3 ], and p-anisidinium acetate [p-Anis][OAc], which were obtained in a solid form. These compounds were characterized by the application of spectroscopic techniques namely nuclear magnetic resonance (NMR) and Fourier Transform Infrared Spectroscopy (FTIR), along with elemental analysis to confirm the structure and purity of the compounds. The compounds were further investigated for their thermal stability using DSC and TGA, and in addition, the melting point temperatures were determined to support the ISs perspective.
MATERIALS AND METHODS
p-Anisidinium chloride (for synthesis, Sigma Aldrich), sodium acetate (anhydrous, AR, Glassworld), sodium nitrate (anhydrous, AR, Glassworld), sodium nitrite (anhydrous, AR, Glassworld), and chloroform (AR, Radchem)
Synthesis of p-anisidinium-based IS
Using a similar counterion metathesis method previously reported by Hassan, Nazir [4], a required amount of a pre-dried sodium acetate, sodium nitrite or sodium nitrate (0.02 mol) was transferred in a 250 mL round bottom flask (RBF), and then 100 mL of deionized water was added forming a solution. Separately, p-anisidinium chloride (0.02 mol) was dissolved in 50 mL of methanol (MeOH) and added to the mixture dropwise. The reaction was maintained at room temperature overnight and monitored by TLC to completion. After completion, the mixture was extracted with 10 mL (3X) of chloroform, and the organic layer was collected, dried in an oven at 40oC overnight, and then desiccated. The solid product obtained was analyzed using FTIR, 1H−NMR, elemental analysis, TGA and DSC (Figures 4).
Figure 4: Schematic route for the ISs synthesized.
Characterization
The synthesized p-anisidinium-based ISs were characterized using a proton nuclear magnetic resonance (1H-NMR, Brucker Avance, 600 MHz, UKZN). A required amount (~5 mg) of a sample was dissolved in 1.5 mL of deuterated solvent (d6 ). Transform Infrared Spectroscopy (FTIR, Agilent Cary 360, DUT) of the compounds were obtained in the range of 4000-650 cm-1 at a resolution of 4 cm-1 and 120 scans per sample, to obtain optimum, smooth spectra. A required amount of a dried sample (~1 mg) was placed on a metallic attenuated total reflectance (ATR) stage fitted with a diamond. EA (Thermoscientific Flash 2000 organic elemental analyzer, UKZN) was conducted by combustion of a pre-weighed amount of sample (~2 mg). Thermal gravimetric analysis and differential scanning colorimetry (TGA and DSC, Mettler Toledo TGA/ DSC ISF Model1346, DUT) were recorded in a temperature range of 25oC – 800oC, under nitrogen atmosphere. Melting point values of the compounds were determined using a Cole-Parmer stuartTM (DUT) electrothermal melting point apparatus, with a digital thermometer where a required amount (± 2 mg) of a powdered sample was inserted into the glass capillary tube and then placed inside the apparatus.
RESULTS AND DISCUSSION
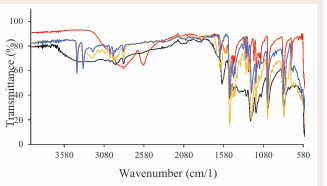
The synthesized ISs were obtained in good yield (87% 93%) and high purity as indicated by the number of protons observed in an 1H-NMR (Figure 5), spectra and EA, and the functional groups (Figure 6), within the compounds were identified using FTIR.
Figure 5: 1H-NMR spectra for the synthesized ISs: (a) [p-Anis][Cl] (Starting Material), (b) [p-Anis][NO2 ], (c) [p-Anis][NO3 ] and (d) [p-Anis][OAc]
Figure 6: FTIR spectra of p-anisidinium based ISs.
Spectroscopic data of the synthesized ISs
p-anisidinium nitrite [p-Anis][NO2 ]
State: Brown solid. Yield: 87%. 1 H-NMR: δH (600 MHz, DMSO, ppm) 3.75 (3H, J = 7.4 Hz), 7.16 (2H), 7.44 (2H), 10.27 (3H). FT-IR: (νmax/cm–1) 3365 (N-H), 3189 (C-H, Ar), 2079 (C=C-H), 1572 (C-N), 1483-1453 (C=C), 1330 (C-O). Elemental Analy.: Estimated: C: 49.41; H:5.92, N: 16.46, Found: C: 45.97, H: 4.94, N: 14.39. Melting point: 147 oC .
p-anisidinium nitrate [p-Anis][NO3 ]
State: Brown solid. Yield: 93%. 1 H-NMR: δH (600 MHz, DMSO, ppm) 3.78 (3H, J = 7.4 Hz), 7.16 (2H), 7.44 (2H), 10.42 (3H). FT-IR: (νmax/cm–1) 3343-3421 (N-H), 3227 (C-H, Ar), 2963 (C=C-H), 1172 (C-N), 1632 (C=C), 2836 (C-O). Elemental Analy.: Estimated: C: 45.16; H:5.41, N: 15.05, Found: C: 42.93, H: 4.06, N: 13.89. Melting point: 167 oC
p-anisidinium acetate [p-Anis][OAc]
State: brown solid. Yield: 90%. 1 H-NMR: δH (600 MHz, DMSO, ppm) 2.56 (3H), 3.89 (3H, J = 7.4 Hz), 4.45 (2H), 6.28 (2H), 8.23 (3H). FT-IR: (νmax/cm–1) 3343-3421 (N H), 3089 (C-H, Ar), 2836 (C=C-H), 1172 (C-N), 1502 (C=C), 2836 (C-O), 1632 (C=O). Elemental Analy.: Estimated: C: 59.04; H:7.20, N: 7.70, Found: C: 57.93, H: 6.76, N: 7.66. Melting point: 97 oC
Thermal properties of the synthesized ISs
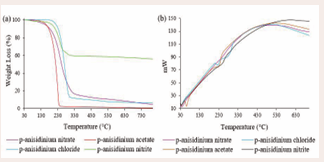
Thermal stability and phase behaviour of the p-anisidinium-based ISs was studied using TGA and DSC, and the thermograms (Figure 7), were recorded and worked out using the STARe software (version 9.20).
Figure 7: TGA (a) and DSC (b) thermograms of p-anisidinium based ISs.
Alkyl substituents, functional groups, coupled anions and cations have an impact on the overall thermal stability or phase behaviour of the resultant ionic compounds, either by decreasing or increasing the decomposition temperatures (Td) [20], and causing a phase change of the compounds to either melt (Tm) or crystallize (Tc) [21]. As per (Figure 7(a)), Td recorded for [p-Anis][Cl], [p-Anis][NO3 ], [p-Anis] [NO2 ], and [p-Anis][OAc], were found to be 301.3, 317.4, 260.5 and 244.2oC, respectively. Thus thermal stabilities of the ISs show the following ascending trend: [p-Anis][OAc] < [p-Anis][NO2 ] < [p-Anis][Cl] < [p-Anis][NO3 ].
In Figure 7(b), all the examined ISs exhibited a glass transition phase (Tg) at 36oC, and upon further heating only [p-Anis][OAc] exhibited a semicrystalline nature while the rest ISs were amorphous. The illustrated heat flow curves at 62oC and 238.1oC for [p-Anis][OAc], 228.0oC and 285.1oC for [p-Anis][Cl], and 252.3oC for both [p-Anis] [NO2 ] and [p-Anis][NO3 ] are associated to the crystalline transition phase tendency.
CONCLUSION
In this study, the perspective of using the term ISs rather than ILs for solid state compounds with higher melting points than common ILs with melting points below 100°C, was proven to be suitable and applicable for NO2 and NO3 −based ionic compounds. Three p-anisidinium based ISs were synthesized using a counterion metathesis approach and characterized to validate their structural C−H framework, purity, and thermal properties. The obtained spectroscopic data for each compound showed a successful preparation, and the obtained melting points are in the range of 97oC to 167oC. From what is commonly known about ILs having melting points below 100oC, it is quite evident from the [p-Anis][NO2 ] and [p-Anis][NO3 ] ionic compounds that the term “ILs” is not applicable but rather “ISs”, except for [p-Anis][OAc] which showed a 97oC melting point. Thermal stabilities and phase behaviour of the compounds were also studied, from the obtained thermograms [p-Anis][NO3 ] was the most thermally stable from all the examined, and all compounds exhibited both a glass transition, amorphous and crystallization phases. Even though the obtained compounds were in a solid state it does not necessarily mean that they can automatically be referred to as ISs because [p-Anis][OAc] which showed a 97oC melting point, the term IL is still applicable. Based on these findings it is imperative to separate the ILs from the ISs despite their physical state. This would be beneficial to researchers that are constantly and continuously exploring these types of material for a variety of applications that require materials to be of specific states for them to be potent.
REFERENCES
- Kinik FP, Uzun A, Keskin S. Ionic liquid/metal-organic framework composites: From synthesis to applications. ChemSusChem. 2017; 10: 2842-2863.
- Philippi F, Welton T. Targeted modifications in ionic liquids - from understanding to design. Phys Chem Chem Phys. 2021; 23: 6993- 7021.
- Stolte S, Steudte S, Igartua A, Stepnowski P. The biodegradation of ionic liquids - The view from a chemical structure. Curr Organ Chem. 2011; 15: 1-28.
- Hassan R, Farzana N, Roosh M, Qaisar A, Habib U, Sajini AA, et al. Synthesis, characterization, biological evaluation, and in silico studies of imidazolium-, pyridinium-, and ammonium-based ionic liquids. Molecules. 2022; 22: 1-20.
- Pernak J, Borucka N, Walkiewicz F, Markiewicz B, Fochtman P, Stolte S, et al. Synthesis, toxicity, biodegradability and physicochemical properties of 4-benzyl-4-methylmorpholinium-based ionic liquids. Green Chemy. 2011; 13: 2901-2910.
- Bizukojc EL, Maton C, Stevens VC. Biodegradation of imidazolium ionic liquids by activated sludge microorganisms. Biodegradation. 2015; 26: 453-463.
- Rajamani S, Santhosh R, Raghunath R, Jadhav AS. Value-added chemicals from sugarcane bagasse using ionic liquids. Chemical Papers. 2021; 75: 5605-5622.
- Ren C, Wang Z, Gao Q, Li Z, Jiang S, Huang Q, et al. Novel Brønsted acidic ionic liquids as high efficiency catalysts for liquid-phase beckmann rearrangement. Catalysts. 2023; 13: 1-16.
- Long G, Wu D, Pan H, Zhao T, Hu X. Imidazolium hydrogen carbonate ionic liquids: Versatile organocatalysts for chemical conversion of CO2 into valuable chemicals. J CO2 Utilization. 2020; 39: 1-6.
- He T, Yan J, Xiao W, Sun J. Latest advances in ionic liquids promoted synthesis and application of advanced biomass materials. Front Chem Sci Engineering. 2023; 17: 798-816.
- Handy ST. Ionic liquids-Classes and Properties. InTech: Janeza Trdine 9, 51000 Rijeka. 2011; 1-313.
- Gazitua M, Fuentealba P, Contreras R , Toledo RO. Lewis acidity/ basicity changes in imidazolium based ionic liquids brought about by impurities. J Phys Chem B. 2015; 119: 13160-13166.
- Duan Z, Gu Y, Zhang J, Zhu L, Deng Y. Protic pyridinium ionic liquids: Synthesis, acidity determination and their performances for acid catalysis. J Mol Catalysis A: Chemical. 2006; 250: 163-168.
- Ajiambo F, Charles N, Namango S. Enhancement of simple sugar production: pretreatment of Gadam sorghum stalks using imidazolium, pyridinium and phosphonium based ionic liquids. Int J Scientific Eng Res. 2017; 8: 471-483.
- Mudzakir A, Anwar B, Sitifatimah S. Ammonium and cholinium based ils as a new chemical modifier for giant bamboo dendrocalamus asper. J Eng Sci Technol. 2021; 16: 1183-1195.
- Jadhav C, Nipate A, Chate A, Gill C. Triethylammonium hydrogen sulfate [Et3NH][HSO4]-Catalyzed rapid and efficient multicomponent synthesis of pyrido[2,3-d]pyrimidine and pyrazolo[3,4-b]pyridine hybrids. ACS Omega. 2021; 6: 18215-18225.
- An YM, Zhuang J, Li Y, Dai JY, Xiu ZL. Pretreatment of Jerusalem artichoke stalk using hydroxylammonium ionic liquids and their influences on 2,3-butanediol fermentation by Bacillus subtilis. Bioresource Technol. 2022; 354: 1-8.
- Nurdin M, Abimanyu H, Putriani H, Setiawan LOMI, Maulidiyah M, Wibowo D, et al. Optimization of OPEFB lignocellulose transformation process through ionic liquid [TEA][HSO4] based pretreatment. Sci Rep. 2021; 11: 11338.
- Nagarajan S, Shaikh TM, Kandasamy E. Synthesis of 1-alkyl triazolium triflate room temperature ionic liquids and their catalytic studies in multi-component Biginelli reaction. J Chem Sci. 2015; 127: 1539-1545.
- Sardar S, Wilfred C, Mumtaz A, Rashid Z, Leveque JM. Synthesis,thermophysical properties, Hammett acidity and COSMO- RS study of camphorsulfonate-based Brönsted acidic ionic liquids. J Mol Liquids. 2018; 271: 621-630.
- Meng Y, Liu J, Li Z, Wei H. Synthesis and physicochemical properties of two SO3H-functionalized ionic liquids with hydrogen sulfate anion. J Chem Engin Data. 2014; 59: 2186-2195.
![Figure 1 Common anions and cations used for producing ILs/ISs: [OAc] = Acetate, [N(CN)2] = Dicyanamide, [OTf] = Trifluoromethanesulfonate, [NTf2] = Bis(trifluoromethylsulfonyl)imide, [CF3CO2] = Trifluoroacetate, [BF4] = Tetrafluoroborate, [PF6] = Hexafluorophosphonate [1,2]](https://www.jscimedcentral.com/public/assets/images/uploads/image-1740742094-1.PNG)
![Figure 5 1H-NMR spectra for the synthesized ISs: (a) [p-Anis][Cl] (Starting Material), (b) [p-Anis][NO2 ], (c) [p-Anis][NO3 ] and (d) [p-Anis][OAc]](https://www.jscimedcentral.com/public/assets/images/uploads/image-1740742214-1.PNG)